Abstract
Studies of mice suggest that pneumococcal proteins, including PspA, pneumolysin, PsaA, and CbpA, are promising vaccine candidates. To determine whether these proteins are good mucosal immunogens in humans, adenoidal lymphocytes from 20 children who had adenoidectomies were isolated and tested by ELISpot for antigen-specific antibody-secreting cells (ASCs). Cells were also cultured for 7 days in the presence of a concentrated culture supernatant (CCS) from a type 14 strain of pneumococcus which contained secreted pneumococcal proteins, including PspA, pneumolysin, PsaA, and CbpA, and then tested by ELISpot. ELISpot assays done on freshly isolated cells detected ASCs to all four antigens in most children studied. However, there were differences both between antigens and between isotypes. The densities of immunoglobulin G (IgG) ASCs against both PsaA and CbpA were significantly higher than those of ASCs for PspA and PdB (pneumolysin toxoid B) (P < 0.001). For all antigens, the numbers of IgA ASCs tended to be lower than those of both IgG and IgM ASCs. The numbers of anti-CbpA and -PsaA IgA ASCs were higher than those of anti-PdB IgA ASCs (P < 0.01). Concentrations of IgA antibodies to PspA and PsaA in saliva correlated with the numbers of IgA ASCs to PspA and PsaA in freshly isolated adenoidal cells, but no such correlation was found between salivary IgG antibody concentrations and IgG ASCs to the four antigens in adenoidal cells. In cultured cells, anti-PspA, -PsaA, and -CbpA IgG ASCs proliferated significantly, but only two of eight samples showed >2-fold increases in anti-CbpA and -PspA IgA ASCs after CCS stimulation. The results suggest that CbpA, PsaA, and PspA may be good upper respiratory mucosal antigens in children. Adenoids may be important inductive sites for memory IgG responses and important sources of salivary IgA. Some protein antigens may also prime for mucosal IgA memory. These data support the effort to explore mucosal immunization against pneumococcal infection.
Streptococcus pneumoniae is a common cause of otitis media, pneumonia, septicemia, and meningitis in children, resulting in significant mortality and morbidity throughout the world. With the prevalence of antibiotic-resistant pneumococci increasing worldwide (20, 26), studies of pneumococcal vaccines have gained much interest. The efficacy of polysaccharide vaccines is limited by poor immunogenicity in high-risk populations, especially young children. Conjugate vaccines have greater immunogenicity than polysaccharide-based vaccines, but serotype coverage is limited. Efforts are being made to find effective pneumococcal protein vaccines which might protect against multiple serotypes and which are immunogenic in children as well as in adults.
Currently, several candidate pneumococcal proteins are under study, including pneumolysin, pneumococcal surface protein A (PspA), pneumococcal surface adhesin A (PsaA), and choline-binding protein A (CbpA; also referred to as PspC, SpsA, or Hic) (11, 19, 21, 44). Of these pneumococcal protein antigens, pneumolysin, PspA, and PsaA have been shown to contribute to the virulence of pneumococci and to be produced by virtually all clinical isolates (35, 39). CbpA is likely to play a role in nasopharyngeal colonization and appears to be expressed by most, if not all, isolates. Preliminary studies of mice have shown that immunization with these proteins can protect against infection with multiple serotypes of pneumococcus (1) and/or prevent nasopharyngeal carriage (9, 10). It has been shown that pneumococcal carriage and infections induce salivary and serum antibodies to PsaA, pneumolysin, and PspA in children (43, 46, 48).
As pneumococci are mucosal pathogens colonizing the nasopharynx, mucosal immunization (e.g., by the intranasal route) is potentially a better way to protect against mucosal carriage than parenteral immunization. Acquisition of pneumococci is generally from nasopharyngeal carriers rather than infected individuals. Therefore, to induce community immunity against S. pneumoniae, it will be necessary to induce protection against carriage. PsaA and CbpA may be good vaccine candidates against carriage, as they have been shown to be associated with pneumococcal adherence (3, 44).
S. pneumoniae gains entry into the host via the epithelium of the upper respiratory tract. Asymptomatic carriage of pneumococci is particularly common in infants and young children (17, 52), age groups also at high risk of invasive disease. Previous studies suggest that natural mucosal infections (or carriage) can be immunizing processes which can prime tonsillar lymphocytes. Natural infection or intranasal immunization with rubella virus vaccine primes tonsillar lymphocytes better than subcutaneous vaccination (34). Natural infection with varicella-zoster virus likewise stimulates tonsillar lymphocytes better than peripheral blood lymphocytes (4).
Adenoids (nasopharyngeal tonsils), which are located in the anatomical area of pneumococcal carriage, are thought to be important immune inductive and effector sites for nasopharyngeal immunity and to act as part of an integrated mucosal immune system (25). Regional mucosal immunity induced by natural carriage or intranasal vaccination most probably involves these immunocompetent tissues in the nasopharynx. Thus, protein-based vaccines against pneumococci should ideally be immunogenic to adenoidal lymphocytes in children if they are candidates for mucosal immunization. Adenoids are rich in lymphocytes, especially B cells, and thus provide a good model to study antigen-specific B-cell responses. Adenoidal lymphocytes have been used in a previous study to investigate the immune responses to the P6 outer membrane protein of nontypeable Haemophilus influenzae in children (23). Specific immunoglobulin-secreting cells were found in freshly isolated and antigen-stimulated adenoidal lymphocytes, suggesting that the P6 protein had primed specific B cells in vivo.
In this study we have investigated the B-cell antibody responses in adenoidal lymphocytes from children to the four pneumococcal protein antigens pneumolysin, PspA, PsaA, and CbpA.
MATERIALS AND METHODS
Adenoids and saliva samples.
Adenoids were obtained from 20 unselected children aged 1 to 10 years (median age, 5 years) with adenoidal hypertrophy who underwent adenoidectomy at the Sheffield Children's Hospital, Sheffield, United Kingdom. Saliva samples were also collected from seven subjects as described previously (54). The study was approved by the local research ethics committee, and informed consent was obtained from the parents in each case.
Isolation of mononuclear cells from adenoids.
The adenoids were transported to the laboratory at room temperature in minimum essential medium (Gibco, Paisley, United Kingdom) supplemented with glutamine and antibiotics (penicillin, 100 U/ml; streptomycin, 100 μg/ml; amphotericin B, 1 μg/ml) and processed within 1 h after the operation. The adenoid tissue was transferred to an 8-mm-diameter petri dish and checked grossly and under phase-contrast microscopy in medium. No grossly inflamed and or necrotic tissue was found in this study. Each sample was minced using a sterile scalpel and teased using sterile steel wire mesh to release cells into the medium. The cell suspension was allowed to sediment for 5 min and was then passed through a nylon mesh (30-μm pore size). Mononuclear cells were isolated using Ficoll (Ficoll-Paque Plus; Pharmacia Biotech, Little Chatfont, United Kingdom) gradient centrifugation (400 × g; 30 min). The cells were washed twice in sterile phosphate-buffered saline (PBS) and resuspended in 2 ml of RPMI medium supplemented with glutamine, penicillin, streptomycin, and 10% fetal bovine serum (FBS). The viability of the cells was assessed by trypan blue dye exclusion, and they were consistently >99% viable. Each cell suspension was adjusted to contain 4 × 106 cells/ml.
Flow cytometric analysis of adenoidal cells.
Mononuclear cells isolated from the adenoids were incubated for 30 min at 4°C with either fluorescein isothiocyanate- or phycoerythrin-labeled monoclonal antibodies, including anti-CD3, anti-CD4, anti-CD-8, anti-CD19, and anti-CD68 (IDS, Boldon, United Kingdom), and washed before analysis by flow cytometry (FACScan; Becton Dickinson).
Antigens.
The pneumococcal protein antigens pneumolysin toxoid B (PdB), PspA, PsaA, and CbpA (provided by James Paton, Adelaide, Australia), purified from recombinant Escherichia coli expressing the respective cloned genes, were used (37, 38, 41, 42). The original source for each gene was a capsular type 2 pneumococcal strain, D39 (NCTC7466). All antigens were >95% pure as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and staining with Coomassie brilliant blue R250 (37).
The PdB antigen was a genetic toxoid of pneumolysin which has a Trp433→Phe mutation reducing cytotoxicity and retains full immunogenicity (41). It is a 53-kDa protein (Fig. 1a) produced by all clinical isolates of pneumococci (40).
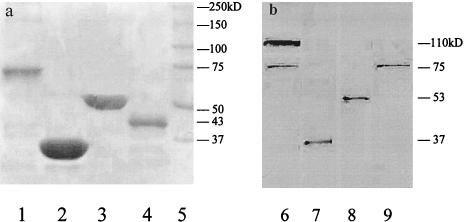
FIG. 1.
(a) SDS-PAGE of the recombinant proteins rPspA, rPdB, rPsaA, and rCbpA; (b) Western immunoblot analysis of the recombinant proteins and the concentrated culture supernatant of S. pneumoniae SSI 14. Lanes 1 to 4, Coomasie blue-stained gel showing the relative mobilities of rCbpA (lane 1), rPsaA (lane 2), rPdB (lane 3), and rPspA (lane 4); lane 5, molecular mass markers; lanes 6 to 9, concentrated culture supernatant-blotted nitrocellulose membrane strips reacted with mouse antisera to CbpA (lane 6) (antiserum to CbpA reacted with both CbpA [∼110 kDa] and PspA [∼75 kDa]), PsaA (lane 7), PdB (lane 8), and PspA (lane 9). The molecular masses of proteins are indicated on the right of each gel.
The recombinant PsaA (rPsaA) was prepared from E. coli expressing the cloned psaA gene and purified as described previously (42). It has a molecular mass of approximately 37 kDa (Fig. 1a), and it is produced by all clinical isolates of S. pneumoniae (45).
PspA was expressed as a His-tagged N-terminally truncated portion protein and purified by Ni-nitrilotriacetic acid affinity chromatography (37). The mass of rPspA was 43 kDa (Fig. 1a), although the native protein has variable molecular mass ranging from 67 to 99 kDa (49). It has been found on every S. pneumoniae strain discovered to date (15). This PspA has been shown to elicit cross-protective immunity against pneumococcal challenge in mice (37, 47, 51).
The CbpA antigen used was an N-terminal His-tagged truncated CbpA fragment, representing amino acids 1 to 445 of the mature CbpA polypeptide, purified from recombinant E. coli (38). The mass of rCbpA was approximately 75 kDa (Fig. 1a). However, the protein in bacterial lysates apparently migrates in SDS-PAGE at higher mass and is variable among different strains (90 to 150 kDa) (11, 44). Polyclonal antiserum to rCbpA has been found to react with both CbpA and PspA proteins in pneumococcal lysates (11), accounting for the two bands seen in Fig. 1b, lane 6 (38).
ELISpot assay for enumeration of antigen-specific ASCs.
Costar plates (96 well) were coated with each pneumococcal protein (5 μg/ml) in PBS at 4°C overnight. After being blocked with 1% bovine serum albumin in PBS (150 μl/well) at 37°C for 30 min, the cells in RPMI medium were added at 4 × 105/well in triplicate and incubated at 37°C in a humid atmosphere with 5% CO2. Alkaline phophatase-conjugated goat anti-human immunoglobulin A (IgA), IgG, or IgM (Sigma, Dorset, United Kingdom) was added after washing, and the plates were incubated overnight at room temperature (RT). The substrate, BCIP (5-bromo-4-chloro-3-indolyl-phosphate; Sigma), was applied in agarose to the plates on a level surface at RT, and spots corresponding to antibody-secreting cells (ASCs) were counted using a phase-contrast microscope under low magnification. The results were expressed as numbers of ASCs (IgA, IgG, or IgM) per 106 cells.
Pneumococcal culture supernatant.
A capsular type 14 pneumococcal strain, SSI 14/1 (Serum Statens Institute [SSI], Copenhagen, Denmark), was cultured in brain heart infusion broth (Oxoid, Basingstoke, United Kingdom) supplemented with 10% FBS in 5% CO2 at 37°C to exponential phase (optical density at 620 nm [OD620], 0.4 to 0.5 [∼108 CFU/ml]). After centrifugation (3,000 × g; 30 min), the culture supernatant was collected and passed through a 0.2-μm-pore-size sterile filter and concentrated (10-fold) using a Vivaspin concentrator (Vivascience, Surry, United Kingdom). The protein concentration of the concentrated pneumococcal culture supernatant (pneumoCCS) was determined by the Bio-Rad (Hemel Hempstead, United Kingdom) protein assay to be 5 mg/ml. Discontinuous SDS-PAGE and Western immunoblotting were performed as described below to determine whether the supernatant contained PspA, PdB, PsaA, and CbpA.
SDS-PAGE and Western immunoblotting.
Purified recombinant proteins (2 μg each) and concentrated culture supernatant (10 μg) from SSI 14 were mixed with sample buffer, heated at 95°C for 5 min, and loaded onto an SDS-PAGE gel (10% separating and 5% stacking gels) and run at 130 V constant voltage for 1 h. The proteins in the gel were transferred to a nitrocellulose membrane (Bio-Rad) by electroblotting following the manufacturer's instructions. The blots were blocked with 10% blotting-grade dry milk (Bio-Rad) in Tris-buffered saline with 0.05% Tween-20 (TBS-T; pH 7.5) for 1 h at RT before incubation with each mouse polyclonal antiserum (gifts of James Paton) (1:5,000) to PspA, pneumolysin (PdB), PsaA, and CbpA for 2 h. After being washed in TBS-T, the blots were incubated with biotinylated goat anti-mouse IgG (1:20,000) (Sigma) for 1.5 h. Extravidin-alkaline phosphatase (1:30,000) (Sigma) was then added, and the blots were incubated for 1.5 h. All of the above-mentioned reagents were diluted in TBS-T, and the incubation steps were performed at RT with gentle shaking on a rocker platform. The blots were developed using color development buffer (Bio-Rad) containing Nitro Blue Tetrazolium and BCIP on a rocker for 10 to 15 min at RT. The color reaction was stopped with deionized water when protein bands became clear. The Western blotting suggested that the pneumoCCS contained PspA, PdB, PsaA, and CbpA (Fig. 1). This pneumoCCS was subsequently used in cell stimulation experiments. Since not all pneumococcal strains express CbpA (11), a cbpA-specific PCR was performed for the SSI type 14 strain showing it to be cbpA gene positive and to belong to clade B (data not shown).
Cell culture.
Mononuclear cells isolated from adenoidal tissue were cultured in RPMI medium supplemented with HEPES and antibiotics in 24-well plates (Costar) in the presence or absence of pneumoCCS. The cells were cultured for up to 9 days. At the end of culture, the cells were harvested. After being washed in RPMI medium, the cells were resuspended in the RPMI culture medium and added to 96-well microtiter plates (4 × 105/well) precoated with antigen and tested for ASCs by ELISpot assay as described above.
Immunoassay for anti-pneumococcal protein antibodies.
In some experiments, cell culture supernatants were collected on days 1, 3, 5, 7, and 9 and assayed for anti-PspA, -PdB, -PsaA, and -CbpA antibodies. The culture supernatants were collected and stored at −20°C until they were assayed. Ninety-six-well Costar plates were coated with each pneumococcal protein as described above. After being blocked with 10% FBS in PBS (150 μl/well) at 37°C for 1 h, cell culture supernatants (1:5 to 1:10) were added in duplicate and incubated at 37°C for 2 h. After the plates were washed, alkaline phophatase-conjugated goat anti-human IgA, IgG, or IgM was added and incubated for 2 h at 37°C. p-Nitrophenyl phosphate was added, and the plates were incubated at RT for 30 min. The OD405 was measured using a microtiter reader (Bio-Rad). Antigen-specific salivary IgA and IgG antibodies were also analyzed by immunoassay as described above.
Statistical analysis.
Median and interquartile ranges were used to express numbers, and the Kruskal-Wallis method was used to analyze differences between numbers of ASCs for different antigen groups. Differences between two antigen groups and differences before and after antigen stimulation were analyzed by the two-related-sample pairwise Wilcoxon test. A P value of <0.05 was taken to indicate statistical significance. Analysis was performed using SPSS software version 10.
RESULTS
Flow cytometry.
Freshly isolated adenoidal mononuclear cells from three subjects were analyzed by flow cytometry; 34 to 45% were CD3+ T cells, 52 to 65% were CD19+ B cells, and 4 to 7% were CD68+ cells (macrophages). Among the T cells, 78 to 85% were CD4+ and 15 to 22% were CD8+.
ASCs in freshly isolated adenoidal cells.
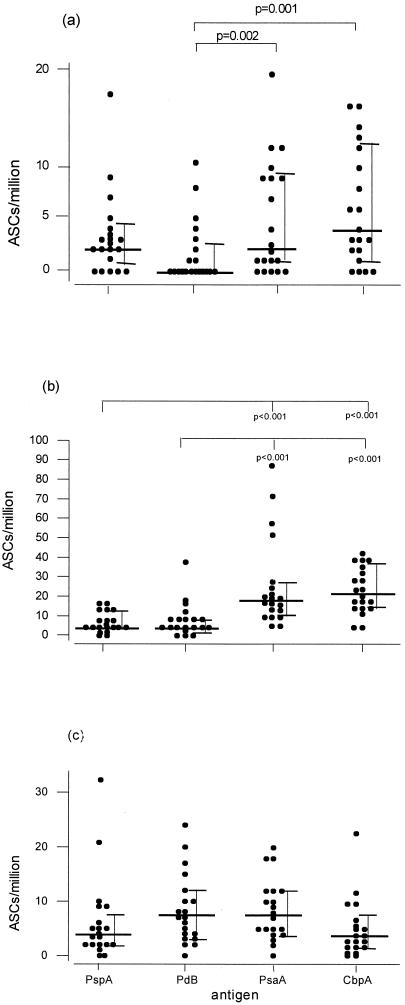
ELISpot assays done on freshly isolated adenoidal cells detected ASCs to all four antigens among most of the children studied (Fig. 2). However, there were some differences both between antigens and between isotypes. The densities of IgG ASCs against both PsaA and CbpA were significantly higher than for those against PspA and PdB (P < 0.001) (Fig. 2b). For all antigens, the numbers of IgA ASCs tended to be lower than those of both IgG and IgM ASCs. Anti-CbpA and -PsaA IgA ASC numbers were significantly higher than anti-PdB IgA ASCs.
FIG. 2.
Numbers of IgA (a), IgG (b), and IgM (c) ASCs to PspA, PdB, PsaA, and CbpA determined by ELISpot assay in freshly isolated adenoidal lymphocytes from 20 children. Median (bold horizontal bars) and interquartile (error bars) ranges are shown, and statistically significant differences between antigens are indicated.
Antigen-specific ASCs and antibody responses after stimulation with pneumoCCS.
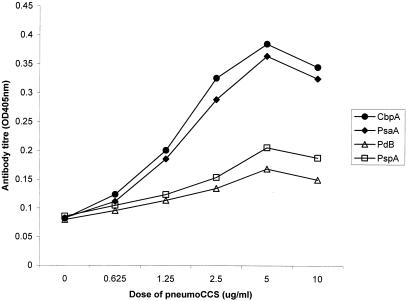
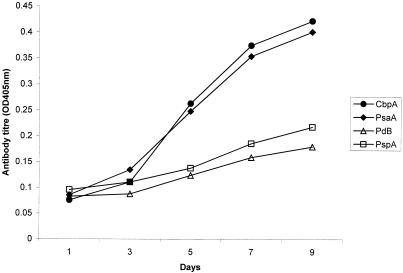
To test whether adenoidal B cells respond in vitro to protein antigen stimulation, adenoidal mononuclear cells from three subjects were cultured in the presence or absence of pneumoCCS. Different concentrations of pneumoCCS were tested, and cell culture supernatants were collected on days 1, 3, 5, 7, and 9 and measured for anti-PspA, -PdB, -PsaA, and -CbpA antibodies. Representative data for IgG concentrations from one experiment are displayed in Fig. 3 and 4. The optimal protein concentration of pneumoCCS for stimulation was approximately 5 μg/ml in cell culture, and this concentration was used for subsequent cell culture experiments.
FIG. 3.
Antigen-specific IgG antibody titers in the culture supernatants of adenoidal lymphocytes cultured for 7 days with different concentrations of pneumoCCS. The results are from a representative experiment among three subjects tested.
FIG. 4.
Kinetics of antigen-specific IgG antibody titers in the culture supernatants of adenoidal lymphocytes cultured with pneumoCCS (5 μg/ml). The results are from a representative experiment among three subjects tested.
The effects of coculture with 5 μg of pneumoCCS/ml for 7 days in vitro upon numbers of ASCs of all three isotypes and to all four antigens were tested in eight subjects. Significant increases were seen only in numbers (per million cells) of IgG ASCs to PspA {[median (interquartile range)], 0 (0, 2) (control without pneumoCCS) to 2.5 (0, 20)}, PsaA [7 (4, 25) to 14 (6.5, 33.5)], and CbpA [22 (13, 31) to 51.5 (31.5, 90)] (P values, 0.043, 0.028, and 0.012, respectively). Although the median numbers of IgA and IgM ASCs did not increase significantly for any antigen, two out of eight samples showed >2-fold increases in the numbers of IgA ASCs (per million cells) to both CbpA (2 to 6 and 2 to 8) and PspA (2 to 10 and 2 to 12).
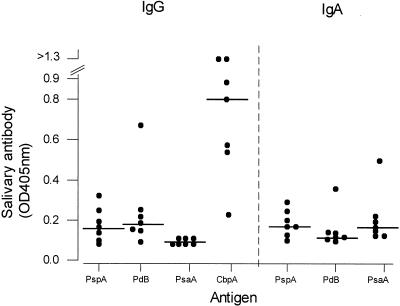
Salivary IgG and IgA antibody concentrations to the four antigens were measured by immunoassay in seven subjects (Fig. 5). There were detectable antibodies of both isotypes to all antigens assayed, with the exception of IgA to PdB in the majority of subjects. Salivary IgA antibody concentrations to PspA and PsaA correlated with the numbers of IgA ASCs to PspA (r = 0.56) and PsaA (r = 0.68) seen in ASC assays done on freshly isolated adenoidal cells. There were no such correlations between salivary IgG antibody concentrations and IgG ASCs in adenoidal cells to the four antigens (r < 0.35). While anti-CbpA salivary IgG concentrations were generally high, as expected, anti-PsaA salivary IgG was notably absent (Fig. 5), which is in contrast with the high numbers of IgG ASCs to PsaA in adenoidal cells in the same subjects. Anti-CbpA IgA antibody concentrations in saliva could not be determined by the immunoassay, as all saliva samples produced very high OD values (possibly due to the interaction between CbpA and the secretory component of secretory IgA (S-IgA) in saliva (19) (see Discussion).
FIG. 5.
Titers of salivary IgG and IgA antibodies to PspA, PdB, PsaA, and CbpA in seven children. Titers of IgA antibody to CbpA were not determined (see Discussion).
DISCUSSION
As pneumococci are mucosal pathogens colonizing the nasopharynx, mucosal immunization (e.g., by the intranasal route) is potentially a better way to protect against mucosal carriage than parenteral immunization. Studies of mice have shown that intranasal immunizations with PspA, pneumolysin, PsaA, and CbpA are effective against invasive disease and/or nasopharyngeal carriage (1, 9, 10, 38). Recent studies of humans have shown that antibodies to pneumolysin, PspA, and PsaA are present in serum in healthy people and patients with invasive disease (43, 48). IgA antibodies are also found in saliva in children, and patients with positive cultures from nasopharyngeal swabs or from middle ear fluid had higher antigen-specific IgA concentrations than culture-negative controls (46). These findings suggest that current carriage, infection, or previous exposure to pneumococci may induce both systemic and local immune responses to these pneumococcal protein antigens. It will be important to find out which proteins are good mucosal immunogens (and so better potential mucosal-vaccine candidates), which antibody isotype (IgA, IgG, or IgM) predominates, how and where these immune responses take place, and whether carriage can induce mucosal memory responses.
Our analysis of cellular components of adenoids by flow cytometry confirms a previous report (6) showing the predominance of B cells over T cells. There were also significant numbers of macrophages, which may be important in antigen presentation, along with dendritic cells, which have also been shown to be present in adenoids (8). The predominance of CD4+ T cells (about fourfold higher than CD8+ cells) supports the notion that adenoidal T cells may provide significant help to B cells for antigen-specific antibody production.
Information on antibody responses to specific antigens in adenoidal cells is limited. To our knowledge, this study is the first description of immune responses in adenoidal lymphocytes from children to pneumococcal protein antigens. In this study, antigen-specific antibody responses to four novel pneumococcal protein antigens in adenoidal B cells from children were investigated, and it was shown that cells secreting antibodies of all three major isotypes (IgA, IgG, and IgM) to the four proteins are present in children. In freshly isolated adenoidal lymphocytes, antigen-specific IgG ASCs are predominant, followed by IgM and IgA ASCs. This is concordant with the general predominance of IgG immunocytes in adenoids (6). The numbers of CbpA- and PsaA-specific IgG ASCs were significantly greater than those of PspA- and PdB-specific ASCs. CbpA- and PsaA-specific IgA ASCs were also more abundant than PdB-specific ASCs. This suggests that CbpA and PsaA may be better mucosal immunogens and supports the findings in a mouse model that PsaA and CbpA (PspC) were the most efficacious protein antigens among those tested in immunizing against nasopharyngeal colonization (10).
The titers of antigen-specific IgA antibodies to the protein antigens in saliva broadly correlated with the numbers of antigen-specific IgA ASCs in adenoidal cells. It is known that adenoidal epithelial cells can produce secretory component, and thus S-IgA can be secreted from adenoids (7, 8). It is thought that salivary IgA, which is predominantly in secretory form, is locally produced (46). Therefore, adenoidal ASCs may be an important source for these protein-specific salivary IgA antibodies. Anti-CbpA IgA titers could not be determined, since all saliva samples measured reacted strongly with the CbpA antigen in the immunoassay. This appears to confirm observations by Hammerschmidt et al. and Zhang et al. that CbpA (SpsA) specifically binds to the secretory component of human S-IgA (19, 53).
There was no significant correlation between antigen-specific IgG in saliva and IgG ASCs in freshly isolated adenoidal cells from the same subjects. Although anti-CbpA salivary IgG levels were generally high, which may be related to high numbers of IgG ASCs in adenoids, anti-PsaA salivary IgG was notably absent, which is in contrast with the high numbers of IgG ASCs to PsaA in adenoidal cells. These data suggest that adenoidal IgG ASC numbers may not reliably predict salivary antibody concentrations. It is generally believed that salivary IgG is largely derived from serum (22, 54). There is no evidence that IgG is actively secreted through epithelium by a polymeric immunoglobulin receptor transport mechanism like S-IgA, and its presence in saliva may simply reflect passive diffusion from serum.
The relative importance of antigen-specific IgA and IgG in the local immune defense against pneumococcus is unknown. It is generally thought that IgA is predominant in mucosal areas (18, 32) and that IgG mainly provides systemic protection. However, recent studies have shown that in both adenoids and tonsils, IgG-secreting cells are predominant (6), which is different from the main induction and effector sites in the gastrointestinal tract (i.e., Peyer's patches and the lamina propria), where the majority of B cells secrete IgA (18, 32). The large numbers of IgG- and IgA-secreting cells in the epithelial and subepithelial compartments of adenoids and tonsils suggest that they have characteristics of effector sites and that IgG could be a significant component of local mucosal responses (6). The predominance of protein antigen-specific IgG-secreting cells in adenoids shown in this study supports the view that both IgA and IgG are important in the immune response in the upper respiratory tract.
It is known that the PspA, PsaA, and CbpA proteins are surface exposed and may be secreted by pneumococci (12, 31, 44, 51). Pneumococcal culture supernatants have been shown to induce interleukin-8 release from cultured human lung epithelial cells, and CbpA has been shown to be responsible for about 35% of this activity (31). Pneumolysin has also been shown to be released into culture supernatants in significant amounts from pneumococci during log-phase growth (2). We used a concentrated culture supernatant of a type 14 strain containing secreted proteins (including PspA, PsaA, CbpA, and pneumolysin, confirmed by Western immunoblotting) to stimulate adenoidal cells in vitro. Significant increases in the numbers of IgG ASCs for CbpA and, to a lesser extent, for PsaA and PspA antigens were observed after stimulation, but increases in IgA ASC numbers were rare, and increases in IgM ASC numbers were absent. The ASCs detected in culture after antigen stimulation (mainly IgG ASCs) are likely to be memory B cells. IgG memory can be induced by immunization and natural colonization. These results suggest that adenoids are an important inductive sites for memory IgG responses. The question of whether mucosal IgA memory responses can be induced is being debated, since several studies have shown that antigen-specific mucosal IgA responses are short-lived and that reimmunization does not reliably induce memory-type responses (5, 24, 36). The nature of the antigen may be an important determinant of whether mucosal IgA memory responses occur. Some studies suggest that some protein antigens, such as cholera toxin, can induce mucosal IgA memory-type rises in antigen-specific IgA ASC numbers in mice (27-30). It is thought that polysaccharide antigens do not induce immunological memory either systemically or at the mucosal level. Conjugate pneumococcal polysaccharide vaccines have been shown to prime for systemic memory IgG responses (13) and may also induce mucosal IgA memory for some serotype-specific responses to capsular pneumococci (14). In this study, two out of eight subjects had >2-fold increases in CbpA- and PspA-specific IgA ASCs following stimulation in culture, but none did for PsaA- and PdB-specific IgA ASCs. Thus, it appears that the former two antigens can prime for mucosal IgA memory responses but that this may not occur reliably following nasal carriage or infection. Differences between individuals may be responsible in part. Mucosal adjuvants, such as cholera toxin, E. coli heat-labile toxin, or their detoxified subunits, could improve or modify antigen-specific mucosal IgA memory responses (16, 33, 50).
Further studies of larger numbers of individuals with more detailed clinical and immunological assessment of prior exposure to pneumococci and more extensive and detailed modeling of immune responses in in vitro culture with purified recombinant proteins will further improve our understanding of the potential of these proteins as mucosal vaccines in humans.
Acknowledgments
We are grateful to Peter Bull, David Chapman, and John McEwan in the ENT department of Sheffield Children's Hospital for providing the adenoid tissues and to the patients who took part in the study. We thank Andrew Heath in the Division of Genomic Medicine, University of Sheffield, for advice and help in phenotypic analysis of adenoidal cells by flow cytometry.
Editor: E. I. Tuomanen
REFERENCES
- 1.Alexander, J. E., R. A. Lock, C. C. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran, P., S. K. Hollingshead, J. C. Paton, and D. E. Briles. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183:3108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogger-Goren, S., J. M. Bernstein, A. A. Gershon, and P. L. Ogra. 1984. Mucosal cell-mediated immunity to varicella zoster virus: role in protection against disease. J. Pediatr. 105:195-199. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, R., A. J. Fox, K. Cartwright, N. T. Begg, and D. M. Jones. 1999. Salivary antibodies following parenteral immunization of infants with a meningococcal serogroup A and C conjugated vaccine. Epidemiol. Infect. 123:201-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyaka, P. N., P. F. Wright, M. Marinaro, H. Kiyono, J. E. Johnson, R. A. Gonzales, M. R. Ikizler, J. A. Werkhaven, R. J. Jackson, K. Fujihashi, S. Di Fabio, H. F. Staats, and J. R. McGhee. 2000. Human nasopharyngeal-associated lymphoreticular tissues. Functional analysis of subepithelial and intraepithelial B and T cells from adenoids and tonsils. Am. J. Pathol. 157:2023-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandtzaeg, P. 1973. Two types of IgA immunocytes in man. Nat. New Biol. 243:142-143. [DOI] [PubMed] [Google Scholar]
- 8.Brandtzaeg, P. 1987. Immune functions and immunopathology of palatine and nasopharyngeal tonsils, p. 63-106. In J. M. Bernstein and P. L. Ogra (ed.), Immunology of the ear. Raven Press, New York, N.Y.
- 9.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briles, D. E., S. Hollingshead, A. Brooks-Walter, G. S. Nabors, L. Ferguson, M. Schilling, S. Gravenstein, P. Braun, J. King, and A. Swift. 2000. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine 18:1707-1711. [DOI] [PubMed] [Google Scholar]
- 11.Brooks-Walter, A., D. E. Briles, and S. K. Hollingshead. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect. Immun. 67:6533-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng, Q., D. Finkel, and M. K. Hostetter. 2000. Novel purification scheme and functions for a C3-binding protein from Streptococcus pneumoniae. Biochemistry 39:5450-5457. [DOI] [PubMed] [Google Scholar]
- 13.Choo, S., L. Seymour, R. Morris, S. Quataert, S. Lockhart, K. Cartwright, and A. Finn. 2000. Immunogenicity and reactogenicity of a pneumococcal conjugate vaccine administered combined with a Haemophilus influenzae type b conjugate vaccine in United Kingdom infants. Pediatr. Infect. Dis. J. 19:854-862. [DOI] [PubMed] [Google Scholar]
- 14.Choo, S., Q. Zhang, L. Seymour, S. Akhtar, and A. Finn. 2000. Primary and booster salivary antibody responses to a 7-valent pneumococcal conjugate vaccine in infants. J. Infect. Dis. 182:1260-1263. [DOI] [PubMed] [Google Scholar]
- 15.Crain, M. J., W. D. Waltman, J. S. Turner, J. Yother, D. F. Talkington, L. S. McDaniel, B. M. Gray, and D. E. Briles. 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58:3293-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czerkinsky, C., M. W. Russell, N. Lycke, M. Lindblad, and J. Holmgren. 1989. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect. Immun. 57:1072-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagan, R., R. Melamed, M. Muallem, L. Piglansky, and P. Yagupsky. 1996. Nasopharyngeal colonization in southern Israel with antibiotic-resistant pneumococci during the first 2 years of life: relation to serotypes likely to be included in pneumococcal conjugate vaccines. J. Infect. Dis. 174:1352-1355. [DOI] [PubMed] [Google Scholar]
- 18.Fujihashi, K., J. R. McGhee, C. Lue, K. W. Beagley, T. Taga, T. Hirano, T. Kishimoto, J. Mestecky, and H. Kiyono. 1991. Human appendix B cells naturally express receptors for and respond to interleukin 6 with selective IgA1 and IgA2 synthesis. J. Clin. Investig. 88:248-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammerschmidt, S., S. R. Talay, P. Brandtzaeg, and G. S. Chhatwal. 1997. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol. Microbiol. 25:1113-1124. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs, M. R., R. Dagan, P. C. Appelbaum, and D. J. Burch. 1998. Prevalence of antimicrobial-resistant pathogens in middle ear fluid: multinational study of 917 children with acute otitis media. Antimicrob. Agents Chemother. 42:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janulczyk, R., F. Iannelli, A. G. Sjoholm, G. Pozzi, and L. Bjorck. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 275:37257-37263. [DOI] [PubMed] [Google Scholar]
- 22.Kauppi, M., J. Eskola, and H. Kayhty. 1995. Anti-capsular polysaccharide antibody concentrations in saliva after immunization with Haemophilus influenzae type b conjugate vaccines. Pediatr. Infect. Dis. J. 14:286-294. [DOI] [PubMed] [Google Scholar]
- 23.Kodama, H., H. Faden, Y. Harabuchi, A. Kataura, J. M. Bernstein, and L. Brodsky. 1996. Adenoid lymphocyte responses to outer membrane protein P6 of nontypable Haemophilus influenzae in children with and without otitis media. Acta Oto-Laryngol. Suppl. 523:153-154. [PubMed] [Google Scholar]
- 24.Korkeila, M., H. Lehtonen, H. Ahman, O. Leroy, J. Eskola, and H. Kayhty. 2000. Salivary anti-capsular antibodies in infants and children immunised with Streptococcus pneumoniae capsular polysaccharides conjugated to diphtheria or tetanus toxoid. Vaccine 18:1218-1226. [DOI] [PubMed] [Google Scholar]
- 25.Korsrud, F. R., and P. Brandtzaeg. 1981. Influence of tonsillar disease on the expression of J chain by immunoglobulin-producing cells in human palatine and nasopharyngeal tonsils. Scand. J. Immunol. 13:281-287. [DOI] [PubMed] [Google Scholar]
- 26.Laurichesse, H., O. Grimaud, P. Waight, A. P. Johnson, R. C. George, and E. Miller. 1998. Pneumococcal bacteraemia and meningitis in England and Wales, 1993 to 1995. Commun. Dis. Publ. Health 1:22-27. [PubMed] [Google Scholar]
- 27.Lycke, N., U. Hellstrom, and J. Holmgren. 1987. Circulating cholera antitoxin memory cells in the blood one year after oral cholera vaccination in humans. Scand. J. Immunol. 26:207-211. [DOI] [PubMed] [Google Scholar]
- 28.Lycke, N., and J. Holmgren. 1986. Intestinal mucosal memory and presence of memory cells in lamina propria and Peyer's patches in mice 2 years after oral immunization with cholera toxin. Scand. J. Immunol. 23:611-616. [DOI] [PubMed] [Google Scholar]
- 29.Lycke, N., and J. Holmgren. 1987. Long-term cholera antitoxin memory in the gut can be triggered to antibody formation associated with protection within hours of an oral challenge immunization. Scand. J. Immunol. 25:407-412. [DOI] [PubMed] [Google Scholar]
- 30.Lycke, N., and J. Holmgren. 1989. Adoptive transfer of gut mucosal antitoxin memory by isolated B cells 1 year after oral immunization with cholera toxin. Infect. Immun. 57:1137-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madsen, M., Y. Lebenthal, Q. Cheng, B. L. Smith, and M. K. Hostetter. 2000. A pneumococcal protein that elicits interleukin-8 from pulmonary epithelial cells. J. Infect. Dis. 181:1330-1336. [DOI] [PubMed] [Google Scholar]
- 32.Mestecky, J., and J. R. McGhee. 1987. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv. Immunol. 40:153-245. [DOI] [PubMed] [Google Scholar]
- 33.Millar, D. G., T. R. Hirst, and D. P. Snider. 2001. Escherichia coli heat-labile enterotoxin B subunit is a more potent mucosal adjuvant than its closely related homologue, the B subunit of cholera toxin. Infect. Immun. 69:3476-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morag, A., B. Morag, J. M. Bernstein, K. Beutner, and P. L. Ogra. 1975. In vitro correlates of cell-mediated immunity in human tonsils after natural or induced Rubella virus infection. J. Infect. Dis. 131:409-416. [DOI] [PubMed] [Google Scholar]
- 35.Morrison, K. E., D. Lake, J. Crook, G. M. Carlone, E. Ades, R. Facklam, and J. S. Sampson. 2000. Confirmation of psaA in all 90 serotypes of Streptococcus pneumoniae by PCR and potential of this assay for identification and diagnosis. J. Clin. Microbiol. 38:434-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nurkka, A., J. MacLennan, V. Jantti, S. Obaro, B. Greenwood, and H. Kayhty. 2000. Salivary antibody response to vaccination with meningococcal A/C polysaccharide vaccine in previously vaccinated and unvaccinated Gambian children. Vaccine 19:547-556. [DOI] [PubMed] [Google Scholar]
- 37.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogunniyi, A. D., M. C. Woodrow, J. T. Poolman, and J. C. Paton. 2001. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect. Immun. 69:5997-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paton, J. C. 1998. Novel pneumococcal surface proteins: role in virulence and vaccine potential. Trends Microbiol. 6:85-87. [DOI] [PubMed] [Google Scholar]
- 40.Paton, J. C., P. W. Andrew, G. J. Boulnois, and T. J. Mitchell. 1993. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu. Rev. Microbiol. 47:89-115. [DOI] [PubMed] [Google Scholar]
- 41.Paton, J. C., R. A. Lock, C. J. Lee, J. P. Li, A. M. Berry, T. J. Mitchell, P. W. Andrew, D. Hansman, and G. J. Boulnois. 1991. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect. Immun. 59:2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pilling, P. A., M. C. Lawrence, A. M. Berry, A. D. Ogunniyi, R. A. Lock, and J. C. Paton. 1998. Expression, purification and preliminary X-ray crystallographic analysis of PsaA, a putative metal-transporter protein of Streptococcus pneumoniae. Acta Crystallogr. D 54:1464-1466. [DOI] [PubMed] [Google Scholar]
- 43.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Kayhty. 2000. Natural development of antibodies to Pneumococcal Surface Protein A, Pneumococcal Surface Adhesin A, and Pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 44.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol 25:819-829. [DOI] [PubMed] [Google Scholar]
- 45.Russell, H., J. A. Tharpe, D. E. Wells, E. H. White, and J. E. Johnson. 1990. Monoclonal antibody recognizing a species-specific protein from Streptococcus pneumoniae. J. Clin. Microbiol. 28:2191-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simell, B., M. Korkeila, H. Pursiainen, T. M. Kilpi, and H. Kayhty. 2001. Pneumococcal carriage and otitis media induce salivary antibodies to Pneumococcal Surface Adhesin A, Pneumolysin, and Pneumococcal Surface Protein A in children. J. Infect. Dis. 183:887-896. [DOI] [PubMed] [Google Scholar]
- 47.Tart, R. C., L. S. McDaniel, B. A. Ralph, and D. E. Briles. 1996. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J. Infect. Dis. 173:380-386. [DOI] [PubMed] [Google Scholar]
- 48.Virolainen, A., W. Russell, M. J. Crain, S. Rapola, H. Kayhty, and D. E. Briles. 2000. Human antibodies to pneumococcal surface protein A in health and disease. Pediatr. Infect. Dis. J. 19:134-138. [DOI] [PubMed] [Google Scholar]
- 49.Waltman, W. D., L. S. McDaniel, B. M. Gray, and D. E. Briles. 1990. Variation in the molecular weight of PspA (pneumococcal surface protein A) among Streptococcus pneumoniae. Microb. Pathog. 8:61-69. [DOI] [PubMed] [Google Scholar]
- 50.Williams, N. A., T. R. Hirst, and T. O. Nashar. 1999. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol. Today 20:95-101. [DOI] [PubMed] [Google Scholar]
- 51.Yother, J., G. L. Handsome, and D. E. Briles. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 174:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zenni, M. K., S. H. Cheatham, J. M. Thompson, G. W. Reed, A. B. Batson, P. S. Palmer, K. L. Holland, and K. M. Edwards. 1995. Streptococcus pneumoniae colonization in the young child: association with otitis media and resistance to penicillin. J. Pediatr. 127:533-537. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, J. R., K. E. Mostov, M. E. Lamm, M. Nanno, S. Shimida, M. Ohwaki, and E. Tuomanen. 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102:827-837. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, Q., S. Choo, J. Everard, R. Jennings, and A. Finn. 2000. Mucosal immune responses to meningococcal group C conjugate and group A and C polysaccharide vaccines in adolescents. Infect. Immun. 68:2692-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]