Abstract
The primary human urethral epithelial cells developed by our laboratory have been immortalized by transduction with a retroviral vector expressing the human papillomavirus E6E7 oncogenes. Analysis of telomerase expression and comparison to that in primary cells revealed detectable levels in the transduced human urethral epithelial cells. Immortalized urethral cells could be passaged over 20 times. Immunofluorescence microscopy studies showed that the immortalized cells were phenotypically similar and responded to gonococcal infection similarly to primary cells. Specifically, positive cytokeratin staining showed that the immortalized cells are keratinocytes; cell surface levels of human asialoglycoprotein receptor increase following gonococcal infection, and, like the primary cells, the immortalized urethral epithelial cells are CD14 negative. Using enzyme-linked immunosorbent assay, we found that interleukin-6 (IL-6) and IL-8 levels in primary urethral epithelial cell supernatants increase after challenge with N. gonorrhoeae. Likewise, the immortalized urethral epithelial cells produced higher levels of IL-6 and IL-8 cytokines in response to gonococcal infection. Cells challenged with a gonococcal lipid A msbB mutant produced reduced IL-6 and IL-8 levels when compared to the parent strain. Additionally, these data suggest that the 1291 msbB lipooligosaccharide may suppress cytokine induction.
Urethral epithelial cells are invaded by Neisseria gonorrhoeae during gonococcal infection in men (2). To further understand the mechanisms of gonococcal entry into host cells, we developed a system to culture primary human urethral epithelial cells (PHUEC) in vitro (8). Studies using the PHUEC have contributed to the understanding of gonococcal pathogenesis (5, 7, 8, 31). However, several factors have limited studies of this primary cell system. The number of cells available for experiments depends on the availability of surgical tissue samples. In addition, the primary nature of these cells results in a limited life span and a limited number of replications before senescence. For this reason the PHUEC were immortalized with a retroviral vector expressing human papillomavirus (HPV) E6E7 oncogenes. A series of experiments to characterize the immortalized cells and to ensure that they provide an environment similar to that for the primary cells for the study of gonococcal pathogenesis were then performed.
In males gonococcal urethritis is characterized by an inflammatory response in the urethral epithelium. Recent awareness that epithelial cells, in addition to serum monocytes and polymorphonuclear leucocytes (PMNs), are a source of cytokines has led to several studies investigating epithelial cell signal transduction and cytokine release during gonococcal infection (11, 16, 17, 19, 21, 26; for reviews see references 12 and 20). It has not been completely defined in vivo which cytokines are involved and which are produced by epithelial cells or by effector cells recruited to the site of infection. Ramsey et al. showed in the human experimental model of gonococcal infection that inflammatory cytokines interleukin-6 (IL-6), IL-8, and tumor necrosis factor alpha (TNF-α) were present in the urine prior to the onset of symptoms, as early as 2 h after challenge with gonococci (26). During this time, IL-6, IL-8, and TNF-α mRNA levels in PMNs present in the urine were unaltered. IL-1β was not detected in the urine until the onset of symptoms. Their findings suggested that IL-6, IL-8, and TNF-α were produced by the urethral epithelium, while IL-1β was released from infiltrating PMNs. One of our present objectives was to study the influence of gonococcal challenge on IL-6 and IL-8 release in the primary and immortalized urethral cells. Similar to other pathogenic bacterial endotoxins (4, 22, 25, 29, 30), gonococcal lipooligosaccharide (LOS) is believed to play a role in the inflammatory response (12, 24, 27).
Previously, an msbB mutant was constructed from N. gonorrhoeae strain 1291 and designated 1291A11K3 (23). This mutant was shown to express a penta-acyl rather than a hexa-acyl lipid A structure (23). Using strains 1291 and 1291A11K3, we investigated the role that the gonococcal lipid A moiety plays in the induction of urethral cell cytokine production.
MATERIALS AND METHODS
Epithelial cell culture.
PHUEC used in these studies were derived from membranous urethral tissue explants obtained from 15 male patients undergoing prostate surgery. PHUEC were grown, maintained, and passaged as described previously (7, 8). Briefly, surgical tissue samples were seeded in prostate epithelial growth medium (PrEGM) (Clonetics, San Diego, Calif.) containing 5% heat-inactivated fetal calf serum (FCS) on 100-mm-diameter tissue culture-treated petri dishes. Five to 7 days following seeding, cultures were maintained in FCS-free PrEGM. Trypsin (0.25%)-0.1% EDTA was used for lifting cells during passaging. Cells were incubated in trypsin solution 4 min at room temperature, followed by removal of trypsin and incubation for 4 min at 37°C. PHUEC were suspended in 5% FCS-PrEGM, centrifuged 2 min at 1,380 × g, and resuspended in the desired volume of PrEGM prior to seeding. HepG2 and Chang epithelial cells were grown in tissue culture flasks with minimal essential medium or RPMI 1640 (Gibco-BRL, Grand Island, N.Y.), respectively, containing heat-inactivated 10% FCS and 1% l-glutamine.
Transduction of PHUEC.
Transduced human urethral epithelial cells (THUEC) originated from PHUEC grown from six different membranous urethral tissue explants obtained from men undergoing prostate surgery. PHUEC were grown and maintained as described above. When PHUEC were 50 to 70% confluent, transductions were performed. Transductions were performed in 100-mm-diameter petri dishes according to previously established protocols (6, 14).
Construction of recombinant retrovirus plasmids containing the HPV E6E7 genes was described previously (6). These constructs were a generous gift from Denise Galloway (Cancer Biology Program, The Fred Hutchinson Cancer Research Center, Seattle, Wash.) and Aloysius Klingelhutz (Department of Microbiology, The University of Iowa, Iowa City, Iowa). Briefly, these constructs consisted of the retrovirus vector pLXSN with a 5′ Moloney murine promoter-enhancer (long terminal repeat), followed by the HPV E6E7 DNA fragment and a downstream gene conferring neomycin resistance driven by the simian virus 40 promoter. Supernatants from mouse fibroblasts (PG-13 and PA317 producer cell lines) infected with retroviral constructs (pLXSN control and HPV E6E7 LXSN, respectively) were used to transduce PHUEC. The PG-13 and PA317 producer cell lines and transduction protocols have been described previously (6, 14).
Prior to transduction, PHUEC medium was changed to PrEGM containing 5% FCS and 4 μg of hexadimethrine bromide (Sigma, St. Louis, Mo.)/ml. One milliliter of PG-13 medium (supernatant containing retrovirus) was then added to each 100-mm-diameter dish, and cells were incubated overnight at 37°C, 5% CO2 (normal growth conditions). The next day urethral cells were rinsed once with normal PrEGM, given fresh PrEGM, and then maintained as usual for up to 1 week following the transduction. Cells were then split 1:3 or 1:4 and maintained in 10 μg of Geneticin (Gibco BRL, Grand Island, N.Y.)/ml. THUEC were maintained and passaged similarly to PHUEC. Cells successfully passaged and grown to confluence greater than 10 times or for more than 6 months were frozen down in aliquots as described below for future use.
For freezing THUEC in liquid nitrogen, THUEC were lifted and centrifuged as described above and then resuspended in 1 ml of 10% dimethyl sulfoxide-10% FCS-PrEGM per 100-mm-diameter tissue culture petri dish containing confluent THUEC growth. One-milliliter aliquots of 105 to 106 cells in cryovials were then kept at −80°C overnight before transfer to liquid nitrogen tank for storage. For subsequent use, THUEC were thawed in a 37°C water bath and 1-ml cell suspensions were added to 3 ml of PrEGM containing 5% FCS and then seeded to 100-mm petri dishes for growth.
Telomerase PCR ELISA.
This photometric enzyme immunoassay uses the telomeric repeat amplification protocol to detect telomerase activity in cells. We used the telomerase PCR enzyme-linked immunosorbent assay (ELISA) kit (Roche Molecular Chemicals, Mannheim, Germany). The assay was performed according to kit instructions.
Karyotyping.
Karyotyping was performed by S. Patil (Cytogenetics Laboratory, Department of Pediatrics, The University of Iowa Hospitals and Clinics). Ten THUEC were examined. Cells were arrested in cell division with Colcemid. Chromosomes were G banded and analyzed for the presence of detectable structural abnormalities. Karyotypes were prepared from computer-assisted images of these metaphases.
Characterization of THUEC.
Growth and morphological characteristics of immortalized urethral cells were monitored daily with a light microscope. We used the Bio-Rad MRC 1024 laser scanning confocal microscope at the University of Iowa Central Microscopy Research Facility for further characterization experiments. Immunofluorescence analysis for cytokeratin was performed as described previously (8) by using a commercially available murine monoclonal antibody immunospecific to cytokeratins 13 and 16 (Sigma). THUEC were analyzed for the presence of CD14 by using mouse monoclonal antibody CD14 Ab-1 (clone B-A8) from NeoMarkers (Union City, Calif.) with a fluorescein-labeled affinity-purified antibody to mouse immunoglobulin G from Kirkegaard & Perry Laboratories Inc. (Gaithersburg, Md.). As a positive control for the CD14 studies, macrophage-derived cell line THP-1 cells were used. Infection of THUEC with N. gonorrhoeae strain 1291 and laser scanning confocal microscopy (LSCM) analysis for the human ASGP-R were performed as described previously (7, 8). As previously described, all samples were counterstained with ethidium bromide prior to mounting (7, 9).
Bacterial strains and infection protocol.
Infections of PHUEC and THUEC were performed as previously described with N. gonorrhoeae strains 1291 and 1291A11K3 (7, 8). Opa+ (opacity-associated protein-positive) P+ (pilus-positive) organisms were selected based on colony morphology. For PHUEC experiments, bacteria were grown overnight on gonococcal (GC) agar, suspended in GC broth, and diluted in antibiotic-free PrEGM. As before, multiplicity-of-infection ratios were approximately 100 gonococci/epithelial cell. Infections of THUEC were performed in media containing gentamicin so that the bacteria would not be allowed to replicate. 1291 and 1291A11K3 have previously been shown to have differences in their abilities to survive intracellularly (23). The MICs of gentamicin for both strains were the same. THUEC were challenged with 1291 and 1291A11K3 at concentrations of 105 or 106 bacteria/ml. 1291 and 1291A11K3 were grown overnight on GC agar, suspended in GC broth, and diluted in PrEGM to the proper cell density. Three milliliters of bacterial suspensions was added per 60-mm-diameter dish. In some experiments, PHUEC and THUEC were challenged with purified, lyophilized LOS from gonococcal strains 1291 and 1291A11K3 suspended in tissue culture medium at concentrations of 0.01, 0.1, 1.0, and 10 μg/ml. All experiments were performed a minimum of three times.
Cytokine assays.
Supernatants were collected from infected or LOS-challenged epithelial cells and frozen at −80°C until analysis. After being thawed on ice, supernatants were concentrated 10-fold with Centricon-10 concentrators (Amicon, Beverly, Mass.) for IL-6, IL-8, and IL-1β analysis and Centricon-30 concentrators for TNF-α analysis. During concentration the samples were maintained at 4°C. They were then analyzed for IL-6, IL-8, TNF-α, and IL-1β content according to a previously established cytokine ELISA protocol (3). Recombinant human cytokine standards were obtained from Pharmingen (San Diego, Calif.). Rat anti-IL-6 capture antibody 20F3.11 and detection antibody 32C11.4 were generously provided by G. Bishop and M. Baccam (The University of Iowa Department of Microbiology), with permission from J. Abrams (DNAX Research Institute, Palo Alto, Calif.). For detection of IL-8, TNF-α, and IL-1β, the appropriate purified mouse anti-human monoclonal antibodies for capture and biotinylated mouse anti-human monoclonal antibodies for detection were obtained from Pharmingen. Samples were read at 490 nm on an EL311 microplate autoreader (Bio-Tek Inc., Winooski, Vt.) using the DeltaSoft 3 software (Biometallics Inc., Princeton, N.J.). Data were analyzed with DeltaGraph software, version 4.0.1 (SPSS, Chicago, Ill.).
RESULTS
Immortalization of PHUEC.
Transduction with recombinant retrovirus vector pLXSN containing the HPV16 E6E7 oncogenes was attempted with primary cells derived from six different patients. Three of the six transductions resulted in immortalized PHUEC, here designated THUEC. Of the three unsuccessful attempts, one transduction became contaminated and two were discarded due to excess growth of fibroblasts. THUEC survived more than 10 passages in selective medium containing 10 μg of Geneticin (neomycin)/ml. THUEC were frozen in liquid nitrogen, thawed, and subsequently recultured at least twice. Following thawing and reculturing, THUEC have been successfully passaged over 20 times. None of the PHUEC transduced with the pLXSN retrovirus control (no E6E7 oncogene insert) survived multiple passaging or the presence of neomycin.
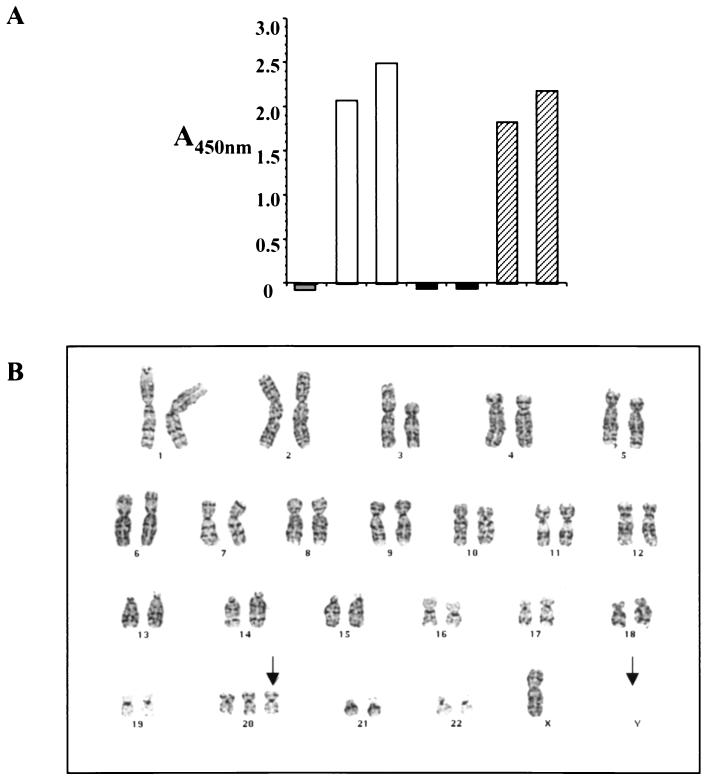
Telomerase PCR ELISA showed that, compared to PHUEC, THUEC produce detectable levels of telomerase (Fig. 1A). Karyotyping demonstrated that the THUEC had a modal number of 46 chromosomes, including the X and Y chromosomes. Abnormalities that were present in more than two cells were the absence of the Y chromosome and an extra copy of chromosome 20 (Fig. 1B). In support of the telomerase PCR ELISA data, telomere association was observed in most of the cells and involved chromosomes 7, 15, 19, and 20.
FIG. 1.
THUEC phenotype characteristics. (A) Telomerase PCR ELISA showed detectable levels of telomerase in THUEC. y axis, absorbance at 450 nm. Grey bar, lysis buffer (negative control); white bars, 1:4 dilution and undiluted positive-control cell lysates; black bars, 1:4 dilution and undiluted PHUEC lysates; hatched bars, 1:4 dilution and undiluted THUEC lysates. (B) Karyotyping results demonstrated that a number of THUEC examined were missing the Y chromosome and contained an extra copy of chromosome 20. Arrows, sites of chromosomal aberrations.
Growth and morphology characteristics of THUEC were similar to those of PHUEC. Immortalization of the PHUEC produced the desired alteration in division characteristics that led to the ability of the THUEC to proliferate indefinitely but, as determined by light microscopy, did not result in diminished cell-cell adhesion or a reduced requirement for growth on a surface. Light microscopy showed no visual differences in morphology between the THUEC and PHUEC.
Phenotypic characterization of THUEC.
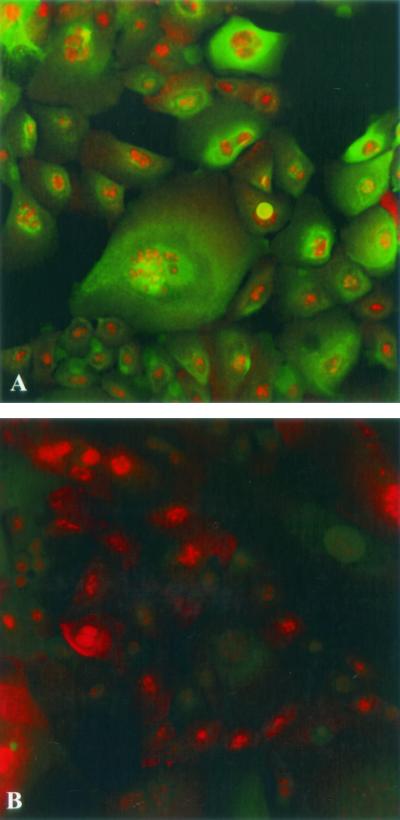
All epithelial cells possess intermediate filament proteins called cytokeratins. When the primary urethral cell tissue culture system was established, these cells were assayed for the presence of cytokeratin to ensure that they were epithelial in nature (8). Studies showed that the PHUEC express cytokeratins 13 and 16 (8). The THUEC were examined for the presence of cytokeratins 13 and 16 by LSCM. The THUEC, like their PHUEC progenitors, express cytokeratins 13 and 16 (Fig. 2).
FIG. 2.
Immunofluorescence confocal microscopy showed that THUEC express squamous epithelial cell markers cytokeratins 13 and 16 (green). Cells were counterstained with the nucleic acid stain ethidium bromide (red). Magnification, ×60. (A) Cytokeratin localized with the fluorescein isothiocyanate-conjugated antibody. (B) Conjugate control (no primary antibody).
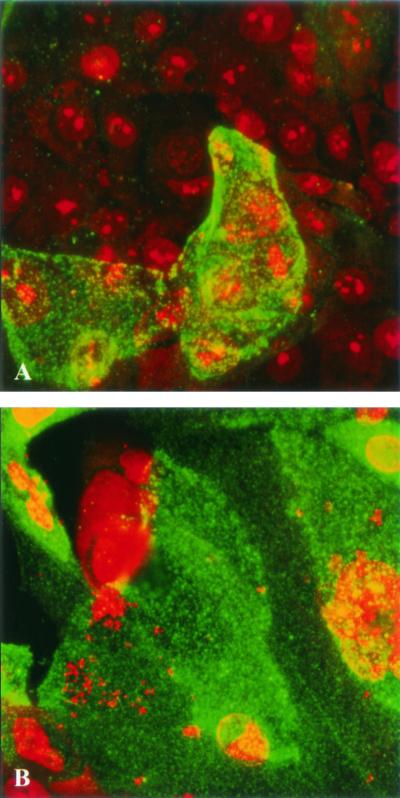
Previous work from our laboratory revealed the presence of the ASGP-R on PHUEC (7, 8). LSCM showed that this receptor is also present on THUEC (Fig. 3). Similar to what has been observed in the PHUEC, challenge of THUEC with N. gonorrhoeae results in increased levels of ASGP-R at the apical cell surface (Fig. 3) (7, 8).
FIG. 3.
LSCM showed that THUEC cell surface expression of ASGP-R is inducible by infection with N. gonorrhoeae. ASGP-R was localized with fluorescein isothiocyanate-conjugated 6C2 (green), and the samples were counterstained with ethidium bromide. Magnification, ×60. (A) Uninfected THUEC. (B) THUEC challenged for 4 h with N. gonorrhoeae.
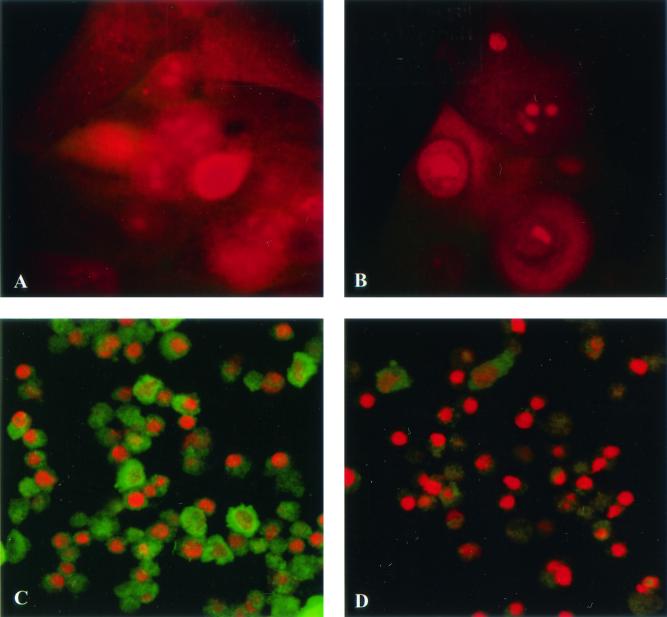
We have also analyzed PHUEC for lipopolysaccharide-binding cell surface antigen CD14 (H. A. Harvey, D. Lubaroff, R. Williams, and M. A. Apicella, Abstr. 11th Int. Pathog. Neisseria Conf., 1998). Although reverse transcriptase PCR showed that PHUEC express CD14 mRNA, CD14 was undetectable by immunofluorescence microscopy in uninfected PHUEC or in PHUEC infected with gonococci (Harvey et al., Abstr. 11th Int. Pathog. Neisseria Conf.). Similarly, LSCM showed that CD14 is undetectable in THUEC (Fig. 4).
FIG. 4.
THUEC do not express CD14. CD14 was localized with a fluorescein isothiocyanate-conjugated antibody (green), and samples were counterstained with ethidium bromide. Magnification, ×60. (A) THUEC. (B) THUEC conjugate control (no primary antibody). (C) THP-1 cells (positive control). (D) THP-1 cell conjugate control.
Gonococcal infection induces inflammatory-cytokine production by urethral epithelial cells.
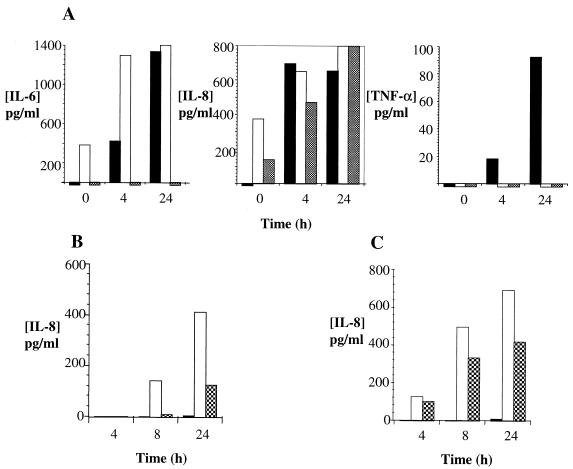
Figure 5A shows PHUEC, Chang cell, and HepG2 cell cytokine production before and after infection with N. gonorrhoeae strain 1291. IL-6 and IL-8 were detectable in PHUEC and Chang cells but not in HepG2. PHUEC produced detectable levels of IL-6, IL-8, and TNF-α following challenge for at least 4 h with N. gonorrhoeae strain 1291. IL-6 and TNF-α levels in PHUEC supernatants increased with increasing infection times. IL-8 levels observed at 4 h of infection in PHUEC did not change significantly after 24 h. PHUEC that were unchallenged or challenged for 15 min to 1 h did not produce detectable levels of IL-6, IL-8, or TNF-α. IL-6 and IL-8 production in Chang cells was inducible after 4 h of infection with gonococci. However, background levels of IL-6 and IL-8 in uninfected Chang cells were considerably higher than in PHUEC. Similar to that by Chang cells, IL-8 production by HepG2 cells was inducible by gonococcal infection, but IL-8 levels in resting cells were higher than those in PHUEC. Interestingly, TNF-α cytokine levels were inducible by gonococcal infection in PHUEC but were undetectable in both HepG2 and Chang cells. IL-1β was not detectable in PHUEC, Chang, or HepG2 supernatants.
FIG. 5.
PHUEC and THUEC produced inflammatory cytokines in response to gonococcal infection. y axes, concentrations of cytokines; x axes, hours of infection with N. gonorrhoeae. (A) PHUEC, Chang cell, and HepG2 cell cytokine production after challenge with N. gonorrhoeae strain 1291. Solid bars, PHUEC; open bars, Chang cells; shaded bars, HepG2 cells. (B and C) THUEC cytokine production in response to gonococcal infection with 105 and 106 bacteria/ml, respectively. Black bars, no challenge; white bars, challenge with 1291 bacteria; cross-hatched bars, challenge with 1291A11K3 bacteria. Data represent at least three separate experiments.
Figure 5B and C show THUEC infected with N. gonorrhoeae strains 1291 and 1291A11K3. Both strains were able to induce a cytokine response in the THUEC. Prior to 8 h, IL-8 was undetectable in THUEC infected with 105 bacteria/ml. IL-8 release was detectable in cells infected with 106 bacteria/ml at all time points. The amount of cytokine release in response to both strains of bacteria was higher at the higher concentrations of bacteria. Additionally, the amount of cytokine released in response to both strains increased over time. Strain 1291 induced a higher level of cytokine release than strain 1291A11K3 under all comparable conditions. Cells that were uninfected did not produce detectable levels of IL-8.
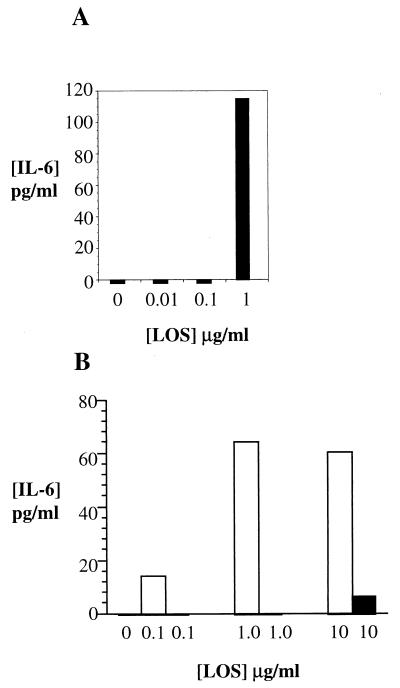
PHUEC were challenged with purified strain 1291 LOS at concentrations ranging from 0.01 to 1 μg/ml. Detectable levels of IL-6 and IL-8 were produced only in response to LOS concentrations greater than or equal to 1 μg/ml (Fig. 6A).
FIG. 6.
PHUEC and THUEC produced IL-6 in response to a 4-h challenge with purified gonococcal LOS. y axes, IL-6 concentrations; x axes, LOS concentrations. (A) PHUEC IL-6 production after challenge with purified 1291 LOS. (B) THUEC IL-6 production after challenge with purified LOS. Gray bars (not visible), no challenge; white bars, challenge with 1291 LOS; black bars, challenge with 1291A11K3 LOS. Data represent at least three separate experiments.
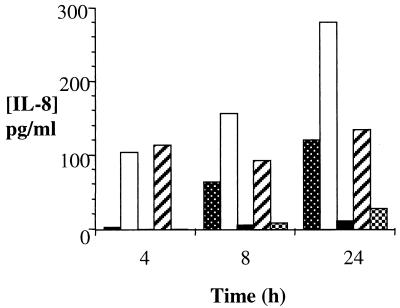
THUEC were challenged with purified LOS isolated from strains 1291 and 1291A11K3 (Fig. 6B and 7). IL-8 challenges were performed for 4, 8, and 24 h. IL-6 challenges were performed for 4 h. Strain 1291 LOS was able to induce both IL-8 and IL-6 responses at all concentrations shown. In the THUEC, strain 1291 LOS was unable to induce a cytokine response below LOS concentrations of 0.1 μg/ml. Strain 1291A11K3 LOS did not induce an IL-8 response above background levels. However, 10 μg of 1291A11K3 LOS/ml induced a detectable IL-6 response.
FIG. 7.
THUEC released IL-8 in response to challenge with purified gonococcal LOS. y axis, IL-8 concentrations; x axis, challenge times. Shown is cytokine production in response to no LOS (dotted bars) and challenge with 1 μg of 1291 LOS/ml (white bars), 1 μg of 1291A11K3 LOS/ml (black bars), 0.1 μg of 1291 LOS/ml (hatched bars), and 0.1 μg of 1291A11K3 LOS/ml (crosshatched bars). Data represent at least three separate experiments.
DISCUSSION
The objective of this work was to modify the primary urethral cell system, which has been shown to be an ideal environment for the study of gonococcal pathogenesis (5, 7, 8, 31). The primary cells were immortalized with E6E7 oncogenes from a tumorigenic virus. In eukaryotic cells telomere length has been shown to be an essential component in determining replicative life span (15). Chromosomes lose telomeric DNA sequence with each round of DNA replication. Shortening of telomeres is hypothesized to contribute to normal cell senescence. Lost telomeric DNA sequence can be restored through the activity of the ribonucleoprotein enzyme telomerase, which uses its RNA component as a template for synthesis of telomeric DNA onto chromosomes. It has been shown that tumor cells and E6E7-transduced cells show no loss of telomere length with cell division and that they have highly detectable levels of telomerase activity, unlike the undetectable levels in normal cells (13, 15). Extended proliferation, detectable levels of telomerase, and the resulting potential for multiple passaging indicated that the urethral cells were successfully immortalized.
Changes in the plasma membrane, adherence properties, growth, and division can occur when normal tissue culture cells are transformed by a virus (1). For this reason characterization of THUEC was important. The comparison studies performed so far demonstrated few phenotypic differences between the PHUEC and THUEC. Upon visual examination, urethral epithelial cell morphology in immortalized cells was found to be unchanged. In addition, similar to the PHUEC, THUEC exhibited the following traits: they were positive for epithelial cell markers cytokeratins 13 and 16, CD14 was undetectable, and cell surface ASGP-R expression was inducible by infection with N. gonorrhoeae. Other studies from our laboratory have shown that THUEC respond comparably to PHUEC when challenged with N. gonorrhoeae (23). THUEC and PHUEC are invaded by gonococci at similar levels (7, 23).
This work demonstrated that PHUEC and THUEC production of inflammatory cytokines is inducible by challenge with gonococci and gonococcal LOS. These studies also showed that some epithelial cell lines do not produce the same cytokines as the primary urethral cells. This demonstrates the importance of using a physiologically relevant cell type (similar to that encountered by gonococci during natural infection) in combination with other cell types to study certain aspects of gonococcal pathogenesis in vitro.
CD14, found on macrophages and PMNs and in soluble form in serum, plays an important role in cell activation by bacterial endotoxin (25, 30). CD14 is undetectable in PHUEC and patient exudates by immunofluorescence microscopy (Harvey et al., Abstr. 11th Int. Pathog. Neisseria Conf.). Stimulation by endotoxin induces gene transcription and expression of proteins that mediate the inflammatory response in activated cells. As mentioned above, the lowest concentration of purified strain 1291 LOS that activated production of IL-6 by the PHUEC was 1 μg/ml. Additionally, the lowest concentration of 1291 LOS that induced an IL-8 or IL-6 response in the THUEC was 0.1 μg/ml. The presence of normal serum may enhance cell activation or response to bacterial challenge. Studies to further explore this possibility are under way.
There are many questions concerning epithelial signal transduction as a result of gonococcal adherence and invasion. Studies with epithelial cells have been done to address this question (20). In vitro studies with Chang and HeLa epithelial cell lines demonstrated that infection with N. gonorrhoeae induces activation of transcription factors nuclear factor κB (NF-κB) and activator protein 1 by a cascade of stress response kinases (19, 21). This leads to the production of inflammatory cytokines TNF-α and IL-8 (19, 21). Another group observed phosphotyrosine accumulation beneath adherent gonococci in A431, HEC-1-B, Chang, and T84 cells (18). They suggested that the phosphotyrosine accumulation may be a part of the signaling pathway that elicits the activation of host transcription factors and cytokine production (18). McGee et al. (16, 17) showed that gonococcal infection induces TNF-α release in the human fallopian tube organ culture model and that the presence of this inflammatory cytokine engenders the resulting damage and sloughing of the epithelial mucosa. This work showed that PHUEC produce and release IL-6, IL-8, and TNF-α as a result of exposure to gonococci or gonococcal LOS. Additionally, data presented here demonstrated that the THUEC developed in this study produce and release IL-6 and IL-8 in response to exposure to 1291 bacteria or purified 1291 LOS. Experiments performed with the 1291A11K3 LOS showed that an intact lipid A structure is necessary for full induction of an IL-6 or an IL-8 response in THUEC. These data correspond to previous findings that demonstrated that a reduction in the acylation of lipid A decreased its ability to stimulate a cytokine response from cells (22, 28). These data also demonstrated that an intact LOS is responsible for the majority of cytokine induction by the THUEC. Additionally, the studies measuring IL-8 induction by 1291 and 1291A11K3 LOS consistently showed that the amount of IL-8 induction in response to 1291A11K3 LOS was less than that in unchallenged cells (Fig. 7). These data suggest that the presence of the 1291A11K3 LOS may suppress cytokine induction.
The following infection scenario incorporates our present findings for the PHUEC and THUEC with previous observations made in vivo during natural infection and human volunteer studies. During the initial stage of gonococcal infection in males prior to the onset of symptoms, urethral epithelial cells produce inflammatory cytokines, including IL-6, IL-8, and TNF-α, resulting in the chemoattraction of neutrophils, which present a further source of cytokines such as IL-1β, to the site of infection. This inflammatory response results in formation of the purulent exudate containing shed epithelial cells and PMNs. These studies support in vivo findings that, during gonococcal infection in males, urethral epithelial cells may be the initial source of cytokine production that results in recruitment of neutrophils and the characteristic local inflammatory response. However, this scenario contrasts with one group's findings that in women with gonococcal cervicitis local levels of IL-1, IL-6, and IL-8 were not elevated (10). These data suggest that the inflammatory response to gonococcal infection differs in males and females.
The characterization studies performed so far have shown that THUEC, in addition to being an unlimited source of tissue culture urethral epithelial cells, provide an ideal environment for the in vitro study of gonococcal pathogenesis. Extension of the tissue culture urethral epithelial cell life span and proliferation capabilities while maintaining growth and phenotype characteristics will have broad research applications. For this reason, a further objective of this work is to make the THUEC available for others studying the pathogenesis of N. gonorrhoeae and other relevant sexually transmitted diseases such as those caused by Chlamydia trachomatis, mycoplasma, and ureaplasma.
Acknowledgments
We thank Richard K. Williams (The Department of Urology, The University of Iowa Hospitals and Clinics) for providing the urethral tissue samples. We thank Denise Galloway (Cancer Biology Program, The Fred Hutchinson Cancer Research Center, Seattle, Wash.) and Aloysius Klingelhutz (Department of Microbiology, The University of Iowa) for providing the PG-13 and PA317 producer cell lines infected with retroviral constructs, pLXSN, and HPV E6E7 LXSN. We thank S. Patil of the Cytogenetics Lab, Department of Pediatrics, The University of Iowa Hospitals and Clinics. We thank the Central Microscopy Research Facility at the University of Iowa. We thank Gail Bishop and Mekhine Baccam (Department of Microbiology, The University of Iowa) and John Abrams (DNAX Research Institute, Palo Alto, Calif.) for their technical advice and for the IL-6 monoclonal antibodies.
This work was supported by NIAID grants AI44642, AI38515, and AI45728. H. A. Harvey's work was in part supported by NIH training grant 5T32 AI07511-04. D. M. B. Post's work was in part supported by NIH training grant T32 A107511.
Editor: E. I. Tuomanen
REFERENCES
- 1.Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. D. Watson. 1989. Molecular biology of the cell, p. 752-761. Garland Publishing, Inc., New York, N.Y.
- 2.Apicella, M. A., M. R. Ketterer, F. K. N. Lee, D. Zhou, P. A. Rice, and M. S. Blake. 1996. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J. Infect. Dis. 173:636-646. [DOI] [PubMed] [Google Scholar]
- 3.Baccam, M., and G. A. Bishop. 1999. Membrane-bound CD154, but not CD40-specific antibody, mediates NF-κB-independent IL-6 production in B cells. Eur. J. Immunol. 29:3855-3866. [DOI] [PubMed] [Google Scholar]
- 4.Curfs, J. H. A. J., J. F. G. M. Meis, and J. A. A. Hoogkamp-Korstanje. 1997. A primer on cytokines: sources, receptors, effects, and inducers. Clin. Microbiol. Rev. 10:742-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giardina, P. C., R. Williams, D. Lubaroff, and M. A. Apicella. 1998. Neisseria gonorrhoeae induces focal polymerization of actin in primary human urethral epithelium. Infect. Immun. 66:3416-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halbert, C. L., G. W. Demers, and D. A. Galloway. 1991. The E7 gene of human papilloma virus type 16 is sufficient for immortalization of human epithelial cells. J. Virol. 65:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey, H. A., M. P. Jennings, C. A. Campbell, R. Williams, and M. A. Apicella. 2001. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol. Microbiol. 42:659-672. [DOI] [PubMed] [Google Scholar]
- 8.Harvey, H. A., M. R. Ketterer, A. Preston, D. Lubaroff, R. Williams, and M. A. Apicella. 1997. Ultrastructural analysis of primary human urethral epithelial cell cultures infected with Neisseria gonorrhoeae. Infect. Immun. 65:2420-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey, H. A., N. Porat, C. A. Campbell, M. Jennings, B. W. Gibson, N. J. Phillips, M. A. Apicella, and M. S. Blake. 2000. Gonococcal lipooligosaccharide is a ligand for the asialoglycoprotein receptor on human sperm. Mol. Microbiol. 36:1059-1070. [DOI] [PubMed] [Google Scholar]
- 10.Hedges, S. R., D. A. Sibley, M. S. Mayo, E. W. Hook III, and M. W. Russell. 1998. Cytokine and antibody response in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J. Infect. Dis. 178:742-751. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis, G. A., J. Li, and K. V. Swanson. 1999. Invasion of human mucosal epithelial cells by Neisseria gonorrhoeae upregulates expression of intercellular adhesion molecule 1. Infect. Immun. 67:1149-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerse, A. E., and R. F. Rest. 1997. Adhesion and invasion by the pathogenic Neisseria. Trends Microbiol. 5:217-221. [DOI] [PubMed] [Google Scholar]
- 13.Kim, N. W., M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. C. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich, and J. W. Shay. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011-2015. [DOI] [PubMed] [Google Scholar]
- 14.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 15.Klingelhutz, A. J. 1997. Telomerase activation and cancer. J. Mol. Med. 75:45-49. [DOI] [PubMed] [Google Scholar]
- 16.McGee, Z. A., C. M. Clemens, R. L. Jensen, J. J. Klein, L. R. Barley, and G. L. Gorby. 1992. Local induction of tumor necrosis factor as a molecular mechanism of mucosal damage by gonococci. Microb. Pathog. 12:333-341. [DOI] [PubMed] [Google Scholar]
- 17.McGee, Z. A., R. L. Jensen, C. M. Clemens, D. Taylor-Robinson, A. P. Johnson, and C. R. Gregg. 1999. Gonococcal infection of human fallopian tube mucosa in organ culture: relationship of mucosal tissue TNF-α concentration to sloughing of ciliated cells. Sex. Transm. Dis. 26:160-165. [DOI] [PubMed] [Google Scholar]
- 18.Merz, A. J., and M. So. 1997. Attachment of piliated, Opa− and Opc− gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect. Immun. 65:4341-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naumann, M., S. Webler, C. Bartsch, B. Wieland, and T. F. Meyer. 1997. Neisseria gonorrhoeae epithelial cell interaction leads to the activation of the transcription factors nuclear factor κβ and activator protein 1 and the induction of inflammatory cytokines. J. Exp. Med. 186:247-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naumann, M., T. Rudel, and T. F. Meyer. 1999. Host cell interactions and signaling with Neisseria gonorrhoeae. Curr. Opin. Microbiol. 2:62-70. [DOI] [PubMed] [Google Scholar]
- 21.Naumann, M., T. Rudel, B. Wieland, C. Bartsch, and T. F. Meyer. 1998. Coordinate activation of activator protein 1 and inflammatory cytokines in response to Neisseria gonorrhoeae epithelial cell contact involves stress response kinases. J. Exp. Med. 188:1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols, W. A., C. R. H. Raetz, T. Clementz, A. L. Smith, J. A. Hanson, M. R. Ketterer, M. Sunshine, and M. A., Apicella. 1997. htrB of Haemophilus influenzae: determination of biochemical activity and effects on virulence and lipooligosaccharide toxicity. J. Endotoxin Res. 4:163-172. [Google Scholar]
- 23.Post, D. M. B., N. Phillips, J. Shao, B. W. Gibson, and M. A. Apicella. 2002. Intracellular survival of Neisseria gonorrhoeae in male urethral epithelial cells: importance of a hexaacyl lipid A. Infect. Immun. 70:909-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preston, A., R. E. Mandrell, B. W. Gibson, and M. A. Apicella. 1996. The lipooligosaccharide of pathogenic gram-negative bacteria. Crit. Rev. Microbiol. 22:139-180. [DOI] [PubMed] [Google Scholar]
- 25.Proctor, R. A., L. C. Denlinger, and P. J. Bertics. 1995. Lipopolysaccharide and bacterial virulence, p. 173-194. In J. A. Roth (ed.), Virulence mechanisms of bacterial pathogens, 2nd ed. ASM Press, Washington, D.C.
- 26.Ramsey, K. H., H. Schneider, A. S. Cross, J. W. Boslego, D. L. Hoover, T. L. Staley, R. A. Kuschner, and C. D. Deal. 1995. Inflammatory cytokines produced in response to experimental human gonorrhea. J. Infect. Dis. 172:186-191. [DOI] [PubMed] [Google Scholar]
- 27.Schneider, H., J. M. Griffiss, J. W. Boslego, P. J. Hitchcock, K. O. McJunkin, and M. A. Apicella. 1991. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J. Exp. Med. 174:1601-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somerville, J. E., Jr., L. Cassiano, B. Bainbridge, M. D. Cunningham, and R. P. Darveau. 1996. A novel Escherichia coli lipid A mutant that produces an anti-inflammatory lipopolysaccharide. J. Clin. Investig. 97:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svanborg, C., M. Hedlund, H. Connell, W. Agace, R. Duan, A. Nilsson, and B. Wullt. 1996. Bacterial adherence and mucosal cytokine responses. Ann. N. Y. Acad. Sci. 797:177-190. [DOI] [PubMed] [Google Scholar]
- 30.Ulevitch, R. J., and P. S. Tobias. 1995. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13:437-457. [DOI] [PubMed] [Google Scholar]
- 31.Zenni, M. K., P. C. Giardina, H. A. Harvey, J. Shao, M. R. Ketterer, D. M. Lubaroff, R. D. Williams, and M. A. Apicella. 2000. Macropinocytosis as a mechanism of entry into primary human urethral epithelial cells by Neisseria gonorrhoeae. Infect. Immun. 68:1696-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]