Abstract
Mycobacterium ulcerans, the cause of Buruli ulcer, is an environmental mycobacterium with a distinct geographic distribution. The reasons why only some individuals who are exposed to M. ulcerans develop ulcers are not known but are likely to reflect individual differences in the immune response to infections with this bacterium. In this study, we investigated cytokine profiles of peripheral blood mononuclear cells (PBMC) from 23 Buruli ulcer patients and 25 household contacts in a region of Australia where Buruli ulcer is endemic. The results showed that following stimulation with M. ulcerans or Mycobacterium bovis BCG, PBMC from Buruli ulcer patients mounted a Th2-type response, which was manifested by the production of mRNA for interleukin 4 (IL-4), IL-5, IL-6, and IL-10, whereas unaffected contacts responded mainly with the Th1 cytokines gamma interferon (IFN-γ) and IL-12. For example, mRNA for IL-4 was detected in 18 of 23 patients but in only 3 of 25 control subjects (P < 0.0001). By contrast, PBMC from 21 of 25 unaffected individuals produced IFN-γ compared with 3 of 23 patients (P < 0.0001). IFN-γ release following stimulation with mycobacteria was markedly reduced in affected subjects. Frequencies of antibodies to M. ulcerans in serum samples from affected and unaffected subjects were similar, indicating that many of the control subjects had been exposed to this bacterium. Together, these findings suggest that a Th1-type immune response to M. ulcerans may prevent the development of Buruli ulcer in people exposed to M. ulcerans, but a Th-2 response does not.
Buruli ulcer is an emerging infectious disease of humans that is caused by the environmental bacterium Mycobacterium ulcerans (reviewed in reference 14). Buruli ulcer has a limited geographic distribution, but in countries where it is endemic, particularly in West Africa, it imposes a major economic burden on health care. In regions where M. ulcerans is endemic, the prevalence of infection may exceed 22% and exposure is likely to be widespread (15), but the reasons why only some exposed individuals develop ulcers are not known (13).
It was recently reported that patients with Buruli ulcer exhibit profound systemic anergy to mycobacterial antigens, compared to control subjects, as evidenced by significantly lower lymphocyte proliferation and production of gamma interferon (IFN-γ) in response to stimulation with live M. ulcerans or Mycobacterium bovis BCG (7). However, patients with Buruli ulcer do mount an antibody response to M. ulcerans, indicating that the immune response of patients is skewed toward humoral immunity and away from cell-mediated immunity (7).
The histopathology of Buruli ulcer is characterized by extensive cutaneous tissue necrosis with large numbers of extracellular bacteria and a paucity of inflammatory cells (14). The tissue necrosis in these cases has been attributed to an immunosuppressive polyketide toxin, mycolactone, which is produced by M. ulcerans (6, 10). However, these immunosuppressive properties are unlikely to account for the systemic mycobacterium-specific anergy that was observed in the previous study (7). It is also unclear if the systemic anergy to mycobacterial antigens in patients with Buruli ulcer develops as a consequence of the infection or if it reflects an intrinsic immune defect which predisposes individuals who are infected with M. ulcerans to develop symptomatic infection (13).
In the previous study, control subjects were selected who were unlikely to have been exposed to M. ulcerans due to the restricted geographic distribution of the disease (7). Accordingly, the differences observed between controls and affected subjects may have reflected different degrees of exposure to M. ulcerans. In the present study, we have examined the Th1 and Th2 responses of subjects with active or healed Buruli ulcer and those of healthy control subjects living in the same environment. Because such controls were likely to have experienced comparable exposure to M. ulcerans, while remaining free of symptoms, evaluation of their immune responses to M. ulcerans may indicate the nature of protective immunity to Buruli ulcer.
MATERIALS AND METHODS
Study subjects.
Two groups of residents in the Douglas Shire of northern Queensland, Australia, where M. ulcerans is endemic (12), were studied. The affected group comprised 23 patients with Buruli ulcer confirmed by culture. One patient had active disease; the remainder had developed an ulcer between 6 months and more than 40 years previously (median, 6 years; mean, 9.4 years; 95% confidence interval [CI], 4.6 to 14.4) (see Fig. 1). The unaffected group was made up of 25 healthy subjects with no history of Buruli ulcer, each of whom lived in the same house as an affected individual or had close, ongoing contact with such an individual. The affected and unaffected groups did not differ from each other in terms of age distribution (for the affected group, the median age was 52 years, the 95% CI was 41.4 to 56.5, and the range was 4 to 76 years; for the unaffected group, the median age was 49 years, the 95% CI was 42.9 to 56.1, and the range was 15 to 77 years), place of residence, or duration of residence in the region of endemicity.
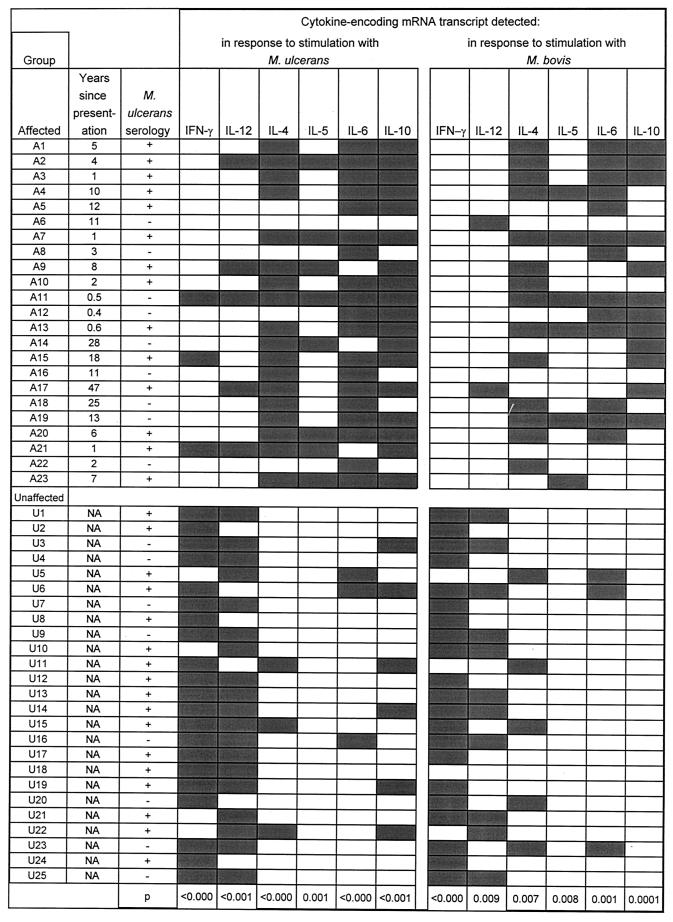
FIG. 1.
Individual cytokine responses of PBMC from affected (A) and unaffected (U) subjects to 6 days of stimulation with M. ulcerans or M. bovis BCG. NA, not applicable; +, M. ulcerans-specific bands detected by immunoblotting; −, no bands detected by immunoblotting; shaded rectangles, positive PCR results (DNA band detected on agarose gels stained with ethidium bromide); open rectangles, negative PCR results (no band detected).
Lymphocyte stimulation and detection of cytokines.
Peripheral blood mononuclear cells (PBMC) were separated from heparinized whole venous blood by using Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation and were stimulated with whole living M. ulcerans (Chant strain, a human isolate) or M. bovis BCG (CSL Limited, Parkville, Victoria, Australia) bacteria as described previously (7). After 6 days, cytokine production was detected by using reverse transcriptase PCR (RT-PCR) as follows. Total RNA was obtained from PBMC by extraction with phenol and chloroform (2). mRNA transcripts for IFN-γ, interleukin-4 (IL-4), IL-5, IL-6, IL-10, and IL-12 were detected by using the Titan One Tube RT-PCR system (Roche Diagnostics, Castle Hill, New South Wales, Australia). RT-PCR for β-actin was used as a control for the presence of intact mRNA in the PBMC extract, and RT-PCR for CD3δ mRNA was used to control for the presence of T lymphocytes. The PCR primers used to amplify the mRNA transcripts encoding specific cytokines are listed in Table 1. Samples were placed in a GeneAmp PCR System 9700 thermocycler (Applied Biosystems) equilibrated at 55°C and were incubated for 30 min. DNA was melted for at 94°C for 30 s, annealed for 30 s at either 50°C (for IFN-γ, IL-4, IL-5, and IL-6), 52°C (for CD3δ and IL-10), or 54°C (for β-actin and IL-12), and extended for 1 min at 68°C. The next 25 cycles followed the same protocol except that 5 s was added to the annealing time after each cycle. After a final elongation step at 68°C for 7 min, products were stored at 4°C until they were analyzed by electrophoresis. IFN-γ production was also assayed directly by enzyme immunoassay in PBMC culture medium after 6 days of stimulation with M. ulcerans or M. bovis BCG as described previously (7).
TABLE 1.
PCR primers used in this study
| Target gene | Orientation | Primer sequence (5′→3′) | Product size (bp) | Source or reference |
|---|---|---|---|---|
| β-Actin | Forward | TGACGGGGTCACCCACACTGTG | 661 | 9 |
| Reverse | CTAGAAGCATTGCGGTGGACGA | |||
| CD3δ | Forward | GTACTGAGCATCATCTCGATG | 309 | 16 |
| Reverse | CTGGACCTGGGAAAACGCATC | |||
| IFN-γ | Forward | AGTTATATCTTGGCTTTTCA | 385 | 16 |
| Reverse | ACCGAATAATTAGTCAGCTT | |||
| IL-12 | Forward | CAGCACAGTGGAGGCCTGTTT | 495 | This study |
| Reverse | CCACATCCTATCAAAGTTTCC | |||
| IL-4 | Forward | TGGACATGAACCTTCTTCGGT | 683 | This study |
| Reverse | GGATCTTTATGACTCTTCG | |||
| IL-5 | Forward | ATGCTTCTGCATTTGAGTTTG | 476 | This study |
| Reverse | TTTCTTGGCGGTCATTCTCAC | |||
| IL-6 | Forward | TGAACTCCTTCTCCACAAGCGC | 608 | 5 |
| Reverse | GAAGACCCCTCAGGCTGGACT | |||
| IL-10 | Forward | CAGGCAACCTGCCTAACATGCTT | 275 | This study |
| Reverse | GCGCCGTAGCCTCAGCCTGGAG | |||
| TNF-α | Forward | CGAGTGACAAGCCTGTAGCCC | 401 | This study |
| Reverse | TGATCCCAAAGTAGACCTGCCC |
Antibody detection.
Serum antibodies to M. ulcerans were detected by immunoblotting as described previously (7). Briefly, 100-μl suspensions of 109 M. ulcerans cells were boiled for 5 min in 2× sodium dodecyl sulfate (SDS) reducing buffer (8). Twenty microliters of the resultant antigen preparations was then separated on 10% polyacrylamide gels at 150 V for 1 h in a Mini-PROTEAN II gel apparatus (Bio-Rad, Hercules, Calif.). The separated material was transferred to nitrocellulose filters (Micron Separations, Westborough, Mass.), which were blocked with phosphate-buffered saline (PBS)-0.1% Tween 20 containing 5% skim milk (PBSTB) and then individually reacted with serum (diluted 1:100 in 5% PBSTB) from each of the affected and unaffected study subjects. Sheep anti-human immunoglobulin conjugated to horseradish peroxidase (Silenus, Melbourne, Australia), diluted 1:50,000, was used as the secondary antibody. Filters were washed with PBS-Tween 20, after which membrane-bound, peroxidase-labeled immunoglobulin was detected by using enhanced chemiluminescence (ECL Western blotting reagents, RPN 2109; Amersham International). A previous study of 11 patients and 10 unexposed control subjects who were sensitive to tuberculin indicated that this test was 100% specific and 82% sensitive for patients with a history of Buruli ulcer, with positive and negative predictive values of 100 and 83%, respectively (7).
Statistical analysis.
All data were analyzed in a blinded fashion with respect to patient category. Statistical analyses were performed by using a two-tailed Student t test and Fisher's exact test. A P value of <0.05 was taken to indicate statistical significance.
RESULTS
Detection of cytokine-specific mRNA.
The cytokine profiles of PBMC from affected and unaffected subjects in response to stimulation with M. ulcerans and M. bovis BCG are shown in Fig. 1. There was close overall agreement between the responses of the two groups to stimulation with M. ulcerans and M. bovis BCG. In general, PBMC from individuals with a history of Buruli ulcer showed a Th2-type cytokine profile in response to stimulation with mycobacterial cells. Specifically, mRNA for the type-2 cytokine IL-4 was detected in 18 of 23 (78%) affected subjects but in only 3 of 25 (12%) unaffected subjects (P < 0.0001), IL-5 was detected in 8 of 23 (35%) affected subjects but in none of the unaffected subjects (P = 0.001), IL-6 was detected in 19 of 23 (83%) affected subjects and 3 of 25 (12%) unaffected subjects (P < 0.0001), and IL-10 was detected in 18 of 23 (78%) affected subjects and 6 of 25 (24%) unaffected subjects (P = 0.0004). By contrast, unaffected subjects showed a distinct Th1-type response following stimulation with M. ulcerans and M. bovis BCG, with the majority producing mRNAs for IFN-γ and IL-12 and only a small number producing the type-2 cytokines, IL-4, IL-5, IL-6, and IL-10. Thus, mRNA for IFN-γ was detected in 21 of 25 (84%) unaffected subjects but in only 3 of 23 (13%) affected subjects (P < 0.0001), and mRNA for IL-12 was found in 19 of 25 (76%) unaffected subjects but in only 5 of 23 (22%) affected subjects (P < 0.001). The cytokine profiles of the 11 patients who presented with symptoms more than 6 years before sampling did not differ from those with more-recent symptoms (Fig. 1).
IFN-γ secretion.
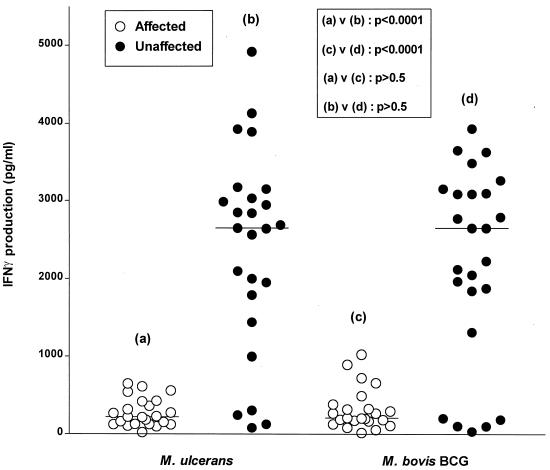
PBMC from unaffected subjects produced significantly greater amounts of IFN-γ in response to stimulation with M. ulcerans or M. bovis BCG than PBMC from affected subjects (P < 0.0001) (Fig. 2).
FIG. 2.
IFN-γ production (measured by enzyme immunoassay) by PBMC from 23 affected and 25 unaffected individuals in response to 6 days of stimulation with M. ulcerans or M. bovis BCG. Horizontal lines indicate the mean for each group.
Antibody responses.
Immunoblot analysis of serum samples from affected and unaffected subjects with extracts of M. ulcerans cells as the antigen revealed antibody responses in 14 of 23 (61%) affected subjects and 17 of 25 (68%) unaffected subjects, respectively (P > 0.5). Among the affected subjects, there was no relationship between the time elapsed since the original presentation and antibody prevalence: 6 of 11 individuals who presented more than 6 years previously were seropositive, compared with 8 of 12 individuals who presented more recently (Table 1).
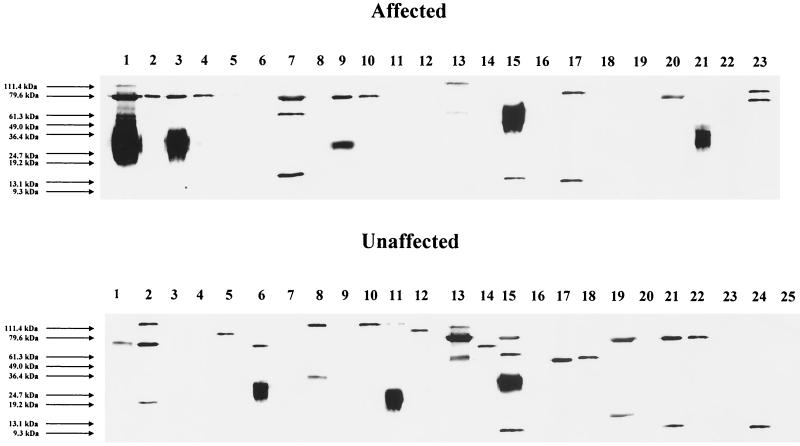
Antibodies were directed against a range of antigens with molecular sizes ranging from approximately 9 to 112 kDa. Antibodies to a 79-kDa antigen were observed most frequently and were present in 11 of 14 (79%) seropositive affected subjects and 7 of 17 (42%) seropositive unaffected subjects (P = 0.07) (Fig. 3). Despite the pronounced differences between the cytokine profiles of the affected and unaffected groups, there were no significant differences between the cytokine patterns of seropositive and seronegative groups, although there was a tendency for the seropositive group overall to be positive for the type-2 cytokines IL-4 (16 of 31 [52%]) and IL-10 (19 of 31 [61%]), compared with those who were seronegative (5 of 17 [29%] for both cytokines).
FIG. 3.
Immunoblots of whole-cell extracts of M. ulcerans with serum samples from subjects with Buruli ulcer and unaffected household controls. Lane numbers correspond to A1 to A23 and U1 to U25 in Fig. 1. The smeared appearance of some of the target antigens suggests that the antibodies are reacting with either partially degraded proteins or glycolipids, or with both.
DISCUSSION
In this study unaffected household contacts exposed to M. ulcerans, as evidenced by the presence of specific antibodies, exhibited a Th1-type cytokine response to M. ulcerans and to M. bovis BCG antigens, which contrasted markedly with the Th2 response found in subjects with active or healed M. ulcerans disease. The reduced Th1 response of affected subjects was further demonstrated by their markedly reduced secretion of IFN-γ in response to live M. ulcerans and M. bovis BCG. It was previously reported that despite their anergy to mycobacteria, the majority of individuals with current or past infection with M. ulcerans develop antibodies to this bacterium (7). In the present study, the prevalence of antibodies in the exposed controls was similar to that in clinically affected subjects. Because these antibodies are not found in unexposed individuals (7), we interpret this observation as indicating that in an environment where M. ulcerans is endemic, subjects who have not developed clinical disease have been exposed to M. ulcerans and developed an immune response to it. Recently, Stienstra et al. (13) hypothesized that a significant proportion of the population residing in an area of M. ulcerans endemicity may be exposed to organisms but not develop disease. Our present results provide the first confirmation of this hypothesis. These findings suggest that a Th1-type immune response to M. ulcerans may prevent the development of Buruli ulcer in subjects exposed to M. ulcerans.
In this study we also show for the first time that M. ulcerans infection is associated with a Th2 response, as evidenced by the increased prevalence of mRNA for Th2 cytokines in affected subjects. Detection of M. ulcerans-induced mRNA for the Th2-type cytokines IL-4, -5, -6, and -10 was significantly greater for subjects with Buruli ulcer. IL-10 is an immunoregulatory cytokine which downregulates both Th1 and Th2 responses and thus could contribute to reduced Th1 immune responses in active disease. This finding is similar to that seen in the multibacillary form of leprosy, where RT-PCR cytokine profiles are Th2 in type, in contrast to the paucibacillary form, where the immune response is characterized by IL-2 and IFN-γ (16).
Possible explanations for the marked reduction in Th1 immunity to mycobacterial organisms in subjects who develop Buruli ulcer include the following: (i) M. ulcerans induces immunosuppression, (ii) M. ulcerans induces immune deviation, resulting in reduced Th1 and increased Th2 responses to mycobacterial antigens, and (iii) those subjects who develop clinical disease have an inherent inability to generate a strong Th1 response to mycobacterial antigens.
Previous studies have shown that M. ulcerans organisms secrete a toxin, mycolactone, which has immunosuppressive activity in vitro and may reduce the inflammatory response to M. ulcerans organisms (10). Such local immunosuppressive activity is consistent with the paucity of inflammatory cells and the large numbers of extracellular bacteria seen in M. ulcerans lesions. However, there is no evidence that M. ulcerans toxins can induce systemic immunosuppression of the type observed in this study. A telling argument against toxin-mediated immunosuppression as an explanation for our findings is the fact that only 1 of the 23 patients had active disease at the time of investigation. This confirms the previous finding that the extents of anergy to mycobacteria are similar in active and healed cases of Buruli ulcer (7), a finding which argues against systemic, toxin-mediated immunosuppression. In addition, it has previously been demonstrated that the proliferative and Th1 cytokine responses to the T-cell mitogen phytohemagglutinin are equivalent in subjects with and without M. ulcerans disease (7), which argues against generalized immunosuppression.
To account for the markedly reduced Th1 responses to both M. bovis and M. ulcerans antigens, it could be postulated that M. ulcerans-induced immune deviation results in a persistent downregulation of the Th1 responses to multiple mycobacterial antigens. However, evidence against a generalized M. ulcerans-induced immune deviation, at least in unaffected contacts, is the finding that the majority of unaffected contacts exhibit both Th1 and Th2 responses, as seen in the release of IFN-γ from PBMC and the production of antibodies in serum. Alternatively, an inherent inability to develop a strong Th1 response to mycobacterial antigens may underlie the development of clinical M. ulcerans disease. This hypothesis accords with the significantly higher Th1 responses to M. ulcerans and M. bovis BCG seen in exposed, but unaffected, subjects. Prospective studies of immunity to M. ulcerans before and after the development of disease would help resolve the issue of an intrinsic immune defect versus M. ulcerans-induced immune deviation.
There are a number of genetic factors which influence the innate immune response to mycobacterial antigens, such as infectious disease susceptibility genes, e.g., SLC11A1 (formerly known as NRAMP1), HLA-DR, vitamin D3 receptor, and mannose binding protein (3, 4). Although the influence of these factors on M. ulcerans infections is unknown (13), it is conceivable that polymorphisms in factors related to the innate immune response may allow the establishment of M. ulcerans infection, which then results in immune deviation from a Th1 to a Th2 immune response. Such polymorphisms may allow the establishment of infection, and the consequent high dose of bacteria may then result in immune deviation. In this context it is of interest that the dose of M. bovis can influence the development of a Th1 or Th2 response, with higher doses of bacteria inducing a Th2-type response (11).
The in vitro finding of reduced Th1 immunity to mycobacteria in patients with Buruli ulcer is consistent with the clinical features of indolent ulcers: a paucity of inflammatory reaction and multiple extracellular bacteria. A disseminated nonulcerative form of M. ulcerans infection has been described previously (1), and although the immune function of these subjects has not been reported, they may be those with the most profound defect in Th1 immunity. Paradoxically, there is no evidence in either developed or developing countries that individuals with M. ulcerans infection are unduly susceptible to other mycobacterial infections, and more-profound acquired immune suppression, such as that observed in AIDS, is not known to be associated with the development of M. ulcerans disease (13). It could therefore be postulated that low levels of mycobacterial Th1 immunity in affected individuals are sufficient to control most mycobacterial infections. However, the unique characteristics of M. ulcerans, such as the local secretion of immunosuppressive toxins, might conceivably require higher levels of Th1 immunity to prevent the development of Buruli ulcer than is the case in infections caused by other species of mycobacteria. The findings of this study relate to a predominantly Caucasian population in an Australian environment, and it is possible that in areas of Buruli ulcer hyperendemicity, such as West Africa, specific host factors or differences in bacterial virulence may also contribute to the development of disease.
Acknowledgments
We are indebted to John McBride for granting us access to the Pathology Laboratory at Cairns Base Hospital.
This study was supported in part by grants from the Department of Human Services, Victoria, Australia, and the Murdoch Children's Research Institute. T. Gooding was supported by an Australian Postgraduate Research Award.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abalos, F. M., J. Aguiar, Sr., A. Guedenon, F. Portaels, and W. M. Meyers. 2000. Mycobacterium ulcerans infection (Buruli ulcer): a case report of the disseminated nonulcerative form. Ann. Diagn. Pathol. 4:386-390. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, and J. A. Smith. 1991. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Bellamy, R., C. Ruwende, T. Corrah, K. P. McAdam, H. C. Whittle, and A. V. Hill. 1998. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N. Engl. J. Med. 338:640-644. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy, R. J., and A. V. Hill. 1998. Host genetic susceptibility to human tuberculosis. Novartis Found. Symp. 217:3-13. [DOI] [PubMed] [Google Scholar]
- 5.Fujihashi, K., M. Yamamoto, T. Hiroi, T. V. Bamberg, J. R. McGhee, and H. Kiyono. 1996. Selected Th1 and Th2 cytokine mRNA expression by CD4+ T cells isolated from inflamed human gingival tissues. Clin. Exp Immunol 103:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George, K. M., D. Chatterjee, G. Gunawardana, D. Welty, J. Hayman, R. Lee, and P. L. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854-857. [DOI] [PubMed] [Google Scholar]
- 7.Gooding, T. M., P. D. R. Johnson, D. E. Campbell, J. Hayman, E. L. Hartland, A. S. Kemp, and R. M. Robins-Browne. 2001. Immune response to infection with Mycobacterium ulcerans. Infect. Immun. 69:1704-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 9.Misra, N., A. Murtaza, B. Walker, N. P. S. Narayan, R. S. Misra, V. Ramesh, S. Singh, M. J. Colston, and I. Nath. 1995. Cytokine profile of circulating T cells of leprosy patients reflects both indiscriminate and polarized T-helper subsets: T-helper phenotype is stable and uninfluenced by related antigens of Mycobacterium leprae. Immunology 86:97-103. [PMC free article] [PubMed] [Google Scholar]
- 10.Pahlevan, A. A., D. J. Wright, C. Andrews, K. M. George, P. L. Small, and B. M. Foxwell. 1999. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-κB function. J. Immunol. 163:3928-3935. [PubMed] [Google Scholar]
- 11.Power, C. A., G. Wei, and P. A. Bretscher. 1998. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect. Immun. 66:5743-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith, M. 1996. Studies on Mycobacterium ulcerans infection in the Douglas Shire of far North Queensland, Australia. M.Sc. thesis. James Cook University, Townsville, Australia.
- 13.Stienstra, Y., W. T. A. van der Graaf, G. J. te Meerman, T. H. The, L. F. de Leij, and T. S. van der Werf. 2001. Susceptibility to development of Mycobacterium ulcerans disease: review of possible risk factors. Trop. Med. Int. Health 6:554-562. [DOI] [PubMed] [Google Scholar]
- 14.van der Werf, T. S., W. T. A. van der Graaf, J. W. Tappero, and K. Asiedu. 1999. Mycobacterium ulcerans infection. Lancet 354:1013-1018. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. 2001. Buruli ulcer. Fact sheet no. 199. [Online.] http://www. who. int/inf-fs/en/fact199. html.
- 16.Yamamura, M., K. Uyemura, R. J. Deans, K. Weinburg, T. H. Rea, B. R. Bloom, and R. L. Modlin. 1991. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254:277-279. [DOI] [PubMed] [Google Scholar]