Abstract
The obligate intracellular bacterium Coxiella burnetii, the agent of Q fever in humans and of coxiellosis in other animals, survives and replicates within large, acidified, phagolysosome-like vacuoles known to fuse homo- and heterotypically with other vesicles. To further characterize these vacuoles, HeLa cells were infected with C. burnetii phase II; 48 h later, bacteria-containing vacuoles were labeled by LysoTracker, a marker of acidic compartments, and accumulated monodansylcadaverine and displayed protein LC3, both markers of autophagic vacuoles. Furthermore, 3-methyladenine and wortmannin, agents known to inhibit early stages in the autophagic process, each blocked Coxiella vacuole formation. These autophagosomal features suggest that Coxiella vacuoles interact with the autophagic pathway. The localization and role of wild-type and mutated Rab5 and Rab7, markers of early and late endosomes, respectively, were also examined to determine the role of these small GTPases in the trafficking of C. burnetii phase II. Green fluorescent protein (GFP)-Rab5 and GFP-Rab7 constructs were overexpressed and visualized by fluorescence microscopy. Coxiella-containing large vacuoles were labeled with wild-type Rab7 (Rab7wt) and with GTPase-deficient mutant Rab7Q67L, whereas no colocalization was observed with the dominant-negative mutant Rab7T22N. The vacuoles were also decorated by GFP-Rab5Q79L but not by GFP-Rab5wt. These results suggest that Rab7 participates in the biogenesis of the parasitophorous vacuoles.
Phagocytosis is responsible for the internalization of microorganisms, damaged cells, and inert particles (1, 29). After internalization, the particles become sequestered in membrane-bound organelles, called phagosomes, which undergo a process of maturation that involves acidification and several fusion events. At early time points after phagocytosis, phagosomes acquire markers of early endosomes such as Rab5 and mannose receptors (1, 4, 15). Subsequently, phagosomes gradually lose these markers and acquire late endosomal markers such as mannose-6-phosphate receptors, Rab7, lysosome-associated membrane glycoproteins (LAMPs), and cathepsin D (1, 4, 15). Finally, phagosomes fuse with secondary lysosomes, acquiring higher concentrations of hydrolytic enzymes and LAMPs and a lower pH (14).
Because many microbes are killed and degraded in the phagosomal compartment, the phagocytic pathway is an important component of host defense against microorganisms. However, some intracellular pathogens inhabit vacuoles that interact with compartments of the biosynthetic pathway, while others escape from the phagosomes or remain in vacuoles which neither acidify nor fuse with lysosomes (for a review see reference 31). In contrast, Coxiella burnetii bacteria live and replicate in acidified compartments with phagolysosomal characteristics (23). Lysosomal membrane markers and enzymes, as well as molecules internalized by fluid phase endocytosis, are easily found in vacuoles containing C. burnetii (23), and. the vacuoles also fuse with other components of the phagocytic/endocytic system (20, 42, 43). The acid environment appears to be essential for C. burnetii replication since raising the lysosomal pH with lysosomotropic amines or proton pump V-ATPase inhibitors reduces the growth of C. burnetii (22, 24).
Lysosomes represent the hydrolytic compartment not only for extracellular substrates (e.g., microorganisms) but also for turnover of cellular components including organelles. Autophagy is probably the main mechanism for degradation of long-lived proteins and the only mechanism for turnover of organelles including mitochondria and peroxisomes (41). Cytoplasmic portions and organelles are sequestered in a membranous structure (i.e., autophagosome) that interacts with the endocytic pathway and finally fuses with the lysosomes, where the incorporated materials are degraded (17, 18). Recently, it has been shown that some intracellular parasites avoid interactions with the endocytic pathway and replicate rapidly in vacuoles with autophagosome features (11, 35, 46).
Rab GTPases belong to the Ras superfamily of small GTPases and play crucial roles in membrane trafficking of eukaryotic cells, regulating tethering, docking, and fusion events among different compartments (48). Rab proteins localized in different intracellular compartments modulate specific vesicular-transport events (48). Rab5, a member of the Rab family, localizes on early endosomes and newly formed phagosomes (14-16), regulating membrane trafficking events in both the endocytic and phagocytic pathways (2, 3, 7, 44). Rab7, in turn, controls transport and fusion among degradative endocytic compartments such as late endosomes and lysosomes (19, 45). Rab7 appears to be a key component for the maintenance of the perinuclear lysosomal compartment by controlling the aggregation and fusion of late endosomes and lysosomes (8). Interestingly, some pathogens reside in phagosomes that exclude Rab7 from their membranes (13, 39, 44) whereas others reside in phagosomes that recruit this protein (10, 30). Nevertheless, both kinds of phagosomes are arrested in their maturation.
Given the diversity of the intracellular compartments targeted and customized by microorganisms, it is likely that modulation of the endocytic and phagocytic pathways allows pathogens to survive and to multiply in their host cells. There is indeed evidence that parasites can disrupt the maturation of the phagosomal pathway by interference with the distribution and function of the Rab protein (2, 3, 10, 13, 30, 39, 44).
Although C. burnetii multiplies in vacuoles with lysosomal features, the molecular mechanisms by which the bacterium controls the biogenesis of the replicative vacuoles are largely unknown. In the present study we further characterized C. burnetii parasitophorous vacuoles. In HeLa cells infected with C. burnetii phase II for 48 h, vacuoles containing these intracellular parasites and labeled with LysoTracker, a marker of acidic compartments, were found to accumulate monodansylcadaverine (MDC) and the LC3 protein, both specific markers of autophagic vacuoles (5, 26, 32). Also, 3-methyladenine (3-MA), an inhibitor of autophagy (40), blocked the development of Coxiella-containing vacuoles. These results suggest that parasitophorous vacuoles interact with the autophagic pathway. Furthermore, we show that vacuoles harboring C. burnetii are heavily labeled with Rab7 and with the GTPase-deficient Rab5 mutant. Interestingly furthermore, overexpression of the dominant-negative Rab7 mutant altered the formation of the replicative vacuole. These results suggest that Rab7 likely participates in the biogenesis of the C. burnetii-containing vacuoles.
MATERIALS AND METHODS
Materials.
Minimal essential medium (α-MEM) and fetal bovine serum (FBS) were obtained from Gibco Laboratories (Grand Island, N.Y.). Rhodamine 6G and LysoTracker were from Molecular Probes (Eugene, Oreg.). All other chemicals were from Sigma Chemical Co. (St. Louis, Mo.). Recombinant Sindbis viruses expressing green fluorescent protein (GFP)-tagged Rab5, Rab7, and their mutants were kindly provided by Philip D. Stahl (Washington University, St. Louis, Mo.). Plasmids encoding enhanced GFP (EGFP)-Rab7 and its mutants were kindly provided by Bo van Deurs (University of Copenhagen, Copenhagen, Denmark).
Cell cultures.
HeLa cells were grown in T25 flasks at 37°C in a 5% CO2 atmosphere in α-MEM supplemented with 10% FBS, 2.2 g of sodium bicarbonate/liter, 2 mM glutamine, and 0.1% penicillin-streptomycin. Stably transfected CHO cells overexpressing the EGFP-LC3 protein (33) were grown in T25 flasks at 37°C in a 5% CO2 atmosphere in α-MEM supplemented with 10% FBS, 2.2 g of sodium bicarbonate/liter, 2 mM glutamine, 0.1% penicillin-streptomycin, and 0.2 mg of Geneticin/ml.
Propagation of C. burnetii phase II.
The clone 4 phase II Nine Mile strain of C. burnetii, which is infective for cells but not for animals, was provided by Ted Hackstadt (Rocky Mountain Laboratories, National Institute for Allergy and Infectious Diseases, National Institutes of Health, Hamilton, Mont.) and was handled in a biosafety level II facility (21). Infective inocula were prepared as described previously (47). Nonconfluent Vero cells were cultured in T25 flasks at 37°C in α-MEM supplemented with 5% FBS, 0.22 g of sodium bicarbonate/liter, and 20 mM HEPES, pH 7 (MfbH). Cultures were infected with C. burnetii phase II suspensions for 2 to 6 days at 37°C in an air atmosphere. After being frozen at −70°C, the flasks were thawed, and the cells were scraped and passed 20 times through a 27-gauge needle connected to a syringe. Cell lysates were centrifuged at 800 × g for 10 min at 4°C. The supernatants were centrifuged at 24,000 × g for 30 min at 4°C, and pellets containing C. burnetii were resuspended with 0.5 ml of phosphate-buffered saline (PBS), aliquoted, and frozen at −70°C.
Infection of cells with C. burnetii.
HeLa cells plated in T25 flasks were washed several times with PBS and detached with trypsin-EDTA. After resuspension with MfbH, cells were plated on coverslips distributed in six-well plates. For infection, a 50-μl aliquot of the C. burnetii suspension was diluted with 550 μl of MfbH, and 100 μl of this dilution was added to each well. Except when indicated otherwise, cells were incubated for 48 h at 37°C in an air atmosphere. The same protocol was used for the infection of CHO cells overexpressing pEGFP-LC3.
Expression of Rab proteins and their mutants in HeLa cells by using the Sindbis virus system.
Coxiella-infected HeLa cell monolayers grown in 35-mm-diameter dishes were incubated with recombinant Sindbis viruses to overexpress GFP-Rab proteins in 300 μl of PBS containing 1% FBS. Virus absorption proceeded at room temperature for 1 h. The medium was replaced by 2 ml of MfbH, and the cells were incubated overnight at 37°C. Cells were mounted and immediately analyzed by fluorescence microscopy using an inverted microscope (Eclipse TE 300; Nikon) equipped with a charge-coupled device camera (Orca I; Hamamatsu). Images were processed with MetaMorph, version 4.5, software (Universal Images Corporation).
Transfection with pEGFP plasmids.
HeLa cells were grown in D-MEM supplemented with 10% FBS, 2.2 g of sodium bicarbonate/liter, 2 mM glutamine, and 0.1% penicillin-streptomycin in a 5% CO2 incubator at 37°C. The cells were transfected with the pEGFP vector alone or pEGFP plasmids encoding wild-type Rab7 (Rab7wt), Rab7T22N, a dominant-negative mutant, and the active Rab7Q67L mutant by using Lipofectamine (GIBCO-BRL) according to the manufacturer's instructions. After 48 h of transfection cells were infected with C. burnetii. The cells were analyzed and processed as described above.
RESULTS
The replication compartment of C. burnetii phase II displays autophagosomal markers.
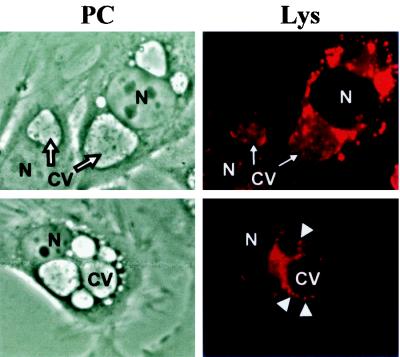
To examine the compartment where C. burnetii phase II survives and multiplies, HeLa cells were infected for 48 h with the bacteria. As previously shown (42), Coxiella-rich large vacuoles can be easily distinguished by phase-contrast microscopy of infected cells (Fig. 1, left). These vacuoles have been shown to be labeled by antibodies against the LAMP-1 protein and to contain the lysosomal hydrolase acid phosphatase (23). To further characterize the bacterial-replication compartment, we labeled Coxiella-infected HeLa cells with LysoTracker, a marker for acidic compartments. As shown in Fig. 1 (right) and in agreement with previous observations (28), the large parasitophorous vacuoles were intensely labeled. We have also observed small vesicles labeled by LysoTracker next to large Coxiella-containing vacuoles.
FIG. 1.
The acidotropic probe LysoTracker red accumulates into vacuoles containing C. burnetii. HeLa cells were incubated with C. burnetii suspended in MfbH for 48 h at 37°C in an air atmosphere. Afterwards, Coxiella-infected HeLa cells were incubated for 15 min at room temperature with LysoTracker red and analyzed by phase-contrast (PC; left) and fluorescence (right) microscopy. Arrows, vacuoles containing C. burnetii (CV) loaded with LysoTracker red (top); arrowheads, clustering of small vacuoles labeled with LysoTracker red around the CV (bottom). N, nucleus.
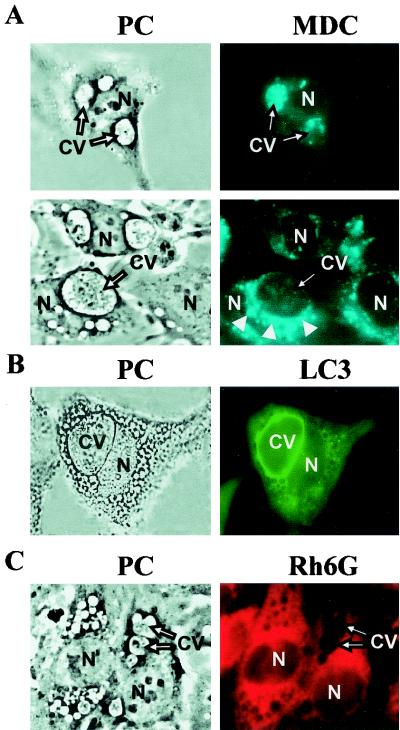
To investigate the possible involvement of an autophagic pathway, we next incubated the cells with MDC, an autofluorescence-specific marker of autophagosomes (5, 32). The Coxiella replication niche was labeled by MDC (Fig. 2A, top), indicating that this compartment has characteristics of an autophagosomal vacuole. The labeling was heterogeneous since some vacuoles accumulated more marker than others. Interestingly, clusters of MDC-labeled vesicles were also observed in close proximity to parasitophorous vacuoles (Fig. 2A, bottom). It has been proposed that autophagosomes originate from an as yet undefined region of the endoplasmic reticulum (ER). Therefore, we were interested in determining if Coxiella colocalizes with ER markers. Cells were labeled with rhodamine 6G, and a typical reticular structure was observed. However, no label surrounding the vacuoles was detected, only empty spaces that look like “holes” where the large Coxiella-containing vacuoles are located (Fig. 2C, right).
FIG. 2.
C. burnetii-containing vacuole colocalizes with MDC and LC3, specific markers of the autophagic pathway. Coxiella-infected HeLa cells were incubated for 10 min at 37°C with MDC. After being washed, cells were analyzed by phase-contrast (PC; left) and fluorescence (right) microscopy. (A) Arrows, Coxiella-containing vacuoles (CV) loaded with MDC; arrowheads, clustering of small vacuoles labeled with MDC (bottom). N, nucleus. (B) Stably transfected CHO cells overexpressing EGFP-LC3 were infected with Coxiella as described in Materials and Methods. Cells were analyzed by phase-contrast (left) and fluorescence (right) microscopy. (C) Coxiella-infected HeLa cells were incubated for 20 min at 37°C with rhodamine 6G (Rh6G). After being washed, cells were analyzed by phase-contrast (left) and fluorescence (right) microscopy. Arrows show that the Coxiella-containing vacuoles are not decorated by the ER marker.
Enzymes of the phosphatidylinositol 3-kinase (PI3K) family has been implicated in the regulation of a multitude of intracellular events (12, 27). It has been shown that wortmannin (WM) inhibits autophagy by blocking PI3K activity (6, 32). Furthermore, 3-MA is a specific inhibitor of autophagy that seems to also block PI3K (6, 40). Therefore, to further investigate the involvement of autophagy in C. burnetii infection, we tested the effect of WM and 3-MA on the development of the large vacuoles. Table 1 shows that both compounds were inhibitory. To confirm the relationship between Coxiella-containing vacuoles and the autophagic pathway, we studied the localization of LC3, a protein that interacts specifically with the autophagosomal compartment (26). However, since the transfection and expression of pEGFP-LC3 in HeLa cells were low, CHO cells, which are easily infected by C. burnetii (42, 43), were used instead. As shown in Fig. 2B (right), after 48 h of infection of stably transfected CHO-LC3 cells, the limiting membrane of the Coxiella-containing vacuole was strongly labeled with protein GFP-LC3. These results clearly indicate that Coxiella interacts with the autophagic pathway.
TABLE 1.
Effect of autophagy inhibitors on the biogenesis of vacuoles containing C. burnetiia
| Inhibitor (concn) | Cells containing CVb
|
|
|---|---|---|
| No. (total cells) | % | |
| Control | 239 (918) | 27 |
| WM (100 nM) | 167 (945)* | 18 |
| 3-MA (2 mM) | 96 (759)* | 12 |
HeLa cells infected with C. burnetii during 12 h were subsequently incubated with the inhibitors. After 24 h, the cells were analyzed by light microscopy.
CV, Coxiella-containing vacuoles. ∗, significantly different (P < 0.001) from control cells as calculated by chi-square test.
C. burnetii localizes in vacuoles labeled with Rab7.
It is well established that Rab proteins function in the tethering and docking of vesicles to their target compartment, leading to membrane fusion. Rab protein activity is regulated by the binding and hydrolysis of GTP, by multiple effectors, and by the signal transduction pathway (48). GTPases have the ability to cycle regularly between GTP- and GDP-bound states. Their on/off regulatory function is restricted to the membrane compartments where they are localized. A useful strategy to explore the function of Rab proteins is the use of GTP-binding-defective (dominant-negative) and GTPase-defective (active) mutants.
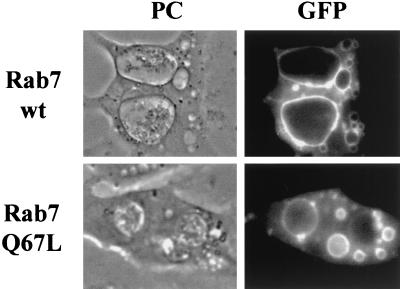
To study the interaction of the endocytic compartments with vacuoles containing C. burnetii, we examined the distribution of Rab5 and Rab7, early and late endosomal markers, respectively, on Coxiella-containing vacuoles formed in HeLa cells. Cells infected with Coxiella for 48 h were superinfected with wild-type and mutant forms of recombinant GFP-Rab5 or -Rab7 constructs of Sindbis virus for another 12 h. As shown in Fig. 3, the large Coxiella-containing vacuoles were clearly decorated with GFP-Rab7wt (top) or with GTPase-defective mutant GFP-Rab7Q67L (bottom). These results suggest that C. burnetii resides in a compartment labeled by Rab7, indicating that this compartment has characteristics of late endosomes and lysosomes.
FIG. 3.
Rab7 decorates the C. burnetii-containing vacuole. Coxiella-infected HeLa cells were incubated for 1 h with recombinant Sindbis viruses encoding the GFP-Rab7wt fusion protein or active mutant GFP-Rab7Q67L. After overnight incubations at 37°C in an air atmosphere, cells were analyzed by phase-contrast (PC; left) and fluorescence (right) microscopy. GFP-Rab7wt and GFP-Rab7Q67L decorate large Coxiella-containing vacuoles (top and bottom, respectively).
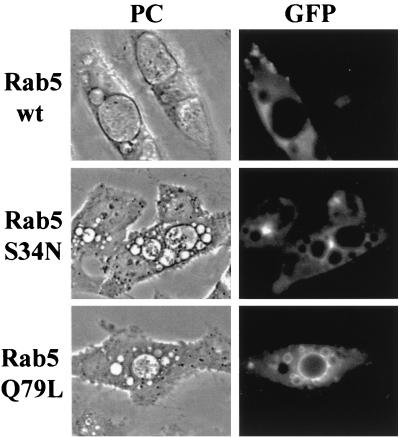
Interestingly, the Coxiella-containing vacuoles were also intensively decorated with the GTPase-deficient Rab5 mutant (i.e., GFP-Rab5Q79L), but they were not stained in cells transfected with Rab5wt or dominant-negative mutant GFP-Rab5S34N (Fig. 4). The presence of the Rab5-positive mutant on Coxiella-containing vacuoles might be explained by the observation that Rab5cQ79L allows the interaction between endosome or phagosome and lysosome compartments (36).
FIG. 4.
The constitutively active form of Rab5 (Rab5Q79L) localizes with the C. burnetii-containing vacuole. Coxiella-infected HeLa cells were incubated for 1 h with recombinant Sindbis viruses encoding the GFP-Rab5wt fusion protein, negative mutant GFP-Rab5S34N, or active mutant GFP-Rab5Q79L. After overnight incubations at 37°C in an air atmosphere, cells were analyzed by phase-contrast (PC; left) and fluorescence (right) microscopy. Coxiella-containing vacuoles did not colocalize with GFP-Rab5wt and GFP-Rab5S34N proteins (top and middle, respectively). Interestingly, the Coxiella-containing vacuole membrane was strongly labeled by GFP-Rab5Q79L (bottom).
Since the Coxiella-containing vacuoles were heavily labeled with GFP-Rab7wt, we next examined the distribution of GFP-Rab7T22N, a dominant-negative mutant. For technical reasons we were unable to generate recombinant Sindbis virus able to overexpress GFP-Rab7T22N. Therefore, we used a different approach to overexpress this protein. HeLa cells were transiently transfected with plasmid pEGFP-Rab7wt and mutants. The expression and distribution of EGFP-Rab proteins were similar to those observed by other authors (8). In the HeLa cells overexpressing EGFP-Rab7wt or dominant-positive mutant EGFP-Rab7Q67L, the GFP signal was associated with vesicles partly distributed throughout the cytoplasm and concentrated in the perinuclear region. As expected, dominant-negative mutant GFP-Rab7T22N showed a diffuse and widespread distribution (data not shown).
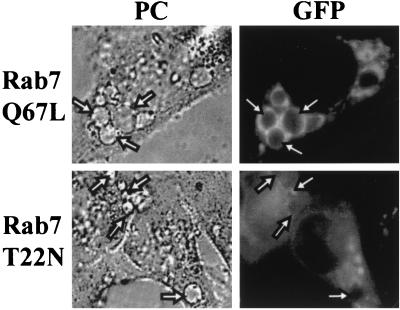
Transiently transfected cells were subsequently infected with C. burnetii, and after 48 h the cells were analyzed by fluorescence microscopy. As shown in Fig. 5, top, similar to the results obtained with the recombinant Sindbis virus, in HeLa cells transfected with pEGFP encoding Rab7Q67L, large Coxiella-containing vacuoles were formed and a noteworthy ring-shaped EGFP signal on the vesicles was observed. In cells transfected with EGFP-Rab7wt, the EGFP-tagged protein showed a distribution essentially similar to that exhibited by mutant Rab7Q67L (data not shown). Conversely, in cells overexpressing the dominant-negative EGFP-Rab7T22N mutant, the protein presented a diffuse pattern and it was not localized on the vacuole-limiting membrane (Fig. 5, bottom). Furthermore, the formation of replication vacuoles seems to be delayed or partly impaired since the transfected cells had few and small Coxiella-containing vacuoles. These results suggest that Rab7-mediated vesicular transport is required for the formation and maintenance of the vacuoles that shelter C. burnetii.
FIG. 5.
The dominant-negative Rab7 mutant does not localize with the Coxiella replication compartment. HeLa cells transfected with pEGFP plasmids encoding Rab7T22N, a dominant-negative mutant, or active mutant Rab7Q67L were incubated with C. burnetii under infection conditions (see Materials and Methods). Cells were analyzed by phase-contrast (PC; left) and fluorescence (right) microscopy. The active Rab7Q67L mutant clearly labels the Coxiella-containing vacuole membrane (arrows, top), whereas the inactive Rab7T22N mutant does not (arrows, bottom).
DISCUSSION
In this study we present evidence that the compartment inhabited by Coxiella displays some of the features of autophagosomes, such as uptake of MDC and presence of the protein LC3 (5). Furthermore, vacuolar development was reduced by 3-MA and WM, known inhibitors of the autophagic pathway (6, 40). Since parasitophorous vacuoles that shelter Coxiella also display lysosomal markers such as LAMPs and lysosomal enzymes, it is possible that C. burnetii resides in an autophagolysosome-like compartment. This is not an unusual observation for an intracellular parasite since Brucella abortus seems to reside within compartments resembling autophagosomes (35). C. burnetii has two cell variants, the large-cell variant (LCV), the more sensitive to environmental stress of the two, and the small-cell variant (SCV), which is considered the dormant, stress-resistant, and less metabolically active form (23). Therefore, it is possible that Coxiella may transit through the autophagic pathway to initially avoid the harsh environment of lysosomes, perhaps during the transition from SCV to LCV. Indeed, Howe and Mallavia (25) have shown that phagosomes containing C. burnetii exhibited a significant delay in fusion with lysosomes. These authors have also shown, in an in vitro assay, increased protein synthesis in C. burnetii incubated at pH 5.5 (endosomal pH) compared to that observed at pH 4.5 (lysosomal pH). These results suggest that C. burnetii may prefer a slightly less acidic compartment perhaps with characteristics of a late endosomal or autophagosomal compartment. The interaction between the autophagic compartments and the Coxiella-containing vacuole which has some lysosomal characteristics could allow Coxiella to obtain metabolites for its intracellular growth and development.
In this report we have shown for the first time that the spacious vacuoles generated by Coxiella are heavily labeled by Rab7, a late endocytic marker. The presence of Rab7 antigen in the vacuolar membranes of C. burnetii phase II-infected mouse primary macrophages has been observed (S. Paul and S. Gomes, personal communication). Other intracellular parasites also appear to reside in vacuoles that become positive for Rab7 (10, 13, 30, 38, 39, 44). It has been shown that Mycobacterium tuberculosis and Legionella pneumophila phagosomes acquire staining for Rab7 and for the constitutively active mutant form of Rab7 (Rab7Q67L) in HeLa cells overexpressing these proteins (10). Also Salmonella enterica serovar Typhimurium seems to inhabit a compartment enriched in lysosomal glycoproteins and Rab7, acquired apparently by fusion with nonlysosomal vesicles (30). The functional importance of Rab7 in regulating membrane trafficking in the endocytic pathway has been established by the demonstration that expression of a dominant-negative Rab7 mutant interrupts the normal endocytic flow from early to late endosomes (19, 36). In addition, overexpression of a GFP-tagged Rab7 dominant-negative mutant has been shown to lead to dispersal of the lysosomal compartment (8). We have reported here not only that the Coxiella-containing vacuoles are labeled by Rab7 but also that this protein seems to regulate vacuole biogenesis since smaller parasitophorous vacuoles were observed in HeLa cells overexpressing dominant-negative mutant Rab7T22N. However, a more exhaustive analysis of the effect of this mutant is necessary to determine the role of Rab7 in the biogenesis of the Coxiella replication compartment. Also, further experiments need to be done to establish whether Rab7 is actively recruited to the Coxiella-containing phagosomes or if the protein is acquired by fusion with late endosomes. Indeed, we have observed large clusters of Rab7-positive vesicles in close proximity to the Coxiella replication compartment.
In agreement with our results it has also been shown that HeLa cells incubated with VacA, a toxin produced by Helicobacter pylori, develop large intracellular vacuoles in a Rab7-dependent fashion through a process that involves fusion between late endosomes (34). Interestingly, in cells overexpressing Rab5Q79L, VacA induces the formation of large vesicles positive for both Rab7 and Rab5Q79L. We have also observed that the large Coxiella-containing vacuoles were labeled by Rab5Q79L but not by Rab5wt, suggesting that the mutant protein is mistargeted or remains associated with the Coxiella replication compartment. Clemens et al. (9) have observed that large vacuoles stained positively for Rab5c and LAMP-1 are formed in HeLa cells overexpressing Rab5cQ79L. In contrast, in cells overexpressing Rab5cwt this colocalization was not observed. Furthermore, M. tuberculosis, which normally resides in a compartment that acquires Rab5 but excludes Rab7 and LAMP-1 and that avoids fusion with latex bead phagosomes (44), resides in a compartment that gets LAMP-1 and that fuses with latex bead phagosomes in HeLa cells overexpressing the Rab5cQ79L mutant. These results suggest that Rab5cQ79L allows the interaction between endosome or phagosome and lysosome compartments. Indeed, in a recent report it has been shown that Rab5aQ79L-positive vesicles colocalize with lysosomal membrane proteins and lysosomal enzymes (37). Therefore, an altered traffic between lysosomes and endosomes might explain the presence of Rab5Q79L on the large lysosome-like Coxiella replication compartment. However, since by fluorescence microscopy we are unable to distinguish the smallest Coxiella-containing vacuoles, we cannot discard the possibility that Rab5wt might be present in the tiny vesicles generated at the early time points of infection.
In summary, in the present report we have further characterized the Coxiella replication compartment and we have clearly shown that Rab7 is present in the parasite-containing vacuoles. Since C. burnetii replicates only when is phagocytosed and transported to the phagolysosome, dissecting this transport pathway at the molecular level will help us to find a therapeutic approach for this infectious agent.
Acknowledgments
We specially thank Bo van Deurs for the generous gifts of plasmids encoding the Rab7 protein and its mutants. We also thank Luis S. Mayorga for critical reading of the manuscript.
This work was partly supported by grants from CONICET (PIP 0695/98), Agencia Nacional de Promoción Científica y Tecnológica (PICT97 no. 01-00111-02072, PICT99 no. 1-6058), and CIUNC (Universidad Nacional de Cuyo) to M.I.C. and W.B.
Editor: J. T. Barbieri
REFERENCES
- 1.Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Dominguez, C., A. M. Barbieri, W. Beron, A. Wandinger-Ness, and P. D. Stahl. 1996. Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J. Biol. Chem. 271:13834-13843. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Dominguez, C., and P. D. Stahl. 1999. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J. Biol. Chem. 274:11459-11462. [DOI] [PubMed] [Google Scholar]
- 4.Beron, W., C. Alvarez-Dominguez, L. Mayorga, and P. D. Stahl. 1995. Membrane trafficking along the phagocytic pathway. Trends Cell Biol. 5:100-104. [DOI] [PubMed] [Google Scholar]
- 5.Biederbick, A., H. F. Kern, and H. P. Elsasser. 1995. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur. J. Cell Biol. 66:3-14. [PubMed] [Google Scholar]
- 6.Blommaart, E. F., U. Krause, J. P. Schellens, H. Vreeling-Sindelarova, and A. J. Meijer. 1997. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 243:240-246. [DOI] [PubMed] [Google Scholar]
- 7.Bucci, C., R. G. Parton, I. H. Mather, H. Stunnenberg, K. Simons, B. Hoflack, and M. Zerial. 1992. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70:715-728. [DOI] [PubMed] [Google Scholar]
- 8.Bucci, C., P. Thomsen, P. Nicoziani, J. McCarthy, and B. van Deurs. 2000. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11:467-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2000. Deviant expression of Rab5 on phagosomes containing the intracellular pathogens Mycobacterium tuberculosis and Legionella pneumophila is associated with altered phagosomal fate. Infect. Immun. 68:2671-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2000. Mycobacterium tuberculosis and Legionella pneumophila phagosomes exhibit arrested maturation despite acquisition of Rab7. Infect. Immun. 68:5154-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coers, J., C. Monahan, and C. R. Roy. 1999. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat. Cell Biol. 1:451-453. [DOI] [PubMed] [Google Scholar]
- 12.Cullen, P. J., G. E. Cozier, G. Banting, and H. Mellor. 2001. Modular phosphoinositide-binding domains—their role in signalling and membrane trafficking. Curr. Biol. 11:R882-R893. [DOI] [PubMed] [Google Scholar]
- 13.Deretic, V., and R. A. Fratti. 1999. Mycobacterium tuberculosis phagosome. Mol. Microbiol. 31:1603-1609. [DOI] [PubMed] [Google Scholar]
- 14.Desjardins, M., J. E. Celis, G. van Meer, H. Dieplinger, A. Jahraus, G. Griffiths, and L. A. Huber. 1994. Molecular characterization of phagosomes. J. Biol. Chem. 269:32194-32200. [PubMed] [Google Scholar]
- 15.Desjardins, M., L. A. Huber, R. G. Parton, and G. Griffiths. 1994. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J. Cell Biol. 124:677-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desjardins, M., N. N. Nzala, R. Corsini, and C. Rondeau. 1997. Maturation of phagosomes is accompanied by changes in their fusion properties and size-selective acquisition of solute materials from endosomes. J. Cell Sci. 110:2303-2314. [DOI] [PubMed] [Google Scholar]
- 17.Dunn, W. A., Jr. 1990. Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J. Cell Biol. 110:1923-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn, W. A., Jr. 1990. Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J. Cell Biol. 110:1935-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng, Y., B. Press, and A. Wandinger-Ness. 1995. Rab 7: an important regulator of late endocytic membrane traffic. J. Cell Biol. 131:1435-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes, M. S., S. Paul, A. L. Moreira, R. Appelberg, M. Rabinovitch, and G. Kaplan. 1999. Survival of Mycobacterium avium and Mycobacterium tuberculosis in acidified vacuoles of murine macrophages. Infect. Immun. 67:3199-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hackstadt, T. 1996. Biosafety concerns and Coxiella burnetii. Trends Microbiol. 4:341-342. [DOI] [PubMed] [Google Scholar]
- 22.Hackstadt, T., and J. C. Williams. 1981. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc. Natl. Acad. Sci. USA 78:3240-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinzen, R. A., T. Hackstadt, and J. E. Samuel. 1999. Developmental biology of Coxiella burnettii. Trends Microbiol. 7:149-154. [DOI] [PubMed] [Google Scholar]
- 24.Heinzen, R. A., M. A. Scidmore, D. D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64:796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howe, D., and L. P. Mallavia. 2000. Coxiella burnetii exhibits morphological change and delays phagolysosomal fusion after internalization by J774A.1 cells. Infect. Immun. 68:3815-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabeya, Y., N. Mizushima, T. Ueno, A. Yamamoto, T. Kirisako, T. Noda, E. Kominami, Y. Ohsumi, and T. Yoshimori. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19:5720-5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin, T. F. 1998. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu. Rev. Cell Dev. Biol. 14:231-264. [DOI] [PubMed] [Google Scholar]
- 28.Maurin, M., A. M. Benoliel, P. Bongrand, and D. Raoult. 1992. Phagolysosomes of Coxiella burnetii-infected cell lines maintain an acidic pH during persistent infection. Infect. Immun. 60:5013-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.May, R. C., and L. M. Machesky. 2001. Phagocytosis and the actin cytoskeleton. J. Cell Sci. 114:1061-1077. [DOI] [PubMed] [Google Scholar]
- 30.Meresse, S., O. Steele-Mortimer, B. B. Finlay, and J. P. Gorvel. 1999. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 18:4394-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meresse, S., O. Steele-Mortimer, E. Moreno, M. Desjardins, B. Finlay, and J. P. Gorvel. 1999. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat. Cell Biol. 1:E183-E188. [DOI] [PubMed] [Google Scholar]
- 32.Munafo, D. B., and M. I. Colombo. 2001. A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J. Cell Sci. 114:3619-3629. [DOI] [PubMed] [Google Scholar]
- 33.Munafo, D. B., and M. I. Colombo. 2002. Induction of autophagy causes dramatic changes in the subcellular distribution of GFP-Rab24. Traffic 3:472-482. [DOI] [PubMed] [Google Scholar]
- 34.Papini, E., B. Satin, C. Bucci, M. de Bernard, J. L. Telford, R. Manetti, R. Rappuoli, M. Zerial, and C. Montecucco. 1997. The small GTP binding protein rab7 is essential for cellular vacuolation induced by Helicobacter pylori cytotoxin. EMBO J. 16:15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pizarro-Cerda, J., E. Moreno, and J. P. Gorvel. 2000. Invasion and intracellular trafficking of Brucella abortus in nonphagocytic cells. Microbes Infect. 2:829-835. [DOI] [PubMed] [Google Scholar]
- 36.Press, B., Y. Feng, B. Hoflack, and A. Wandinger-Ness. 1998. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J. Cell Biol. 140:1075-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenfeld, J. L., R. H. Moore, K. P. Zimmer, E. Alpizar-Foster, W. Dai, M. N. Zarka, and B. J. Knoll. 2001. Lysosome proteins are redistributed during expression of a GTP-hydrolysis-defective rab5a. J. Cell Sci. 114:4499-4508. [DOI] [PubMed] [Google Scholar]
- 38.Roy, C. R., K. H. Berger, and R. R. Isberg. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663-674. [DOI] [PubMed] [Google Scholar]
- 39.Scianimanico, S., M. Desrosiers, J. F. Dermine, S. Meresse, A. Descoteaux, and M. Desjardins. 1999. Impaired recruitment of the small GTPase rab7 correlates with the inhibition of phagosome maturation by Leishmania donovani promastigotes. Cell. Microbiol. 1:19-32. [DOI] [PubMed] [Google Scholar]
- 40.Seglen, P. O., and P. B. Gordon. 1982. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc. Natl. Acad. Sci. USA 79:1889-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stromhaug, P. E., and D. J. Klionsky. 2001. Approaching the molecular mechanism of autophagy. Traffic 2:524-531. [DOI] [PubMed] [Google Scholar]
- 42.Veras, P. S., C. de Chastellier, M. F. Moreau, V. Villiers, M. Thibon, D. Mattei, and M. Rabinovitch. 1994. Fusion between large phagocytic vesicles: targeting of yeast and other particulates to phagolysosomes that shelter the bacterium Coxiella burnetii or the protozoan Leishmania amazonensis in Chinese hamster ovary cells. J. Cell Sci. 107:3065-3076. [DOI] [PubMed] [Google Scholar]
- 43.Veras, P. S., C. Moulia, C. Dauguet, C. T. Tunis, M. Thibon, and M. Rabinovitch. 1995. Entry and survival of Leishmania amazonensis amastigotes within phagolysosome-like vacuoles that shelter Coxiella burnetii in Chinese hamster ovary cells. Infect. Immun. 63:3502-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Via, L. E., D. Deretic, R. J. Ulmer, N. S. Hibler, L. A. Huber, and V. Deretic. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272:13326-13331. [DOI] [PubMed] [Google Scholar]
- 45.Vitelli, R., M. Santillo, D. Lattero, M. Chiariello, M. Bifulco, C. B. Bruni, and C. Bucci. 1997. Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 272:4391-4397. [DOI] [PubMed] [Google Scholar]
- 46.Vogel, J. P., and R. R. Isberg. 1999. Cell biology of Legionella pneumophila. Curr. Opin. Microbiol. 2:30-34. [DOI] [PubMed] [Google Scholar]
- 47.Zamboni, D. S., R. A. Mortara, and M. Rabinovitch. 2001. Infection of Vero cells with Coxiella burnetii phase II: relative intracellular bacterial load and distribution estimated by confocal laser scanning microscopy and morphometry. J. Microbiol. Methods 43:223-232. [DOI] [PubMed] [Google Scholar]
- 48.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107-117. [DOI] [PubMed] [Google Scholar]