Abstract
The effects of Escherichia coli O157:H7 (strains E30480 and PM601) and the associated verotoxins (VTs), VT1 and VT2, on stress-activated protein kinase and nuclear factor kappa B (NF-κB) signaling were investigated with Vero cells, which are extremely sensitive to the cytotoxic effects of E. coli O157:H7 in vitro. Cell-free supernatants prepared from E30480 and PM601 cultures and purified VT1 and VT2 stimulated a strong and prolonged (>4-h) activation of both c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase. However, JNK activity stimulated in response to E30480 supernatants was substantially reduced following pretreatment with anti-VT1 and anti-VT2 antibodies, while a VT1 and VT2 gene knockout mutant of PM601 was unable to stimulate JNK activity. E30480 supernatants also caused a sustained activation of NF-κB DNA binding, degradation of inhibitory kappa B alpha (IκBα), and an increase in inhibitory kappa B kinase α activity, although PM601 supernatants and VT1 and VT2 had no effect. However, preincubation with VTs prolonged the transient activation of NF-κB and IκBα degradation stimulated by either tumor necrosis factor alpha or interleukin 1β, while preincubation with anti-VT antibodies prevented the prolonged loss of IκBα and partially reduced DNA binding in response to E30480 supernatants. These results strongly suggest that in Vero cells, VT plays an essential role in sustained JNK and NF-κB signaling in response to E. coli O157:H7 and that this action may underpin their cell-selective cytotoxic effects. These studies also suggest that another component released by strain E30480 contributes to the early activation of JNK and NF-κB.
Infection with Escherichia coli O157:H7 (verotoxigenic E. coli [VTEC]) can lead to diseases such as hemorrhagic colitis and hemolytic-uremic syndrome, mediated in part via the production and release of toxins termed verotoxins (VTs) (3). The two major VTs produced by VTEC, VT1 and VT2, have been well described (34, 35). Following initial infection, E. coli O157:H7 adheres to intestinal epithelial cells, inducing characteristic attaching and effacing lesions (1) and leading to the development of bloody diarrhea, one of the hallmarks of hemorrhagic colitis. It is likely that this disruption of the epithelial barrier contributes to the VTs entering the bloodstream and spreading to target organs such as the kidneys (24, 40) in addition to their reported ability to translocate directly through epithelium (39). Both VT1 and VT2 bind to globotriaosylceramide (Gb3 [CD77]) receptors (21) and exert a profound cytotoxic effect on target cells such as microvascular endothelial cells, renal tubular epithelial cells, renal cortical cells, and African green monkey kidney cells (Vero cells) (24, 26, 28, 57). The VTs are known protein synthesis inhibitors and inducers of apoptosis (2, 19, 35). Renal endothelial and epithelial cells are at particular risk (24, 49, 50); the development of the hemolytic-uremic syndrome, characterized by microangiopathic anemia, thrombocytopenia, and acute renal failure, is a common cause of mortality among infants and the elderly following infection with E. coli O157:H7 (9, 54, 55). Although VT1 and VT2 have been shown to be cytotoxic to tubular epithelial cells, Vero cells, and neurons (2, 19, 27, 49), they are not cytotoxic to monocytes, despite their possession of Gb3 receptors (41). Instead, monocytes respond to VT stimulation via the release of cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 1 (51, 56). These findings suggest differences in the mechanisms by which different cellular responses are initiated.
To date, very little is known about the cellular signaling events which regulate either the release of cytokines by E. coli O157:H7 and its VTs or the selective cytotoxicity of VT1 and VT2 in kidney epithelial cells. However, two pathways can play important roles in mediating responses to infection and toxic challenge, namely, the stress-activated protein (SAP) kinases and the family of transcription factors known as nuclear factor kappa B (NF-κB). The SAP kinases consist of homologues of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein (MAP) kinase and are strongly activated by cytokines, including interleukin 1 and TNF-α; cytokine inducers, such as lipopolysaccharide (LPS); and physical trauma induced by osmotic stress and UV irradiation (53). While JNK and p38 MAP kinase have been consistently implicated in the release of cytokines and in the enhanced expression of inflammatory proteins (4, 14, 48), both proapoptotic and cytoprotective roles have been suggested for these kinases, depending on cell type, their relative expression, the nature of the stimulus, and their kinetics of activation (22, 33, 59, 62).
NF-κB (also known as Rel) proteins exist as homo- or heterodimers of p50 (NF-κB1), p65 (RelA), c-Rel, RelB, and p52 (NF-κB2), which are all related through a conserved sequence of ∼300 amino acids known as the Rel homology domain. NF-κB binding sites are found within the promoter sequences of many inflammatory genes, including those for TNF-α, inducible nitric oxide synthase, and cyclooxygenase-2 (7). At rest, NF-κB is retained within the cytosol bound to inhibitory kappa B (IκB); following cellular activation, NF-κB dissociates from IκB and translocates to the nucleus. Dissociation is initiated by the phosphorylation of IκBα, which is mediated by isoforms of IκB kinase (IKK) (10, 42, 61). Although NF-κB is well recognized as regulating inflammatory responses, its role in cellular survival is still poorly defined, and both proapoptotic and protective effects have been proposed (reviewed in reference 32).
In this study, we examined the ability of E. coli O157:H7 and the associated VTs, VT1 and VT2, to stimulate SAP kinase and NF-κB signaling events in African green monkey kidney cells (Vero cells), which are extremely sensitive to the cytotoxic effects of E. coli O157:H7 in vitro. In Vero cells, supernatants from E30480 and PM601 caused a sustained activation of JNK that was dependent on the presence of either VT1 or VT2. Furthermore, while VT alone had no effect on the NF-κB signaling pathway, the toxin was required for a sustained NF-κB response to E30480, primarily due to an inhibition of the resynthesis of IκB. These toxin-mediated responses, which distinguish E. coli O157:H7 from other, non-VT-producing E. coli serotypes in Vero cells, may underpin the cell-selective cytotoxic effects previously observed with this infective agent.
MATERIALS AND METHODS
All chemicals and reagents were obtained from appropriate commercial sources. E. coli expression plasmids for glutathione S-transferase (GST)-MAP kinase-activated protein kinase 2 (MAPKAPK-2) and GST-c-Jun(5-89) were kind gifts from J. Woodgett (Toronto, Ontario, Canada) and C. J. Marshall (Chester Beatty Laboratories, London, United Kingdom), respectively. E. coli O157:H7 (strain E30480: VT1 and VT2 producing) (47) was purchased from the Public Health Laboratory Service, National Collection of Type Cultures and Pathogenic Fungi, Central Public Health Laboratory, London, United Kingdom. The clinical isolate of E. coli O157:H7 (strain 00:BA152345) was obtained from the University of Rochester clinical microbiology laboratory, Rochester, N.Y., and was termed PM601.
All bacterial cultures were grown to stationary phase in tryptic soy broth at 37°C with continuous agitation. Control cultures in all assays were incubated in tryptic soy broth alone. Supernatants were obtained from the cultures following centrifugation at 17,000 × g and were filtered through a 0.22-μm-pore-size filter (Millipore). VT1, VT2, and monoclonal antibody to VT1 (SLT-13C4) were purchased from Toxin Technology (Sarasota, Fla.). Both VT1 and VT2 were purified (at Toxin Technology) by DEAE anion-exchange and Sephacryl S-100 gel filtration chromatography and were 50 and 25% pure, respectively, as determined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). The relatively low purity was due to the high potency and instability of the holotoxin (alpha and beta subunits), yet a 1-mg/ml concentration of either toxin was verotoxigenic to at least a dilution of 10−7. Lipopolysaccharide (LPS) contamination of the VTs was routinely found to be less than 1%. Monoclonal antibody to VT2 was a kind gift from T. G. Obrig (University of Virginia). The IKKα, IκBα, c-Rel, p50, and p65 antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Human recombinant TNF-α was purchased from R&D Systems, Inc. (Oxon, United Kingdom).
Construction of VT1 and VT2 gene knockout strains.
VT1 and/or VT2 gene knockout strains of E. coli O157:H7 (PM601) were constructed by using a suicide vector system as described by Donnenberg and Kaper (11). Deletion derivatives of genes sltA1 and sltB1 and of genes sltA2 of sltB2, encoding VT1 and VT2, respectively, were created by PCR from E. coli O157:H7 (PM601) genomic DNA. The primer sequences were as follows: VT1a forward, 5′-ACAAAGCGGAGCTCATTGTTGAAGG-3′, and VT1a reverse, 5′-CTAAAAATACGCGTTTTCATATTAC-3′; VT1b forward, 5′-GTTATTACGCGTTGAGTCAGAATAG 3′, and VT1b reverse, 5′-ATCACCTTCTAGAGTAATCGCCTCC-3′; VT2a forward, 5′-CAGCATTGAGCTCTACGAGTTTGA-3′, and VT2a reverse, 5′-CCCATTTAAATAAACGCGTCTTCATATA-3′; and VT2b forward, 5′-AGTGCAGTTTAATACGCGTTGAGGCAT-3′, and VT2b reverse, 5′-CGCAAGTCTAGAGGCGACGCCG-3′.
The resulting deletion constructs lacked all but the initial coding region of the toxin gene, but with the start and stop codons replaced with a unique MluI site. The deletion fragments were flanked with a 5′ SacI site and a 3′ XbaI site. With these sites, the deletion derivatives were cloned into suicide vector pCVD442. The plasmids containing the gene deletion mutations of VT1 or VT2 were then conjugated into E. coli O157:H7, and transcripts were selected on minimal medium containing ampicillin. Ampicillin-resistant, sucrose-sensitive prototrophs were subjected to further selection on modified Luria-Bertani (LB) medium at 30°C as previously described (5). Sucr Amps clones were then screened by PCR to confirm deletion of the toxin genes. The resulting strains were PM676 (lacking VT1 [VT1−] but containing VT2 [VT2+]), PM635 (VT1+ VT2−), and PM678 (VT1− VT2−). In addition, PM678 was transformed with plasmids bearing both the VT1 and the VT2 genes, giving rise to two new strains: PM678/pMP208/pMP216 (ΔsltA1 ΔsltB1, ΔsltA2 ΔsltB2, sltA1+ ΔsltB1+, ΔsltA2+ ΔsltB2+; VT1+ [high], VT2+ [low]) and PM678/pMP211/pMP215 (ΔsltA1 ΔsltB1, ΔsltA2 ΔsltB2, sltA1+ ΔsltB1+, ΔsltA2+ ΔsltB2+; VT1+ [low], VT2+ [high]), where slt is Shiga-like toxin, high is high-copy-number plasmid, and low is low-copy-number plasmid). Hence, functional verotoxicity was restored, mimicking that of the wild-type PM601 isolate, to ensure that any resulting inactivity of PM676 was due to the removal of the VT genes and not damage by insertion of plasmids.
Cell culture.
African green monkey kidney epithelial cells (Vero cells) were maintained in M199 medium with Earle's salts and supplemented with 5% (vol/vol) fetal calf serum, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml in a humidified atmosphere containing 5% CO2 at 37°C.
Western blotting.
Detection of IκBα by SDS-PAGE was conducted as outlined previously (29). The titers of all antibodies were determined for optimal conditions.
JNK and p38 MAP kinase assays.
Following termination by washing in ice-cold phosphate-buffered saline (PBS), cells were lysed in the appropriate solubilizing buffer, and precleared supernatants were added to the relevant substrate as previously described (38). Briefly, following solubilization, lysates were clarified by centrifugation at 13,000 × g for 5 min at 4°C, and supernatants were retained. For the JNK assay, precleared supernatants (10 μg of protein) were added to 20 μl of a slurry of GST-c-Jun(5-89)—glutathione-Sepharose beads and mixed for 3 h at 4°C; for the p38 MAP kinase assay, full-length GST-MAPKAPK-2 was used. The precipitates were resuspended in 25 μl of kinase buffer (38), and the reaction was started by the addition of [γ-32P]ATP (1 to 2 μCi, 25 μM) and incubated for 20 min at 30°C. The addition of 10 μl of 4× Laemmli sample buffer terminated the reaction. Samples were boiled for 5 min, resolved by SDS-11% PAGE, and fixed in 20 ml of fixer solution (20% [vol/vol] methanol, 10% [vol/vol] acetic acid) for 30 min. Gels were dried and subjected to autoradiography overnight.
Electrophoretic mobility shift assay (EMSA).
Following termination by washing in ice-cold PBS, agonist-stimulated cells were scraped, pelleted, resuspended in 400 μl of buffer 1 (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride, 0.5 mg of leupeptin/ml, 0.5 mg of aprotinin/ml), and incubated on ice for 15 min. Twenty-five microliters of 10% (vol/vol) NP-40 was then added, followed by brief vortexing. Detergent extracts were collected by centrifugation, and the pellet was extracted in 50 μl of buffer 2 (20 mM HEPES [pH 7.9], 25% [vol/vol] glycerol, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT) at 4°C with shaking for 15 min. The final nuclear extract was obtained by sonication on ice in a bath-type sonicator (twice for 30 s each time) followed by centrifugation at 13,000 × g for 15 min at 4°C.
NF-κB DNA binding was assayed by an EMSA with an NF-κB DNA probe, 5′-AGTTGAGGGGACTTTCCCAGGC-3′, which had been previously labeled with 32P by using T4 polynucleotide kinase in accordance with the manufacturer's instructions (Promega). Samples were run on 5% nondenaturing polyacrylamide gels, and binding was identified by autoradiography.
IKK assay.
Cells were incubated with vehicle or agonist as appropriate, washed twice in ice-cold PBS, and then lysed with solubilization buffer (20 mM Tris-HCl [pH 7.6], 1 mM EDTA, 0.5 mM EGTA, 150 mM NaCl, 0.1% [wt/vol] Brij 35, 1% [wt/vol] Triton X-100, 20 mM sodium fluoride, 0.5 mM sodium orthovanadate, 20 mM β-glycerophosphate, 10 μg of aprotinin/ml, 10 μg of pepstatin A/ml, 10 μg of leupeptin/ml, 1 mM phenylmethylsulfonyl fluoride). Extracts were clarified by centrifugation at 13,000 × g for 5 min at 4°C, equalized for protein, and incubated with 1.5 μg of an anti-IKKα antibody precoupled to protein G-Sepharose (2 h, 4°C) with rotation. Immunocomplexes were collected by centrifugation (13,000 × g, 1 min) and washed once with solubilization buffer and once with 25 mM HEPES buffer (pH 7.6) containing 25 mM β-glycerophosphate, 25 mM NaF, 15 mM MgCl2, and 1 mM DTT before incubation in the same buffer containing [γ-32P]ATP (25 μM, 5 μCi) and 1 μg of a recombinant fusion protein consisting of GST and the N terminus of IκBα (final volume, 30 μl) for 30 min at 30°C. Samples were boiled with 4× Laemmli sample buffer (5 min). Aliquots of each sample were then subjected to SDS-10% PAGE and fixed in 20 ml of fixer solution for 30 min. After drying, phosphorylated IκB was visualized by autoradiography overnight.
RESULTS
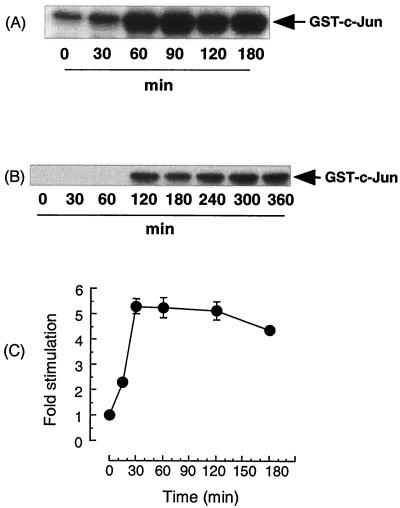
In Vero cells, supernatants from E. coli O157:H7 strain E30480 strongly stimulated JNK activity (Fig. 1A). Maximal activation was observed at between 30 and 60 min, was approximately 20- to 25-fold basal levels, and was sustained for at least 5 h (Fig. 1B). E. coli O157:H7 also strongly activated p38 MAP kinase (Fig. 1C) with biphasic kinetics, in that a 5- to 10-fold increase in the activity of the kinase was observed as early as 15 min and before a return to basal levels by 60 min (Fig. 1D). A second phase of activity was maximal by 2 h and remained elevated for the rest of the time course. This effect was also reflected at the level of MAPKAPK-2, the immediate downstream target of p38 MAP kinase, which was also activated following stimulation, confirming that E30480 prolonged the activation of SAP kinase signaling (results not shown). In contrast, extracellular signal-regulated kinase was not activated by E30480 supernatants (results not shown). Furthermore, the effects of E30480 were not mimicked by LPS (50 μg/ml), which gave a negligible response (results not shown). This finding was significant, as E30480 supernatants contained 22 ng of LPS/ml.
FIG. 1.
E30480-stimulated SAP kinase activity in Vero cells. (A and C) Vero cells were exposed to E30480 supernatants for the times indicated. Samples were assayed for JNK (A) and p38 MAP kinase (C) activities as outlined in Materials and Methods. Each autoradiograph represents at least three experiments. (B and D) JNK and p38 activities were quantified by scanning densitometry, respectively; each value represents the mean and standard error of the mean for three experiments.
The cytotoxic agents of E30480 and PM601, VT1 and VT2, were also examined for their ability to activate JNK in Vero cells. Both toxins stimulated a sustained activation of JNK, with kinetics similar to those observed with the supernatants (Fig. 2A for VT2; results not shown for VT1). Although the concentrations of VT1 and VT2 in the E30480 supernatants (570 and 930 ng/ml, respectively) and the purified VTs (2 μg/ml) used to stimulate JNK activity were high relative to the amount of toxin required to initiate cytotoxicity in Vero cells (50% cytoxic dose, 5 ng/ml) (results not shown), VT2 at a 400-fold-reduced concentration (5 ng/ml) still caused a substantial and sustained increase in JNK activity (Fig. 2B); however, this activation was further delayed by approximately 1.5 h, reaching a maximum at 2 h.
FIG. 2.
Activation of JNK by VT2 in Vero cells. (A and B) Vero cells were stimulated with VT2 at 2 μg/ml (A) or 5 ng/ml (B) for the times indicated. Samples were assayed for JNK activity as outlined in Materials and Methods. Each autoradiograph represents at least three experiments. (C) JNK activity from panel A (2 μg/ml) was quantified by scanning densitometry; each value represents the mean and standard error of the mean for three experiments.
Furthermore, we found that the activation of JNK by E30480 supernatants was substantially reduced by preincubation of Vero cells with antibodies directed against VT1 and VT2 (Fig. 3). Cells were incubated with 5 μg of either anti-VT1 or anti-VT2 antibody/ml, as this concentration was previously found to completely inhibit either VT1- or VT2-stimulated JNK activity (results not shown). Anti-VT1 antibody reduced JNK activity stimulated by E. coli O157:H7 supernatants by approximately 10%; however, anti-VT2 antibody was much more effective, reducing the response by at least 50%. Treatment of cells with both antibodies in combination essentially abolished activity (Fig. 3).
FIG. 3.
Effects of anti-VT-1 and anti-VT-2 antibodies on E30480-stimulated JNK activity in Vero cells. (A) Vero cells were preincubated with 5 mg of either anti-VT1 or anti-VT2 antibody/ml alone or in combination for 60 min and then stimulated with VT1, VT2, or E. coli O157: H7 supernatants for a further 60 min. Samples were assayed for JNK activity as outlined in Materials and Methods. The autoradiograph represents at least three experiments. (B) JNK activity from panel A was quantified by scanning densitometry; each value represents the mean and standard error of the mean for three experiments. Asterisks indicate P of <0.05 compared to E30480 stimulation.
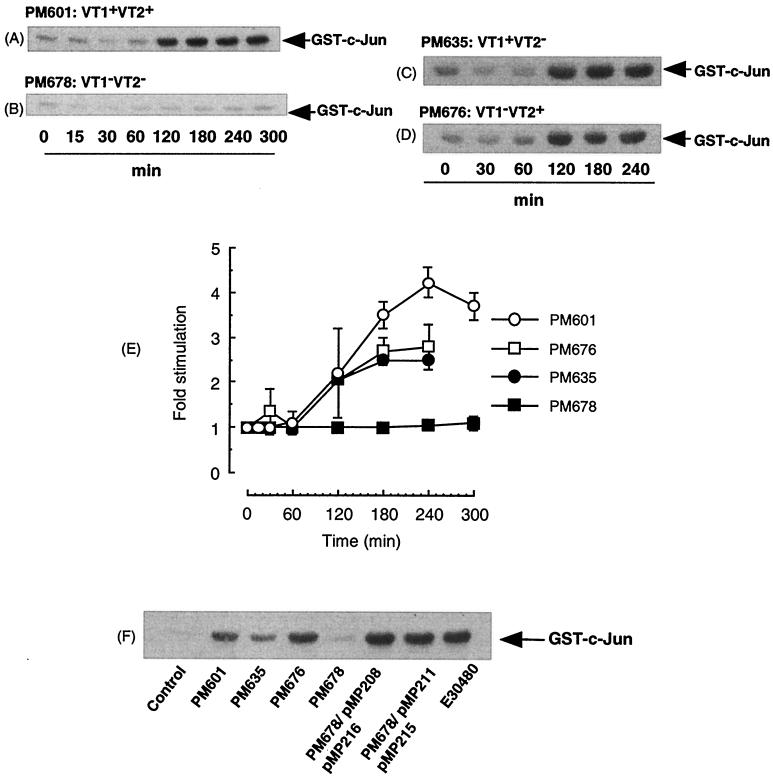
In order to further determine the obligatory role of VTs in the effects of E. coli O157:H7, we also tested the ability of E. coli O157:H7 VT gene knockout bacteria derived from another strain, PM601, to activate JNK signaling in Vero cells (Fig. 4). Wild-type PM601 supernatants stimulated a strong and sustained increase in JNK activity which was similar to that observed with E30480 supernatants (Fig. 4A). However, the kinetics of activation were slightly delayed, such that maximal activity was not reached until 2 h, similar to the results obtained with purified VT2. The VT1 knockout strain, PM676, and the VT2 knockout strain, PM635, induced similar sustained increases in JNK activity, which was also maximal by 2 h (Fig. 4C and D). The nonverotoxigenic double-VT knockout strain, PM678, was unable to stimulate JNK activity at any time point examined (Fig. 4B).
FIG. 4.
Effect of VT1 and VT2 gene knockout strain PM601 on JNK activity in Vero cells. (A to D and F) Vero cells were incubated with supernatants from PM601 (A), PM678 (B), PM635 (C), and PM676 (D) for the times indicated. Cells were incubated with PM601, PM635, PM676, PM678, PM678/pMP208/pMP216, PM678/pMP211/pMP215, and E30480 for 3 h (F). Samples were assayed for JNK activity as outlined in Materials and Methods. Each autoradiograph represents at least three experiments. (E) Values obtained in panels A to D were quantified by scanning densitometry; each value represents the mean and standard error of the mean for three experiments.
Furthermore, retransformation of PM678 with plasmids bearing the VT1 and VT2 genes restored JNK activity to a level which was not significantly different from that obtained with the original wild type (Fig. 4F). This result suggested that the effect of VT deletion from the original strain, PM601, was not due to any artifacts associated with the deletion procedure.
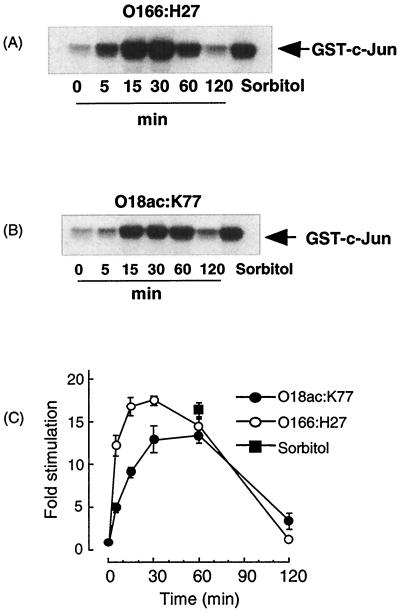
While these experiments clearly suggested that the presence of VTs is essential for a large component of JNK signaling in response to E. coli O157:H7, some qualitative differences could be observed between the E30480 and the PM601 supernatants (Fig. 1A versus Fig. 4A), suggesting the presence in E30480 of a component which resulted in a more rapid activation of JNK signaling. To further examine this phenomenon, we used supernatants derived from the nonverotoxigenic E. coli serotypes O166:H27 and O18ac:K77 (Fig. 5). Both supernatants stimulated a rapid transient activation of JNK signaling, which was maximal at 15 to 30 min and then returned to basal levels by 2 h.
FIG. 5.
Effects of non-VTEC serotypes on JNK activity in Vero cells. (A and B) Vero cells were incubated with supernatants from O166:H27 (A) and O18ac:K77 (B) for the times indicated. Samples were assayed for JNK activity as outlined in Materials and Methods. Each autoradiograph represents at least three experiments. (C) Values obtained in panels A and B were quantified by scanning densitometry; each value represents the mean and standard error of the mean for three experiments.
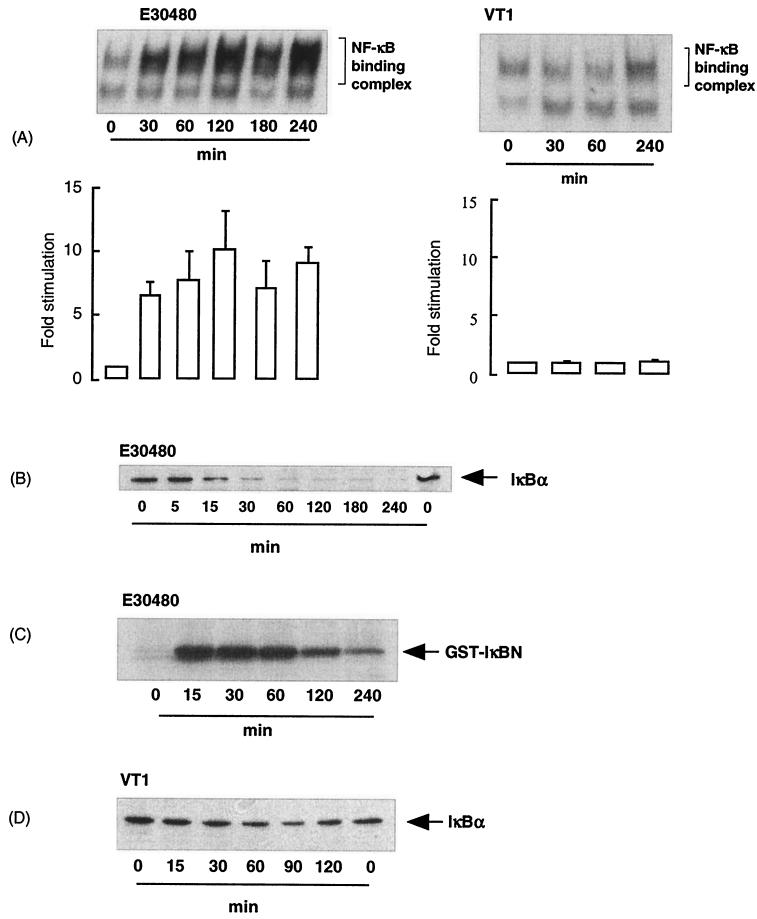
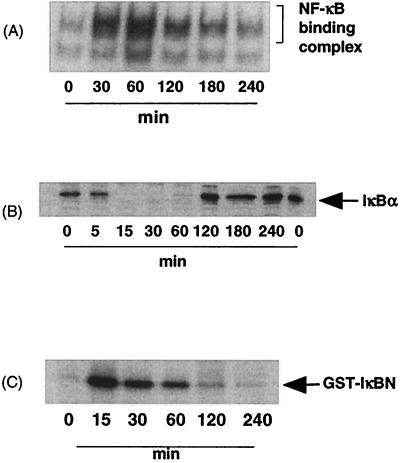
In addition to novel effects on JNK signaling, we also found unusual effects on NF-κB signaling in response to E30480 supernatants and the toxins (Fig. 6). Supernatants from E30480 stimulated a rapid increase in NF-κB DNA binding activity, which was maximal by 60 min and sustained for a further 4 h. Interestingly, this activity was accompanied by a sustained decrease in the expression of IκBα (Fig. 6B), rather than the normal transient decrease observed in other cell types in response to cytokines (16, 52). Furthermore, we found that E30480 supernatants also stimulated a strong increase in IKKα activity (Fig. 6C), which was maximal by 30 min and then returned to basal levels by 2 h. Additionally, and in contrast to their effects on JNK signaling, VT1, VT2, and PM601 supernatants at concentrations that strongly activated JNK signaling had no significant effects on any intermediate of the NF-κB signaling cascade (Fig. 6A and D). Again, the activation of NF-κB by E30480 supernatants was not mimicked by LPS, suggesting that the effects of E. coli O157:H7 are not mediated by this contaminant (results not shown).
FIG. 6.
Effects of E30480 and VT1 on NF-κB DNA binding, IκBα degradation, and IKKα activity in Vero cells. Vero cells were stimulated with E30480 supernatants and VT1 for the times indicated and then assayed for DNA binding activity (A), IκBα content (B and D), and IKKα activity (C) as outlined in Materials and Methods. Each autoradiograph represents at least three experiments. Error bars in graphs indicate standard errors of the mean.
Using supernatants derived from other E. coli serotypes, we observed further differences in the regulation of NF-κB signaling (Fig. 7). In contrast to the sustained activation in response to E30480, incubation of Vero cells with O166:H27 generated a much more transient increase in NF-κB DNA binding activity; while peaking at 30 min at a level of stimulation comparable to that observed with E30480 (20-fold), this activity returned to basal levels by 2 h (Fig. 7A). This increase was accompanied by a very transient decrease in IκBα expression, which returned to basal levels by 60 min (Fig. 7B). This result was not due to a more transient activation of IKKα, because the activation was very similar to that observed with E30480 (compare Fig. 7C with Fig. 6C), suggesting that the differences in the responses to the supernatants are likely to be manifested at the level of IκB degradation.
FIG. 7.
Effect of E. coli O166:H27 on the activation of NF-κB DNA binding activity, IκBα degradation, and IKKα activation in Vero cells. Vero cells were stimulated with E. coli O166:H27 for the times indicated and then assayed for DNA binding activity (A), IκBα content (B), and IKKα activity (C) as outlined in Materials and Methods. Each autoradiograph represents at least three experiments.
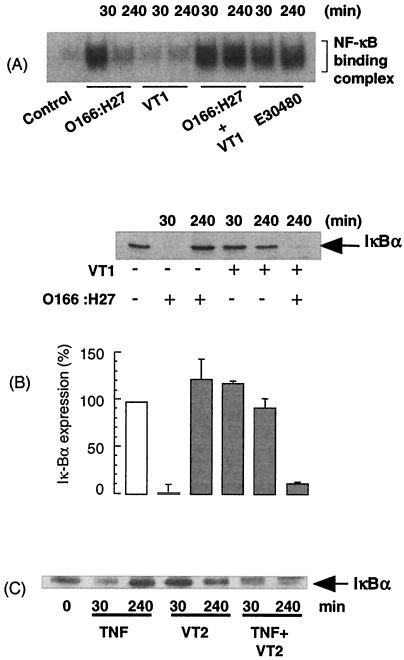
To determine further the effects of VTs on the kinetics of NF-κB signaling, we incubated cells with either VT1 or VT2 prior to the addition of O166:H27 supernatants or TNF-α (40 ng/ml) (Fig. 8). While VTs alone had little effect on NF-κB DNA binding in response to O166:H27 at 30 min, they markedly prolonged the response at 4 h, such that the signal was sustained and comparable to that observed with E30480 (Fig. 8A). This effect was also mimicked at the level of IκBα expression, where the presence of VTs maintained both O166:H27- and TNF-α-stimulated decreases in IκBα expression for up to 4 h (Fig. 8B and C).
FIG. 8.
Conditional sustained activation of NF-κB DNA binding and IκBα degradation by VTs in Vero cells stimulated with E. coli O166:H27 and TNF-α. Vero cells were preincubated with vehicle or VT1 for 60 min before further stimulation with E. coli O166:H27 supernatants or TNF-α (40 ng/ml) for 30 min or 4 h. Samples were assessed for NF-κB DNA binding (A) or IκBα levels (B and C) as outlined in Materials and Methods. Each autoradiograph represents at least three independent experiments. Error bars in the graph indicate standard errors of the mean.
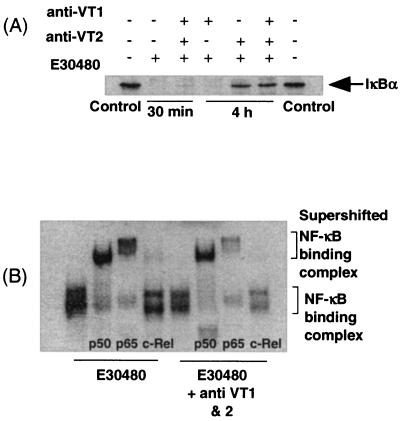
Figure 9 shows the effects of anti-VT antibodies on NF-κB signaling in response to E30480. Pretreatment of cells with anti-VT antibodies did not affect the initial decrease in cellular IκBα expression stimulated by the supernatants (Fig. 9A). However, in the presence of both anti-VT1 and anti-VT2 antibodies, there was a complete reversal of the prolonged decrease in IκBα expression in response to the supernatants. However, NF-κB DNA binding was only partially affected under similar conditions, with a decrease of only 30% in E30480-stimulated EMSA activity in the presence of the antibodies in combination (results not shown). Therefore, additional experiments were carried out with a supershift assay to determine whether a selective decrease in the DNA binding of one isoform of NF-κB occurred (Fig. 9B). In nuclear extracts prepared from E30480-stimulated cells, NF-κB DNA binding was attributed to the p50 and p65 isoforms (Fig. 9B, E30480). However, pretreatment with both anti-VT1 and anti-VT2 antibodies did not selectively diminish the binding of either isoform; rather, there was a consistent, approximately 30 to 50% decrease in both p50 and p65 binding (Fig. 9B, E30480 plus antibodies).
FIG. 9.
Effect of anti-VT antibodies on E30480-stimulated IκBα degradation and NF-κB supershift activity. (A) Vero cells were incubated with anti-VT antibodies for 60 min before exposure to E30480 supernatants for 30 min or 4 h. Samples were assayed for IκBα levels as outlined in Materials and Methods. (B) Nuclear extracts from cells stimulated with E30480 supernatants in the presence or absence of anti-VT1 and anti-VT2 antibodies were incubated with 1 μg of antibodies against p50, p65, or c-Rel/tube for 15 min prior to the EMSA. Each autoradiograph represents at least three independent experiments.
DISCUSSION
In this study, we examined the intracellular signaling events potentially relevant to the cytotoxic actions of E. coli O157:H7. Our studies revealed differences in the actions of supernatants derived from cultures of E. coli O157:H7, the associated verotoxins, VT1 and VT2, and supernatants from other serotypes. E. coli O157:H7 strain E30480 stimulated a remarkably strong and sustained activation of JNK and p38 MAP kinase that was comparable to that seen with several other forms of extreme cellular stress (8, 17). This response was distinct from that observed with other nonverotoxigenic E. coli serotypes, suggesting that VTs mediate a portion of this signal, particularly at later time points. This suggestion was confirmed by further experiments, which showed that either VT1 or VT2 alone stimulated a sustained activation of JNK and that antibodies directed against either toxin limited the degree of stimulation obtained with E. coli O157:H7 supernatants. Additionally, a gene knockout strain derived from PM601 was constructed and was unable to activate JNK.
The ability of a toxin to sustain the activation of JNK may be critical in determining whether the toxin and thus E. coli O157:H7 are able to initiate cell death. This area of study is controversial, and both protective and proapoptotic roles for JNK have been identified (15, 43, 59, 62). Recent studies have indicated that sustained JNK activity is more likely to result in cellular apoptosis in different cell types (8, 17), including endothelial cells, which are known target cells for VTs. However, until studies are able to effectively limit the kinetics of JNK through the use of either dominant-negative forms of JNK or overexpression of the relevant deactivating phosphatase, then this idea remains unproven. Nevertheless, it is interesting that decreasing the concentrations of VTs not only delayed the onset of JNK activation (Fig. 2) but also delayed the onset of cell death (results not shown). Thus, while JNK alone may not be responsible for VT-induced cytotoxicity, it may determine the rate of cell death by acting in synergy with other events. These events may include activation of caspases and inhibition of protein synthesis, both of which have been shown to be regulated by VTs (12, 23, 26, 36).
Although VTs were responsible for a large proportion of the JNK signal induced by E. coli O157:H7, it was clear that other nonverotoxigenic serotypes, for example, O166:H27, could also stimulate the activation of JNK. This finding suggested that a component of the supernatants common to these serotypes was able to rapidly activate the JNK signaling pathway. Thus, for E30480, the observed JNK signal was likely to comprise an early, non-toxin-dependent phase and a late, sustained phase which was toxin dependent. This scenario was difficult to determine without a very detailed analysis of the early kinetics of the JNK response. However, a biphasic response was much more apparent for p38 MAP kinase activity, with an initial peak at 15 min followed by later sustained activity.
The rapid onset of SAP kinase activation in response to E30480 and O166:H27 is consistent with a receptor-mediated effect similar to that observed with cytokines and other G-protein-coupled receptors (29, 38). LPS is not likely to be involved, as this agent, when added exogenously to Vero cells, did not initiate JNK or p38 MAP kinase activation. Various investigators have shown that LPS is able to activate MAP kinase homologues in numerous cells types (37, 46) through the activation of Toll-like receptors (6, 45, 60). Since Vero cells are unresponsive to LPS, this finding suggests that these cells lack the relevant Toll-like receptor subtypes or the adaptor proteins required to activate signaling responses. Soluble factors (outer membrane proteins, glycolipids, polysaccharides, and secreted proteins) other than LPS are released from both verotoxigenic and non-VT-producing strains of E. coli; some of these could activate epithelial cells (25). Thus, it is possible that an additional factor present in some strains of E. coli is able to activate Vero cells. Intriguingly, the American isolate PM601 did not have the same profile of JNK activity as E30480, and a VT double-knockout strain did not give a JNK response. These results suggest differences in the products that different strains of E. coli O157:H7 can produce in cultures.
Differences in the regulation SAP kinase signaling responses by different supernatants were also manifested at the level of another signaling cascade, the NF-κB pathway. Supernatants from E30480 but not PM601 generated strong and sustained increases in DNA binding and IκBα degradation and, as shown for the first time, an increase in the activity of the upstream intermediate IKK. Surprisingly, VT1 and VT2 had little effect on any of these intermediates. These results reveal that despite the presence on Vero cells of CD77, the putative VT receptor, VTs are also unlikely to activate NF-κB in a manner similar to that observed for cytokine receptors, i.e., involving receptor-associated intermediates such as NF-κB-inducing kinase, MyD88, and TNF receptor-associated factors (31, 33); rather, they suggest an indirect activation of these pathways. However, once again, the fact that both E30480 and O166:H27 are able to rapidly initiate NF-κB signaling is consistent with the notion that a component common to these organisms is likely to involve the activation of a receptor which couples simultaneously to both NF-κB and SAP kinase.
Despite the inability of VTs to directly activate the IKK/IκBα/NF-κB cascade, an important and additional action of VTs was the conditional, sustained activation of NF-κB signaling in Vero cells. This action was not due to effects on the initiation of the NF-κB signaling pathway per se, since there were no differences in the abilities of E. coli O157:H7 strain E30480, which contains VTs, and O166:H27, which does not, to activate the upstream intermediate IKK. Rather, the effect of VTs was manifested at the level of IκBα degradation, which was prolonged in response to E30480 but not other supernatants. Additional results were consistent with these findings; the presence of VTs prolonged the activation of NF-κB signaling and IκBα degradation in response to either O166:H27 or the cytokine TNF-α. Furthermore, the prolongation of TNF-α-induced degradation of IκBα was observed following incubation of cells with wild-type strain PM601 but not with double-knockout strain PM678 (data not shown). Thus, irrespective of the agent used to generate the initial early NF-κB signal, VTs are essential for that signal to be prolonged.
The effect of VTs on NF-κB signaling is likely to be due to inhibition of the resynthesis of IκBα, a process which normally occurs following NF-κB activation due to the presence on the promoter of the IκBα gene of an NF-κB binding site (20). This effect would in turn be consistent with the well-described ribotoxic effect of VTs (36). Attempts to address this question with protein synthesis inhibitors, such as puromycin and actinomycin D, were only partially successful, as these agents alone caused the degradation of IκBα (results not shown). Nevertheless, this mechanism is most likely to explain the actions of VTs.
It should be noted, however, that despite the substantial reversal of the loss of IκBα, preincubation of Vero cells with antibodies to VTs did not substantially affect E. coli O157:H7-stimulated NF-κB DNA binding. This result implies that other isoforms of IκB undergo phosphorylation and degradation, allowing sufficient translocation of NF-κB isoforms to the nucleus. Our experiments consistently failed to detect IκBβ; thus, it is possible that IκBɛ is involved in sustaining NF-κB activity. However, if this is the case, then the implication is that there is another component in the supernatants which has additional effects on NF-κB signaling and is manifested even when the actions of the toxins are inhibited. Nevertheless, the fact that E. coli O157:H7 can sustain NF-κB DNA binding activity is likely to have profound implications for the physiological outcome for the cell, since NF-κB is implicated in both induction of and protection from apoptosis (reviewed in reference 32). However, it still remains unclear whether this effect contributes to the cytotoxicity induced by VTs.
The sustained effects of VT on JNK, p38 MAP kinase, and NF-κB signaling may directly relate to the cell-selective cytotoxic effects of the VTs. In support of this notion, a recent report (18) described a sustained activation of p38 in response to VT1 stimulation in Vero cells which, when inhibited with the specific p38 inhibitor SB203580, partially reduced cell death. Cells of myeloid origin are not susceptible to VT-induced cytotoxicity, and in neutrophils, VT is cytoprotective (30). In preliminary experiments with human peripheral blood monocytes, we found that VTs are unable to activate JNK or prolong NF-κB activation and that the transient kinetics of JNK and NF-κB activation by E. coli O157:H7 strain E30480 are far more consistent with those observed in response to O166:H27 (P. Cameron, D. Rotondo, and R. Plevin, unpublished data). In a recent study, Shiga toxin type 1 was shown to directly activate both AP-1 and NF-κB in THP-1 cells (44), suggesting variations in signaling responses even between different cells of myeloid origins. The difference between Vero cells and monocytes with regard to VT responsiveness is unclear, as both express Gb3 receptors, but it is likely to involve coupling of the receptors to distinct intracellular signaling intermediates. As mentioned above, very recently, caspases, particularly isoforms 1 and 3, were implicated in VT-induced cell death (23, 26). Therefore, since caspase 3 activation, apoptosis, and prolonged JNK activity have been shown to be linked through the cleavage and sustained activation of upstream MEKK-1 (13, 22, 58), this action may constitute a possible pathway by which the cell-selective effects of VTs are mediated. This pathway may also represent a new site for therapeutic intervention.
Acknowledgments
We acknowledge the kind gifts of constructs and reagents from various laboratories.
This work was sponsored by SHERT grant RG6/01.
Editor: J. T. Barbieri
REFERENCES
- 1.Agin, T. S., and M. K. Wolf. 1997. Identification of a family of intimins common to Escherichia coli causing attaching-effacing lesions in rabbits, humans, and swine. Infect. Immun. 65:320-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arab, S., M. Murakami, P. Dirks, B. Boyd, S. L. Hubbard, C. A. Lingwood, and J. T. Rutka. 1998. Verotoxins inhibit the growth of and induce apoptosis in human astrocytoma cells. J. Neuro-Oncol. 40:137-150. [DOI] [PubMed] [Google Scholar]
- 3.Arbus, G. S. 1997. Association of verotoxin-producing E. coli and verotoxin with hemolytic uremic syndrome. Kidney Int. 51:S-91-S-96. [PubMed] [Google Scholar]
- 4.Birrell, M., E.-B. Haddad, K. McCluskie, D. Hele, S. Phipps, S. E. Webber, M. Foster, and M. G. Belvisi. 1998. Effect of the p38 inhibitor, SB203580, in a model of airway inflammation. Br. J. Pharmacol. 125:89. [Google Scholar]
- 5.Blomfield, I. C., V. Vaughn, R. F. Rest, and B. I. Eisenstein. 1991. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol. Microbiol. 5:1447-1457. [DOI] [PubMed] [Google Scholar]
- 6.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Beck, H. C. Reinecker, and D. K. Podolsky. 2000. Lipopolysaccharide activates distinct signalling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966-972. [DOI] [PubMed] [Google Scholar]
- 7.Chen, F., V. Castranova, X. Shi, and L. M. Demers. 1999. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 45:7-17. [PubMed] [Google Scholar]
- 8.Chen, Y.-R., C. F. Meyer, and T.-H. Tan. 1996. Persistent activation of c-jun N-terminal kinase 1 (JNK1) in γ radiation-induced apoptosis. J. Biol. Chem. 271:631-634. [DOI] [PubMed] [Google Scholar]
- 9.Coia, J. 1998. Clinical, microbiological and epidemiological aspects of Escherichia coli O157 infection. FEMS Microbiol. Immunol. 20:1-9. [DOI] [PubMed] [Google Scholar]
- 10.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 11.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endo, Y., K. Mitsui, M. Motizuki, and K. Tsurugi. 1987. The mechanisms of action of ricin and related toxic lectins on eukaryotic ribosomes. J. Biol. Chem. 262:5908-5912. [PubMed] [Google Scholar]
- 13.Gibson, S., C. Widmann, and G. L. Johnson. 1999. Differential involvement of MEK kinase-1 (MEKK1) in the induction of apoptosis in response to microtubule-targeted drugs versus DNA damaging agents. J. Biol. Chem. 274:10916-10922. [DOI] [PubMed] [Google Scholar]
- 14.Guan, Z., S. Y. Buckman, B. W. Miller, L. D. Springer, and A. R. Morrison. 1998. Interleukin-1β-induced cyclooxygenase expression requires activation of both c-Jun NH2-terminal kinase and p38 MAPK signal pathways in rat renal mesangial cells. J. Biol. Chem. 273:28670-28676. [DOI] [PubMed] [Google Scholar]
- 15.Harada, J., and M. Sugimoto. 1999. An inhibitor of p38 and JNK MAP kinases prevents activation of caspase and apoptosis of cultured cerebellar granule neurons. Jpn. J. Pharmacol. 79:369-378. [DOI] [PubMed] [Google Scholar]
- 16.Haskill, S., A. A. Beg, S. M. Tompkins, J. S. Morris, and A. D. Yurochko. 1991. Characterisation of an immediate-early gene induced in adherent monocytes that encodes IκB-like activity. Cell 65:1281-1288. [DOI] [PubMed] [Google Scholar]
- 17.Hu, Y.-L., S. Li, J. Y.-J. Shyy, and S. Chien. 1999. Sustained JNK activation induces endothelial apoptosis: studies with colchicine and shear stress. Am. J. Physiol. 277:H1593-H1599. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda, M., Y. Gunji, S. Yamasaki, and Y. Takeda. 2000. Shiga toxin activates p38 MAP kinase through cellular Ca(2+) increase in Vero cells. FEBS Lett. 485:94-98. [DOI] [PubMed] [Google Scholar]
- 19.Inward, C. D., J. Williams, I. Chant, D. V. Milford, P. E. Rose, and C. M. Taylor. 1995. Verocytotoxin-1 induces apoptosis in Vero cells. J. Infect. 30:213-218. [DOI] [PubMed] [Google Scholar]
- 20.Ito, C. Y., A. G. Kazantsev, and A. S. Baldwin. 1994. Three NF-kappa B sites in the I kappa B-alpha promoter are required for induction of gene expression by TNF alpha. Nucleic Acids Res. 22:3787-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacewicz, M. S., M. Moassaleth, S. K. Gross, K. A. Balasubramanian, P. F. Daniel, S. Raghavan, R. H. McCluer, and G. T. Keusch. 1994. Pathogenesis of Shigella diarrhea. XVII. A mammalian cell membrane glycolipid, Gb3, is required but not sufficient to confer sensitivity to Shiga toxin. J. Infect. Dis. 169:538-546. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, N. L., A. M. Gardner, K. M. Diener, C. A. Lange-Carter, J. Gleavy, M. B. Jarpe, A. Minden, M. Karin, L. I. Zon, and G. L. Johnson. 1996. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J. Biol. Chem. 271:3229-3237. [DOI] [PubMed] [Google Scholar]
- 23.Jones, N. L., A. Islur, R. Haq, M. Mascarenhas, M. A. Karmali, M. H. Perdue, B. W. Zanke, and P. M. Sherman. 2000. Escherichia coli Shiga toxins induce apoptosis in epithelial cells that is regulated by the Bcl-2 family. Am. J. Gastrointest. Liver Physiol. 278:G811-G819. [DOI] [PubMed] [Google Scholar]
- 24.Karpman, D., A. Hakansson, M.-T. R. Perez, C. Isaksson, E. Carlemalm, A. Caprioli, and C. Svanborg. 1998. Apoptosis of renal cortical cells in the hemolytic-uremic syndrome: in vivo and in vitro studies. Infect. Immun. 66:636-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenny, B., and B. Finlay. 1995. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc. Natl. Acad. Sci. USA 92:7991-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyokawa, N., T. Mori, T. Taguchi, M. Saito, K. Mimori, T. Suzuki, T. Sekino, N. Sato, H. Nakajima, Y. U. Katagiri, T. Takeda, and J. Fujimoto. 2001. Activation of the caspase cascade during Stx1-induced apoptosis in Burkitt's lymphoma cells. J. Cell. Biochem. 81:128-142. [DOI] [PubMed] [Google Scholar]
- 27.Kiyokawa, N., T. Taguchi, T. Mori, H. Uchida, N. Sato, T. Takeda, and J. Fujimoto. 1998. Induction of apoptosis in normal human renal tubular epithelial cells by Escherichia coli Shiga toxins 1 and 2. J. Infect. Dis. 178:178-184. [DOI] [PubMed] [Google Scholar]
- 28.Konowalchuk, J., J. I. Spiers, and S. S. Stavric. 1977. Vero response to a cytotoxin of Escherichia coli. Infect. Immun. 18:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laird, S. M., A. Graham, A. Paul, G. W. Gould, C. Kennedy, and R. Plevin. 1998. Tumour necrosis factor stimulates stress-activated protein kinases and the inhibition of DNA synthesis in cultures of bovine aortic endothelial cells. Cell. Signal. 10:473-480. [DOI] [PubMed] [Google Scholar]
- 30.Liu, J., T. Akahoshi, T. Sasahana, H. Kitasato, R. Namai, T. Sasaki, M. Inoue, and H. Kondo. 1999. Inhibition of neutrophil apoptosis by verotoxin 2 derived from Escherichia coli O157:H7. Infect. Immun. 67:6203-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malinin, N. L., M. P. Boldin, A. V. Kovalenko, and D. Wallach. 1997. MAP3K-related kinase involved in NFκB induction by TNF, CD95 and IL-1. Nature 385:540-544. [DOI] [PubMed] [Google Scholar]
- 32.May, M. J., and S. Ghosh. 1998. Signal transduction through NF-κB. Immunol. Today 19:80-88. [DOI] [PubMed] [Google Scholar]
- 33.Muzio, M., J. Ni, P. Feng, and V. M. Dixit. 1997. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278:1612-1615. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien, A. D., G. D. LaVeck, M. R. Thompson, and S. R. Formal. 1982. Production of Shigella dysenteriae type-1 cytotoxin by Escherichia coli. J. Infect. Dis. 146:763-769. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien, A. D., and R. K. Holmes. 1987. Shiga and Shiga-like toxins. Microbiol. Rev. 51:206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pallanca, A., R. Mazzaracchio, M. Brigotti, D. Carnicelli, P. Alvergna, S. Sperti, and L. Montanaro. 1998. Uncompetitive inhibition by adenine of the RNA-N-glycosidase of ribosome-inactivating proteins. Biochim. Biophys. Acta 1384:277-284. [DOI] [PubMed] [Google Scholar]
- 37.Paul, A., A. Cuenda, C. E. Bryant, J. Murray, E. R. Chilvers, P. Cohen, G. W. Gould, and R. Plevin. 1999. Involvement of mitogen-activated protein kinase homologues in the regulation of lypopolysaccharide-mediated induction of cyclo-oxygenase-2 but not nitric oxide synthase in RAW 264.7 macrophages. Cell. Signal. 11:491-497. [DOI] [PubMed] [Google Scholar]
- 38.Paul, A., L. Torrie, G. McLaren, C. Kennedy, and R. Plevin. 2000. P2Y-receptor mediated inhibition of tumour necrosis factor-α-stimulated stress-activated protein kinase in EAhy926 endothelial cells. J. Biol. Chem. 275:13243-13249. [DOI] [PubMed] [Google Scholar]
- 39.Philpott, D., J., C. A. Acerly, A. J. Iliaan, M. A. Karmali, M. H. Perdue, and P. M. Sherman. 1997. Translocation of verotoxin-1 across T84 monolayers: mechanism of bacterial toxin penetration of epithelium. Am. J. Microbiol. 273:G1349-G1358. [DOI] [PubMed] [Google Scholar]
- 40.Proulx, F., J. P. Turgeon, G. Delage, G. Lior, L. Lafleur, and L. Chiconne. 1995. Immune response to verotoxin 1 and 2 in children with Escherichia coli O157:H7 hemorrhagic colitis and classic hemolytic syndrome. Can. J. Infect. Dis. 6:136-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramegowda, B., and V. L. Tesh. 1996. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to the Shiga-like toxins in human monocytes and monocytic cell lines. Infect. Immun. 64:1173-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regnier, C. H., H. Y. Song, X. Gao, D. V. Goeddel, Z. Cao, and M. Rothe. 1997. Identification and characterization of an IκB kinase. Cell 90:373-383. [DOI] [PubMed] [Google Scholar]
- 43.Roulston, A., C. Rienhardt, P. Amiri, and L. T. Williams. 1998. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor α. J. Biol. Chem. 273:10232-10239. [DOI] [PubMed] [Google Scholar]
- 44.Sakiiri, R., B. Ramegowda, and V. L. Tesh. 1998. Shiga Toxin type 1 activates tumour necrosis factor a gene transcription and nuclear translocation of the transcriptional activators nuclear factor κB and activator protein-1. Blood 92:558-566. [PubMed] [Google Scholar]
- 45.Schumann, R. R., D. Pfeil, N. Lamping, C. Kirschning, G. Scherzinger, P. Schlag, L. Karawajew, and F. Herrmann. 1996. Lipopolysaccharide induces the rapid tyrosine phosphorylation of the mitogen-activated protein kinases erk-1 and p38 in cultured human vascular endothelial cells requiring the presence of soluble CD14. Blood 87:2805-2814. [PubMed] [Google Scholar]
- 46.Schumann, R. R., D. Pfeil, D. Freyer, W. Buerger, N. Lamping, C. J. Kirschning, U. B. Goebel, and J. R. Weber. 1998. Lipopolysaccharide and pneumococcal cell wall components activate the mitogen activated protein kinases (MAPK) erk-1, erk-2, and p38 in astrocytes. Glia 22:295-305. arsid6291543 [DOI] [PubMed] [Google Scholar]
- 47.Scotland, S. M., H. R. Smith, and B. Rowe. 1985. Two distinct toxins active on Vero cells from Escherichia coli O157. Lancet ii:885-886. arsid6291543 [DOI] [PubMed]
- 48.Swantek, J. L., M. H. Cobb, and T. D. Geppert. 1997. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol. Cell. Biol. 17:6274-6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taguhi, T., H. Uchida, N. Kiyokawa, T. Mori, N. Sato, H. Horie, T. Takeda, and J. Fujimoto. 1998. Verotoxins induce apoptosis in human renal tubular epithelium derived cells. Kidney Int. 53:1681-1688. [DOI] [PubMed] [Google Scholar]
- 50.Tesh, V. L., and A. D. O'Brien. 1991. The pathogenic mechanisms of Shiga toxin and the Shiga-like toxins. Mol. Microbiol. 5:1817-1822. [DOI] [PubMed] [Google Scholar]
- 51.Tesh, V. L., B. Ramegowda, and J. E. Samuel. 1994. Purified Shiga-like toxins induce expression of proinflammatory cytokines from murine macrophages. Infect. Immun. 62:5085-5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson, J. E., R. J. Phillips, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1992. IκBβ regulates the persistent response in a biphasic activation of NFκB. Cell 80:573-582. [DOI] [PubMed] [Google Scholar]
- 53.Tibbles, L. A., and J. R. Woodget. 1999. The stress-activated protein kinase pathways. Cell. Mol. Life Sci. 55:1230-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuttle, J., T. Gomez, M. P. Doyle, J. G. Wells, T. Zhao, R. V. Tauxe, and P. M. Griffin. 1999. Lessons from a large outbreak of Escherichia coli O157:H7 infections: insights into the infectious dose and method of widespread contamination of hamburger patties. Epidemiol. Infect. 122:185-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van de Kar, N. C. A. J., L. A. H. Monnems, M. A. Karmali, and V. W. M. van Hinsbergh. 1992. Tumour necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the haemolytic uraemic syndrome. Blood 80:2755-2764. [PubMed] [Google Scholar]
- 56.van Setten, P. A., L. A. H. Monnens, R. G. G. Verstraten, L. P. W. J. van den Heuvel, and V. W. M. van Hinsbergh. 1996. Effects of verocytotoxin-1 on nonadherent monocytes: binding characteristics, protein synthesis, and induction of cytokine release. Blood 88:174-183. [PubMed] [Google Scholar]
- 57.van Setten, P. A., V. W. M. van Hinsbergh, T. J. A. N. van der Velden, N. C. A. J. van de Kar, M. Vermeer, J. D. Mahan, K. J. M. Assamann, L. P. W. J. van den Heuvel, and L. A. H. Monnens. 1997. Effects of TNFα on verocytotoxin cytotoxicity in purified human glomerular microvascular endothelial cells. Kidney Int. 51:1245-1256. [DOI] [PubMed] [Google Scholar]
- 58.Widmann, C., P. Gerwins, N. L. Johnson, M. B. Jarpe, and G. L. Johnson. 1998. MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis. Mol. Cell. Biol. 18:2416-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 60.Yang, H., D. W. Young, F. Gusovsky, and J. C. Chow. 2000. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J. Biol. Chem. 275:20861-20866. [DOI] [PubMed] [Google Scholar]
- 61.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]
- 62.Zanke, B. W., C. Lee, S. Arab, and I. F. Tannock. 1998. Death of tumor cells after intracellular acidification is dependant on stress-activated protein kinases (SAPK/JNK) pathway activation and cannot be inhibited by Bcl-2 expression or interleukin 1β-converting enzyme inhibition. Cancer Res. 58:2801-2808. [PubMed] [Google Scholar]