Abstract
Immunization of mice with pneumococcal surface adhesin A (PsaA) emulsified in complete Freund's adjuvant (CFA) provides protection against systemic infection with Streptococcus pneumoniae. Because the use of CFA is not acceptable in humans, we sought to develop alternative means of enhancing the immunogenicity of protein antigens of potential use in pneumococcal vaccines. We designed a series of genetic constructs in which coding sequences for PsaA were linked to sequences encoding either murine interleukin-2 (mIL-2), mIL-4, or two copies of an immunostimulatory nonapeptide derived from mIL-1β. The PsaA-cytokine constructs were cloned and expressed in Escherichia coli. Mice immunized twice with PsaA-IL-2, or PsaA-IL-4 responded with PsaA-specific antibody production comparable in magnitude to that of mice primed with PsaA in CFA and boosted with PsaA in incomplete Freund's adjuvant (PsaA-Adj). Antibodies elicited by PsaA-Adj were predominantly of the immunoglobulin G1 (IgG1) subclass, while PsaA-IL-2 and PsaA-IL-4 elicited substantial amounts of IgG2a in addition to IgG1. Mice immunized with PsaA-Adj or PsaA-IL-4 were partially protected against intraperitoneal challenge with virulent S. pneumoniae (30% overall survival beyond 15 days postchallenge). Mice immunized with PsaA and no adjuvant or PsaA-IL-2 exhibited 0 or 5% survival rates, respectively, following challenge. In contrast, mice immunized twice with capsular polysaccharide were 100% protected. The modest levels of protection seen in mice immunized with PsaA and its more immunogenic derivatives may be explained in part by the relative inaccessibility of antibody to PsaA on the surface of encapsulated S. pneumoniae.
Streptococcus pneumoniae is the cause of infections that manifest clinically as bacterial pneumonia, meningitis, otitis media, and sinusitis. Each year S. pneumoniae causes approximately 1.2 million deaths worldwide from pneumonia (28). Antibiotics offer effective treatment for many cases of pneumococcal disease. However, the rapid emergence of multiple-drug-resistant strains of S. pneumoniae (15, 16) has limited the effectiveness of antibiotics and stimulated renewed interest in the prevention of pneumococcal infections with vaccines.
Of the currently licensed pneumococcal vaccines, the 23-valent PS vaccine is poorly immunogenic in children younger than 2 years (14) while the 7-valent conjugate vaccine covers only a minority of pneumococcal strains (17). To address these and other shortcomings, several investigators have identified protein antigens widely expressed among pneumococcal strains for possible use as third-generation pneumococcal vaccines (18, 21, 26, 31). Several of these proteins elicit protection against pneumococcal bacteremia and nasopharyngeal carriage. Such proteins are attractive targets for pneumococcal vaccine development because, unlike capsular polysaccharide (PS), proteins are highly immunogenic in young children and are potentially cross-reactive among different serotypes.
Pneumococcal surface adhesin A (PsaA) is an approximately 33-kDa lipoprotein found on the surface of S. pneumoniae and is thought to be attached to the bacterial membrane via an N-terminal cysteine-linked lipid tail. This protein is highly conserved in 90 strains of S. pneumoniae tested to date, including all clinically relevant strains (11, 25, 27). Immunization of mice with PsaA is protective against nasopharyngeal carriage (8, 12) and systemic infection (30). On this basis, PsaA has been advanced as a suitable target for pneumococcal vaccine development.
Many animal models of vaccine efficacy involve the use of purified protein antigens mixed with adjuvants, such as complete Freund's adjuvant (CFA) and incomplete Freund's adjuvant (IFA) (7), that are not approved for use in humans. Alum is commonly used as an adjuvant in licensed human vaccines but is a less potent adjuvant than CFA (33), usually requiring multiple inoculations of vaccine to elicit protective antibody responses. To circumvent the requirement for potentially toxic adjuvants in vaccine formulations, investigators have studied a variety of host molecules potentially able to enhance the immunogenicity of selected target antigens. Host-encoded immune potentiators such as cytokines (10, 32) and complement 3d (C3d) (13) have been reported to enhance the immunogenicity of covalently linked target antigens.
In these studies, we constructed fusion proteins consisting of PsaA linked to interleukin-2 (IL-2), IL-4, or immunogenic peptides derived from IL-1β (9, 22). Fusion to IL-2 and IL-4 was found to enhance the immunogenicity of PsaA comparably to that for administration with CFA and IFA and, in the case of fusion to IL-4, to provide partial protection against fatal sepsis.
MATERIALS AND METHODS
Mice.
CBA/J, C3H/HeJ, and BALB/c mice, 6 to 8 weeks old, were housed under specific-pathogen-free conditions and given sterile food and water ad libitum. CBA/J and C3H/HeJ were purchased from the Jackson Laboratory, Bar Harbor, Maine, and BALB/c mice were purchased from Taconic Farms (Germantown, N.Y.). The Case Western Reserve University Institutional Animal Care and Use Committee approved all animal experiments.
Immunizations.
Mice were inoculated intraperitoneally (i.p.) with 50 pmol (or 250 pmol) of recombinant PsaA (rPsaA), rPsaA-IL-2, rPsaA-IL-4 or rPsaA-IL-1β. Antigens were administered in 100 μl of phosphate-buffered saline (PBS) containing 1% globulin-free mouse serum albumin (MSA). Animals were inoculated on days 0 and 21 and bled on days 14 and 35 unless otherwise indicated. For adjuvant inoculations, rPsaA was mixed 1:1 with either CFA or IFA (both from Sigma Chemical Co., St. Louis, Mo.). Mice inoculated i.p. with 1% MSA in PBS were used as negative controls. Sera were prepared from blood collected from mice via the tail vein. Serum samples were stored at −20°C until used for assays.
Bacteria.
Escherichia coli DH5α (Life Technologies, Gaithersburg, Md.) was used for plasmid construction. Recombinant proteins were expressed in E. coli BL21(DE3)pLysS (Novagen, Inc., Madison, Wis.). Bacteria were cultured in Luria broth supplemented with antibiotics. Virulent S. pneumoniae strain A66.1 (a gift from David Briles, University of Alabama, Birmingham, Ala.) was used for challenge experiments and as a source of genomic DNA. S. pneumoniae were routinely grown on Trypticase soy agar plates supplemented with 5% sheep blood (blood agar) or in Todd-Hewitt broth supplemented with 0.5% yeast extract (Difco, Detroit, Mich.). Salmonella enterica serovar Typhimurium strain χ4064 (catalog no. 53648) purchased from the American Type Culture Collection was cultured in Luria broth supplemented with 40μg of nalidixic acid per ml.
PCR amplification.
Oligonucleotide primers used in the PCR experiments (Table 1) were all purchased from Life Technologies. The gene encoding PsaA was amplified from the genomic DNA of S. pneumoniae strain A66.1 using the high-fidelity thermostable DNA polymerase, Platinum Pfx (Life Technologies) with primers PsaA 21(F) and PsaA 308(R). Plasmids encoding cDNAs for mouse IL-2 (catalog no. 39892) and mouse IL-4 (catalog no. 736430) were purchased as bacterial stocks from the American Type Culture Collection. Mouse IL-2 was amplified from the IL-2 plasmid using primers IL-2: 21(F) and IL-2: 169(R), and mouse IL-4 was amplified from the IL-4 plasmid using primers IL-4: 22(F) and IL-4: 140(R).
TABLE 1.
Sequence of oligonucleotide primers used for amplification and cloning
| Gene | Primera | Sequence (5′-3′)b |
|---|---|---|
| psaA | 21(F) | AATCGTCATATGGCCATGGgcgctagcggaaaaaaagatgcagcttc |
| 308(R) | ATTCCCCTCGAGAAGCTTGGATCCtgccaatccttcagcaatctt | |
| IL-2 | 21(F) | AATCGTCATATGGCCATGGGCGGATCCgcacccacttcaagctccacttc |
| 169(R) | ATTCCCCTCGAGttgagggcttgttgagatgatgctttgaGagaaggctatcc | |
| IL-4 | 22(F) | AATCGTCATATGGCCATGGGCAAGCTTatccacggatgcgacaaaaatcac |
| 140(R) | ATTCCCCTCGAGcgagtaatccatttgcatgatgc | |
| IL-1β | Sense | CATTTTAAGCTTgtacaaggagaaccaagcaacgacaaaatacctgtggcc |
| ttgggcgtacaaggagaaccaagcaacgacaaaGAATTCCTCGAGCACG | ||
| Antisense | CGTGCTCGAGGAATTCtttgtcgttgcttggttctccttgtacgcccaa | |
| ggccacaggtattttgtcgttgcttggttctccttgtacAAGCTTAAAATG |
Numbers represent the first amino acid encoded by the forward (F) primers or the last amino acid encoded by the reverse (R) primers. All numbering is based on pre-pro amino acid sequences for each gene. Underlined nucleotides represent coding sequences for amino acids 163 to 171 of murine pre-pro IL-1β.
Restriction sites in each primer relevant to this study are in bold; nucleotides in lowercase are derived from sequences of each gene deposited in GenBank.
Plasmid construction and expression.
The various genes and gene fragments used in the plasmid construction and expression experiments were cloned into plasmid pET29b+ (Novagen, Inc., Madison, Wis.) using E. coli DH5α as the bacterial host. The PsaA amplicon was cloned at NcoI and XhoI of pET29b+ to generate p29-psaA. PsaA was genetically linked to IL-2 by digesting the IL-2 amplicon with BamHI and XhoI and cloning it into p29-psaA at BamHI and XhoI. The PsaA-IL-4 fusion was generated by digesting the IL-4 amplicon with HindIII and XhoI and ligated into p29-psaA at HindIII and XhoI. Oligonucleotides encoding two tandem copies of an immunostimulatory peptide of mouse IL-1β peptide (amino acids 163 to 171) (5) were annealed to each other, digested with HindIII and XhoI, and ligated directly into p29-psaA at the corresponding sites. For recombinant protein expression, the p29-psaA plasmids were trans cloned into the E. coli expression strain BL21(DE3)pLysS. Recombinant proteins were purified from the soluble fraction of recombinant E. coli lysates using metal affinity resin and buffers (Novagen, Inc.) as specified by the manufacturers. Protein concentrations were estimated using the Bradford kit from Bio-Rad (Hercules, Calif.). Proteins were stored at 4°C.
Hyperimmune mouse sera and PsaA-specific MAb.
Hyperimmune mouse sera specific for PsaA (anti-PsaA) or type 3 capsular PS (anti-PS) were generated by repeated inoculation of mice with rPsaA-IL-4 or type 3 capsular PS, respectively. At 2 weeks after the final immunization, blood was collected from the mice by cardiac puncture. Sera prepared from the blood were pooled and stored at −20°C until used for assays. The PsaA-specific monoclonal antibody (MAb) 1E7A3D7C2 (1E7) was a gift from Jacquelyn Sampson, Centers for Disease Control and Prevention, Atlanta, Ga. This MAb has been previously described and shown to bind PsaA from all 90 serotypes of S. pneumoniae tested (11).
Polyacrylamide gel electrophoresis and Western blot analysis.
Recombinant proteins or whole-cell lysates of S. pneumoniae or S. enterica serovar Typhimurium strain χ4064 were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue or electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad) for Western blot analysis. Blots were reacted with MAb 1E7, PsaA-specific hyperimmune antiserum (anti-PsaA), goat anti-mouse IL-2 (Sigma), or goat anti-mouse IL-4 (Sigma). Membranes were subsequently incubated in alkaline phosphatase (AP)-conjugated goat anti-mouse immunoglobulin G (IgG) to detect binding by PsaA-specific antibodies or in AP-conjugated donkey anti-goat IgG to detect specific binding by anti-IL-2 or anti-IL-4. The blots were developed by incubation in 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (BCIP-NBT) chromogenic phosphatase substrate (Sigma).
Competitive Western blot analysis for detection of native PsaA.
For competitive Western blot analysis, whole-cell lysates of S. pneumoniae, S. enterica serovar Typhimurium χ4064, purified rPsaA, and a prestained molecular weight marker (Life Technologies) were subjected to SDS-PAGE. The proteins were transferred to PVDF membranes and blocked. The blots were reacted with anti-PsaA polyclonal serum. To determine the specificity of binding for PsaA, PsaA hyperimmune serum was depleted of PsaA-specific antibodies by preincubation with 50 μg of rPsaA. An equivalent sample of PsaA hyperimmune serum was also preincubated with 50 μg of the E. coli protein thioredoxin (Trx) purified from the soluble fraction of recombinant E. coli harboring pET32a+ (Novagen, Inc.) by affinity chromatography as described above. Blots were reacted with the pretreated PsaA antisera and developed as described above.
Biological assays for recombinant cytokines.
A colorimetric CTLL-2 bioassay was used to demonstrate the ability of recombinant PsaA-IL-2 or PsaA-IL-4, in comparison to either rIL-2 or rIL-4, to stimulate proliferation of the CTLL-2 cell line as previously described (2, 4).
ELISA for antibodies against PsaA and type 3 PS.
Microtiter plates (Immulon I; Dynatech, Chantilly, Va.) were coated with recombinant PsaA protein (10μg/ml, 100 μl/well) overnight at 4°C. Serial dilutions of sera were added to the wells in duplicate. AP-conjugated antibodies specific for murine immunoglobulins were used as the secondary reagent. The plates were developed by adding p-nitrophenyl phosphate (Sigma) and read at 405 nm using a spectrophotometer (Molecular Devices, Inc.). Antibodies were quantified by calculating the reciprocal of the lowest serum dilution that gave detectable color above background (sera from MSA-inoculated mice diluted 1:100). Isotype-specific antibody titers were determined by the same means and used to calculate IgG1-to-IgG2a ratios. To detect type 3 PS-specific antibodies, Polysorp plates (Nunc, Roskilde, Denmark) were coated with type 3 PS (10μg/ml, 100 μl/well) overnight at 4°C. Enzyme-linked immunosorbent assays (ELISAs) were then performed as previously described (23). AP-conjugated antibodies specific for mouse immunoglobulin κ light chains, mouse IgG, mouse IgG1, IgG2a, IgM, and IgA were all purchased from Southern Biotechnologies, Birmingham, Ala.
Indirect immunofluorescence and flow cytometry.
Indirect immunofluorescence was carried out on intact S. pneumoniae bacteria to demonstrate the binding of antibodies to bacterial surface components (proteins and polysaccharides). Bacteria from frozen stock (cryopreserved in 20% glycerol in Todd-Hewitt broth supplemented with 0.5% yeast extract, at −70°C) were streaked onto blood agar plates and incubated at 37°C overnight. Bacteria were washed in sterile PBS and resuspended in staining buffer (PBS with 0.05% sodium azide and 1% bovine serum albumin). Then 2 × 107 bacteria were incubated with 10% serum from mice inoculated with MSA as negative controls or with anti-PS or anti-PsaA. After incubation at 4°C, the bacteria were washed in staining buffer and incubated with a 1:50 dilution in staining buffer of an F(ab′)2 fragment of goat anti-mouse IgG conjugated to the fluorescent dye Alexa-488 (Molecular Probes, Inc., Eugene, Oreg.). The bacteria were then washed in PBS and subjected to flow cytometry using a Beckton-Dickinson bench top flow cytometer. Data were collected and analyzed using Cell-Quest software (Beckton-Dickinson).
Protection studies.
Virulent type 3 S. pneumoniae organisms were prepared for challenge of immunized mice by streaking the bacteria onto blood agar plates and incubating the plates overnight at 37°C. The next day, the culture was used to inoculate 20 ml of Todd-Hewitt broth supplemented with 0.5% yeast extract, and this culture was incubated at 37°C for 4 to 5 h with gentle agitation. Logarithmic-phase bacteria were adjusted to a density of 500 to 5,000 CFU per ml in sterile PBS. BALB/c mice to be used in challenge experiments were inoculated i.p. on days 0 and 11 with 50 or 250 pmol of various PsaA or PsaA-cytokine fusion proteins. For adjuvant-free immunizations, mice were inoculated with either PsaA, PsaA-IL-2, or PsaA-IL-4 in PBS with 1% MSA. For immunizations with adjuvant, mice were primed with 50 or 250 pmol of PsaA in CFA (1:1 ratio) on day 0 and boosted with the same concentration of PsaA in IFA (1:1 ratio) on day 11. BALB/c mice inoculated with 0.5 μg of type 3 PS in sterile PBS on days 0 and 11 served as positive controls. All mice were bled on days 10 and 21 and challenged on day 25. Mice were injected i.p. with 50 to 500 CFU of virulent S. pneumoniae type 3. The actual number of CFU administered was determined retrospectively by plating serial dilutions of S. pneumoniae inocula on blood agar. Survival of mice was monitored for 15 days, at which time the experiments were terminated.
RESULTS
Expression and characterization of PsaA-cytokine fusion proteins.
The genes encoding PsaA, mouse IL-2, and mouse IL-4 were amplified by PCR using the primers shown in Table 1 and a high-fidelity thermostable DNA polymerase. All the recombinant proteins were retained in the soluble fraction of the E. coli expression strains and were purified to near homogeneity by metal affinity chromatography.
Recombinant PsaA fusion proteins were characterized by SDS-PAGE. Each protein migrated in SDS-polyacrylamide gels, visualized by staining with Coomassie blue, according to their predicted molecular masses (Fig. 1 and 2A).
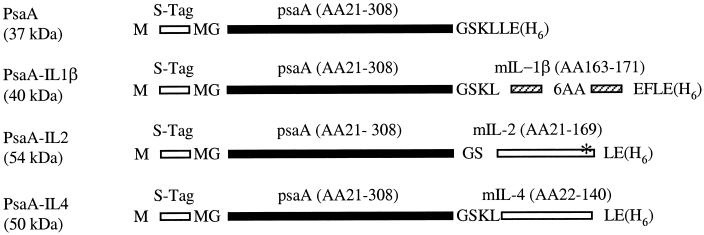
FIG. 1.
Schematic representation of recombinant PsaA proteins cloned and expressed in E. coli. Amino acids (AA) not encoded by PsaA or murine (m) cytokine genes are shown in single-letter code. Each construct is flanked by vector-encoded S-Tag and His Tag (H6) sequences. Predicted molecular masses in kilodaltons are indicated in parentheses. The asterisk in PsaA-IL-2 represents an introduced point mutation changing the C residue at position 159 to S. Diagrams are not drawn to scale. Numbering of AA for PsaA and cytokines is based on their respective pre-pro sequences in GenBank.
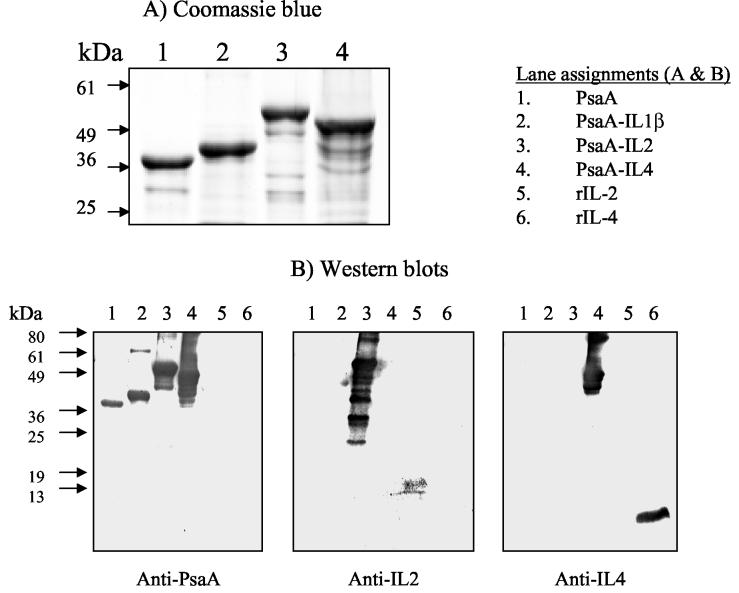
FIG. 2.
Recombinant PsaA proteins cloned, expressed, and purified in E. coli were subjected to SDS-PAGE. Proteins were detected by direct staining with Coomassie blue (A) or transferred to PVDF membranes and reacted with the indicated antibody preparations (B). Specific binding was visualized by using the Sigma fast BCIP-NBT chromogenic substrate. rIL-2 and rIL-4 were loaded as controls. Apparent molecular mass markers in kilodaltons are indicated.
The recombinant proteins were characterized by Western blotting using polyclonal anti-PsaA or commercial preparations of goat anti-mouse IL-2 or goat anti-mouse IL-4. (Fig. 2B). Anti-PsaA reacted with recombinant PsaA, PsaA-IL-1β, PsaA-IL-2, and PsaA-IL-4 (Fig. 2B, lanes 1 to 4, respectively), goat anti-mouse IL-2 reacted specifically with PsaA-IL-2 and mouse rIL-2 (lanes 3 and 5, respectively), and goat anti-mouse IL-4 reacted specifically with PsaA-IL-4 and mouse rIL-4 (Fig. 2B, lanes 4 and 6, respectively). The identity of PsaA was confirmed by Western blotting using a previously described PsaA-specific MAb, 1E7 (11) (data not shown).
We used a nonradioactive proliferation assay to assess the retention of biological activities characteristic of IL-2 and IL-4 by PsaA-IL-2 and PsaA-IL-4, respectively. Both PsaA-IL-2 and PsaA-IL-4 were able to stimulate the proliferation of CTLL-2 cells in culture in a fashion similar to commercial preparations of recombinant E. coli-derived mIL-2 and mIL-4, respectively (data not shown).
Immunogenicity of PsaA-cytokine fusion proteins.
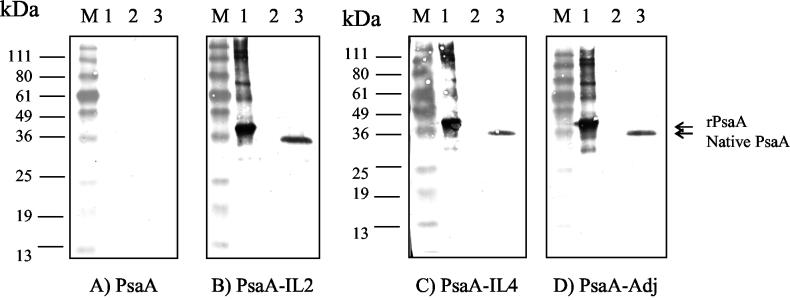
Mice of different strains were inoculated with PsaA, PsaA-IL-1β, PsaA-IL-2, or PsaA-IL-4, respectively. Levels in serum of PsaA-specific antibodies generated in response to these inoculations were used as a measure of immunogenicity. Mice inoculated twice with PsaA elicited low to undetectable responses (Table 2). These responses were dramatically enhanced by inoculation of mice with PsaA emulsified in CFA and boosted with PsaA in IFA (Table 2). PsaA-IL-2 and PsaA-IL-4 fusion proteins elicited amounts of PsaA-reactive antibodies substantially greater than those elicited in response to PsaA without adjuvant and comparable to those elicited by mice inoculated with PsaA-Adj in all three mouse strains tested (Table 2). Although CBA/J mice inoculated with PsaA-IL-1β elicited detectable PsaA-specific antibody titers (Table 2), these titers were not as high as those elicited by PsaA-IL-2, PsaA-IL-4, or PsaA-Adj. Since PsaA-IL-1β proved to be inconsistently immunogenic, it was eliminated from evaluation in BALB/c mice. We performed immunizations in lipopolysaccharide nonresponder C3H/HeJ mice to address the possibility that the enhanced immunogenicity of the PsaA-cytokine fusions was due to contamination by endotoxin, since all the recombinant proteins were expressed and purified from E. coli lysates. The antibody titers in C3H/HeJ mice inoculated twice with PsaA-IL-2, or PsaA-IL-4 were substantially elevated compared to the titers in C3H/HeJ mice inoculated twice with PsaA or PsaA-IL-1β. These results indicate that the enhanced immunogenicity of PsaA-IL-2 and PsaA-IL-4 is not attributable to any endotoxin contaminating the recombinant protein preparations. We examined the isotype distribution characteristics of the PsaA-specific antibodies in mice. CBA/J mice inoculated twice with rPsaA in CFA/IFA, PsaA-IL-2, or PsaA-IL-4 produced PsaA-specific antibodies almost exclusively of the IgG class (Table 2). The reciprocal titers of IgM and IgA were below the limit of detection (<100). We used Western blotting to confirm that the elevated ELISA antibody titers for CBA/J mice inoculated twice with PsaA-IL-2, PsaA-IL-4, or PsaA-Adj (Table 2) were indeed able to recognize both the recombinant and native PsaA proteins (Fig. 3B to D, respectively) but failed to react with proteins in S. enterica serovar Typhimurium lysates (data not shown). We further demonstrated that preincubation of PsaA-specific antisera with rPsaA prior to performing Western blot analyses (competitive Western blots) abolished the detection of bands corresponding to native PsaA in lysates of S. pneumoniae (data not shown). We also performed titrations to determine the relative amounts of IgG1 and IgG2a subclasses in the pooled sera of CBA/J, C3H/HeJ, and BALB/c mice collected after two inoculations with the various PsaA antigens (Table 2). Pooled sera from mice inoculated with PsaA-Adj had a preponderance of PsaA-specific IgG1 (Table 2), as did sera from mice inoculated with PsaA or PsaA-IL-1β (Table 2). In contrast, sera from mice inoculated with PsaA-IL-2, or PsaA-IL-4 had substantial amounts of PsaA-specific IgG2a in addition to PsaA-specific IgG1 (Table 2).
TABLE 2.
Serum antibody responses to PsaA in different mouse strains
| Strain | Antigen | Amt (pmol) | Antibody titerc | IgG1/IgG2a ratio |
|---|---|---|---|---|
| CBA/Ja | PsaA | 50 | NAd | |
| PsaA-IL-1β | 50 | 1,697 (1,026) | 11.8 | |
| PsaA-IL-2 | 50 | 12,548 (3,331) | 1.4 | |
| PsaA-IL-4 | 50 | 17,152 (2,324) | 2.1 | |
| PsaA-Adj | 50 | 19,989 (4,737) | 12.5 | |
| C3H/HeJa | PsaA | 50 | 1,021 (759) | 9.5 |
| PsaA-IL-1β | 50 | 1,294 (1,023) | 7.8 | |
| PsaA-IL-2 | 50 | 14,316 (4,604) | 1.0 | |
| PsaA-IL-4 | 50 | 32,763 (13,763) | 1.0 | |
| BALB/cb | PsaA | 250 | <100 | NA |
| PsaA-IL-2 | 250 | 10,545 (2,422) | 1.3 | |
| PsaA-IL-4 | 250 | 6,745 (2,933) | 1.5 | |
| PsaA-Adj | 250 | 123,578 (47,477) | 9.2 |
Groups of six CBA/J mice or C3H/HeJ mice were inoculated i.p. with the indicated antigens on days 0 and 21 and bled on day 35.
Groups of 9 or 10 BALB/c mice were inoculated i.p. with the indicated antigens on days 0 and 11 and bled on day 21.
Total anti-PsaA immunoglobulin titers were measured by ELISA for individual sera. Standard errors of the mean are indicated in parentheses.
NA, not applicable.
FIG. 3.
Native PsaA was delected by Western blot analysis. SDS-polacrylamide gels were loaded with equivalent amounts of prestained molecular mass markers (lanes M), purified rPsaA (lanes 1), purified thioredoxin (lanes 2), or S. pneumoniae whole-cell lysate (lanes 3). Each gel was transferred to a PVDF membrane and reacted with pooled serum obtained from mice inoculated twice with 50 pmol of the indicated antigens. Specific reactivity for each blot was visualized by incubation in the BCIP-NBT chromogenic substrate for the same time for direct comparison between the blots. Apparent molecular mass markers in kilodaltons are indicated. The blots are representative of three similar experiments performed.
Protection of BALB/c mice from lethal infection with S. pneumoniae.
Challenge experiments (summarized in Table 3) were carried out to determine the protective efficacy of PsaA immunization to a lethal challenge with virulent S. pneumoniae type 3. In these experiments, mice inoculated twice with type 3 PS served as positive experimental controls. These mice were 100% protected against challenge with 50 to 500 CFU of S. pneumoniae. Control mice (inoculated with MSA) or mice inoculated with PsaA did not survive challenge with 300 to 500 CFU. This result is not surprising, given that these two immunization protocols did not result in the production of detectable PsaA-specific antibodies. Groups of mice inoculated with PsaA-Adj or PsaA-IL-4 exhibited survival rates of 30% at challenge doses of 300 or 500 CFU. Mice inoculated with PsaA-IL-2 did not survive challenge with 300 or 500 CFU. This result was of interest since these mice had relative concentrations of PsaA-specific antibodies in serum comparable to those in the serum of mice inoculated with PsaA-IL-4 (Table 3).
TABLE 3.
Protection of BALB/c mice following challenge with S. pneumoniae type 3
| Dosea (CFU) | Antigen | Amtb | No. alive/total no.c |
|---|---|---|---|
| 300-500 | Ctrl | NAd | 0/9 |
| PsaA | 50 pmol | 0/10 | |
| PsaA-IL-2 | 50 pmol | 0/10 | |
| PsaA-IL-4 | 50 pmol | 3/10 | |
| PsaA-Adj | 50 pmol | 3/10 | |
| T3-PS | 0.5 μg | 9/9 | |
| 50 | Ctrl | NA | 2/5 |
| PsaA | 250 pmol | 1/9 | |
| PsaA-IL-2 | 250 pmol | 1/10 | |
| PsaA-IL-4 | 250 pmol | 3/10 | |
| PsaA-Adj | 250 pmol | 3/5 | |
| T3-PS | 0.5 μg | 5/5 |
Mice were challenged with live S. pneumoniae type 3 strain A66.1 injected i.p. 2 weeks after the second immunization.
Mice were immunized with the indicated antigen on days 0 and 11. T3-PS is type 3 capsular polysaccharide Control mice (Ctrl) mice were immunized twice with MSA.
Survival of mice was monitored for 15 days following challenge with S. pneumoniae.
NA, not applicable.
In a second series of experiments, we increased the immunizing dose of antigen fivefold to 250 pmol per mouse for all antigens. The mice were inoculated with antigens twice and subsequently challenged with 50 CFU of S. pneumoniae. The results of this experiment were more difficult to interpret because 40% of the control mice survived this challenge dose. This survival rate was higher than that seen for mice that had received PsaA (11% survival), PsaA-IL-2 (10% survival), or PsaA-IL-4 (30% survival). Only the survival rate of mice inoculated with PsaA-Adj was higher than that of the control mice (60 and 40%, respectively). Survival rates of mice did not correlate with levels of PsaA-specific antiserum in individual mice (data not shown).
Indirect immunofluorescence to determine surface accessibility of PsaA.
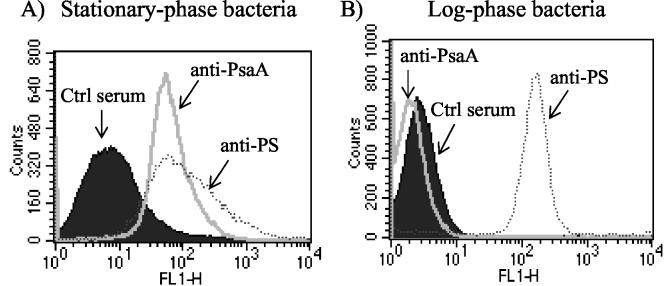
Because of the modest protective responses observed to lethal S. pneumoniae challenge in mice with elevated levels of PsaA-specific antibodies, we performed indirect-immunofluorescence experiments to determine the accessibility of antibody to surface antigens (PsaA and PS) on intact S. pneumoniae bacteria. For this purpose, bacteria from two distinct physiological states were analyzed. Bacteria obtained from log-phase cultures are typically highly encapsulated, whereas bacteria obtained from stationary-phase cultures are much less highly encapsulated. Hyperimmune serum from mice inoculated with type 3 PS was able bind to bacteria from both log- and stationary-phase cultures (Fig. 4). Serum from MSA-inoculated mice did not bind to bacteria from either culture (Fig. 4). PsaA-specific antiserum (anti-PsaA) was incapable of binding to bacteria from log-phase cultures (Fig. 4B) but was capable of significant binding to bacteria from stationary-phase cultures (Fig. 4A).
FIG. 4.
Flow cytometry was used to demonstrate antigens on the surface of intact S. pneumoniae bacteria. Bacteria grown to log or stationary phase were incubated in 10% serum from naive mice (Ctrl), PsaA-immune mice (anti-PsaA), or type 3 PS-immune mice (anti-PS). Specific binding was visualized using an F(ab′)2 fragment of goat anti-mouse IgG (H+L) conjugated to Alexa-488 fluorochrome. Bacterium-sized particles were detected using logarithmic side scatter as the threshold, and specific binding was recorded as green fluorescence (FL1). A total of 105 events were used to generate each histogram. The histograms are representative of five or three similar experiments performed with log- or stationary-phase bacteria, respectively.
DISCUSSION
This study compared the immunogenicity of PsaA-cytokine fusion proteins to that of PsaA administered in Freund's adjuvants. We demonstrate that inoculation of mice with PsaA linked to IL-2 or IL-4 elicited levels of PsaA-specific antibodies comparable in magnitude to those in mice inoculated with PsaA in adjuvant. We also reproducibly demonstrate that PsaA-IL-2 and Psa-IL-4 induce substantial amounts of IgG2a. While this result is to be expected for IL-2 fusions, it would not be expected on the basis of the simplest, dichotomous notions of CD4+ T-cell differentiation for IL-4 fusions (1, 29). However, a number of studies convincingly demonstrate that the presence of biologically active IL-4 at the time of primary antigenic stimulation results in the production of substantial amounts of gamma interferon (6, 19, 24). Thus, the ability of IL-4 to stimulate gamma interferon secretion might account for efficient class switching to the IgG2a subclass which we observed in mice inoculated with PsaA-IL-4.
Evidence provided to date has demonstrated that protection against S. pneumoniae bacteremia is mediated by phagocytosis (3). S. pneumoniae is not normally phagocytosed efficiently in the mammalian host, but the efficiency of phagocytosis can be dramatically enhanced by opsonization (via binding of PS-specific antibodies to the pneumococcal surface followed by the deposition of complement). For antibodies to a pneumococcal protein to mediate protection via direct opsonophagocytosis, the target protein must be accessible to circulating antibody.
Immunization of mice with native PsaA emulsified in CFA has previously been reported to be highly protective against systemic challenge with S. pneumoniae (30). We therefore performed challenge experiments to evaluate the protective efficacy of immunization with PsaA. Immunization of BALB/c mice with type 3 PS resulted in solid protection of all mice against a 50- to 500-CFU challenge with viable S. pneumoniae. In contrast, mice immunized with PsaA or PsaA-IL-2 resulted in no protection. Mice immunized with PsaA-Adj or with PsaA-IL-4 were partially protected from challenge with virulent S. pneumoniae. This partial protection did not appear to correlate with levels of antibody, in that mice immunized with PsaA-IL-2 or PsaA-IL-4 that had the largest amounts of PsaA-specific antibody were not protected. These results can be partially reconciled with what is known about the physical structure of S. pneumoniae. These bacteria are heavily encapsulated, with PS situated external to the bacteria cell wall, which in turn is situated external to the bacterial plasma membrane. PsaA is believed to be anchored to the outer surface of the bacterial plasma membrane, underneath both the cell wall and the capsule (20). Thus, while PS is highly accessible to circulating antibody, PsaA may not be readily accessible. Consistent with this notion, we demonstrated the binding of PsaA-specific antisera to bacteria obtained from stationary-phase cultures but not from log-phase cultures. These results and observations do not explain the mechanism for the modest but consistent levels of protection observed in mice immunized with PsaA in this study and by others (26) or the more robust protection against systemic pneumococcal infection afforded by parenteral immunization with PsaA in other studies (12, 30). It is possible that PsaA is more accessible to antibodies in the strains used for challenge in those studies or that our challenge strain exhibited greater virulence. Alternatively, our inability to detect PsaA on the surface of S. pneumoniae derived from log-phase cultures in this study may be due to the low level of expression of PsaA in this physiological state.
PsaA remains an important vaccine target because immunization of mice with PsaA appears to be highly effective at providing protection against nasopharyngeal carriage (8). Indeed, the combination of PsaA linked to IL-2 or to IL-4, either alone or in conjunction with a suitable mucosal adjuvant such as cholera toxin, might form the basis of a highly effective vaccine against pneumococcal carriage.
Acknowledgments
This study was supported by NIH grants R01-AI41657 to N.S.G. and R01-AI32596 and R01-AI46667 to J.R.S. D.O.G. is supported by an NIH training grant in immunology (T32-AI07427).
We thank John McLay for critical advice in establishing the mouse pneumococcal sepsis challenge model used in this study, and we thank Jacquelyn Sampson for the gift of the PsaA-specific MAb 1E7A3D7C2.
Editor: J. D. Clements
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, S. A., R. M. Gogal, Jr., and J. E. Walsh. 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170:211-224. [DOI] [PubMed] [Google Scholar]
- 3.Alonso De Velasco, E., B. A. Dekker, A. F. Verheul, R. G. Feldman, J. Verhoef, and H. Snippe. 1995. Anti-polysaccharide immunoglobulin isotype levels and opsonic activity of antisera: relationships with protection against Streptococcus pneumoniae infection in mice. J. Infect. Dis. 172:562-565. [DOI] [PubMed] [Google Scholar]
- 4.Askew, D., R. S. Chu, A. M. Krieg, and C. V. Harding. 2000. CpG DNA induces maturation of dendritic cells with distinct effects on nascent and recycling MHC-II antigen-processing mechanisms. J. Immunol. 165:6889-6895. [DOI] [PubMed] [Google Scholar]
- 5.Beckers, W., L. Villa, S. Gonfloni, L. Castagnoli, S. M. Newton, G. Cesareni, and P. Ghiara. 1993. Increasing the immunogenicity of protein antigens through the genetic insertion of VQGEESNDK sequence of human IL-1 beta into their sequence. J. Immunol. 151:1757-1764. [PubMed] [Google Scholar]
- 6.Bemer, V., I. Motta, R. Perret, and P. Truffa-Bachi. 1996. Opposite effects of interleukin-4 on memory T helper cell development depend on interleukin-2. Res. Immunol. 147:139-147. [DOI] [PubMed] [Google Scholar]
- 7.Bennett, B., I. J. Check, M. R. Olsen, and R. L. Hunter. 1992. A comparison of commercially available adjuvants for use in research. J. Immunol. Methods 153:31-40. [DOI] [PubMed] [Google Scholar]
- 8.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, I., M. Pizza, R. Rappuoli, and S. M. Newton. 1998. Effects of the insertion of a nonapeptide from murine IL-1beta on the immunogenicity of carrier proteins delivered by live attenuated Salmonella. Arch. Microbiol. 169:113-119. [DOI] [PubMed] [Google Scholar]
- 10.Chen, T. T., M. H. Tao, and R. Levy. 1994. Idiotype-cytokine fusion proteins as cancer vaccines. Relative efficacy of IL-2, IL-4, and granulocyte-macrophage colony-stimulating factor. J. Immunol. 153:4775-4787. [PubMed] [Google Scholar]
- 11.Crook, J., J. A. Tharpe, S. E. Johnson, D. B. Williams, A. R. Stinson, R. R. Facklam, E. W. Ades, G. M. Carlone, and J. S. Sampson. 1998. Immunoreactivity of five monoclonal antibodies against the 37-kilodalton common cell wall protein (PsaA) of Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 5:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De, B. K., J. S. Sampson, E. W. Ades, R. C. Huebner, D. L. Jue, S. E. Johnson, M. Espina, A. R. Stinson, D. E. Briles, and G. M. Carlone. 2000. Purification and characterization of Streptococcus pneumoniae palmitoylated pneumococcal surface adhesion A expressed in Escherichia coli. Vaccine 18:1811-1821. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey, P. W., M. E. Allison, S. Akkaraju, C. C. Goodnow, and D. T. Fearon. 1996. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271:348-350. [DOI] [PubMed] [Google Scholar]
- 14.Douglas, R. M., J. C. Paton, S. J. Duncan, and D. J. Hansman. 1983. Antibody response to pneumococcal vaccination in children younger than five years of age. J. Infect. Dis. 148:131-137. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein, F. W., and J. Garau. 1997. 30 years of penicillin-resistant S. pneumoniae: myth or reality? Lancet 350:233-234. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein, F. W., and J. Garau. 1994. Resistant pneumococci: a renewed threat in respiratory infections. Scand. J. Infect. Dis. Suppl. 93:55-62. [PubMed] [Google Scholar]
- 17.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 18.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, L. R., F. L. Chen, Y. T. Chen, Y. M. Lin, and J. T. Kung. 2000. Potent induction of long-term CD8+ T cell memory by short-term IL-4 exposure during T cell receptor stimulation. Proc. Natl. Acad. Sci. USA 97:3406-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence, M. C., P. A. Pilling, V. C. Epa, A. M. Berry, A. D. Ogunniyi, and J. C. Paton. 1998. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 6:1553-1561. [DOI] [PubMed] [Google Scholar]
- 21.Lock, R. A., D. Hansman, and J. C. Paton. 1992. Comparative efficacy of autolysin and pneumolysin as immunogens protecting mice against infection by Streptococcus pneumoniae. Microb. Pathog. 12:137-143. [DOI] [PubMed] [Google Scholar]
- 22.Maecker, H. T., D. T. Umetsu, R. H. DeKruyff, and S. Levy. 1997. DNA vaccination with cytokine fusion constructs biases the immune response to ovalbumin. Vaccine 15:1687-1696. [DOI] [PubMed] [Google Scholar]
- 23.McCool, T. L., C. V. Harding, N. S. Greenspan, and J. R. Schreiber. 1999. B- and T-cell immune responses to pneumococcal conjugate vaccines: divergence between carrier- and polysaccharide-specific immunogenicity. Infect. Immun. 67:4862-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris, S. C., W. C. Gause, and F. D. Finkelman. 2000. IL-4 suppression of in vivo T cell activation and antibody production. J. Immunol. 164:1734-1740. [DOI] [PubMed] [Google Scholar]
- 25.Morrison, K. E., D. Lake, J. Crook, G. M. Carlone, E. Ades, R. Facklam, and J. S. Sampson. 2000. Confirmation of psaA in all 90 serotypes of Streptococcus pneumoniae by PCR and potential of this assay for identification and diagnosis. J. Clin. Microbiol. 38:434-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson, J. S., Z. Furlow, A. M. Whitney, D. Williams, R. Facklam, and G. M. Carlone. 1997. Limited diversity of Streptococcus pneumoniae psaA among pneumococcal vaccine serotypes. Infect. Immun. 65:1967-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinefield, H. R., and S. Black. 2000. Efficacy of pneumococcal conjugate vaccines in large scale field trials. Pediatr. Infect. Dis. J. 19:394-397. [DOI] [PubMed] [Google Scholar]
- 29.Snapper, C. M., and W. E. Paul. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236:944-947. [DOI] [PubMed] [Google Scholar]
- 30.Talkington, D. F., B. G. Brown, J. A. Tharpe, A. Koenig, and H. Russell. 1996. Protection of mice against fatal pneumococcal challenge by immunization with pneumococcal surface adhesion A (PsaA). Microb. Pathog. 21:17-22. [DOI] [PubMed] [Google Scholar]
- 31.Wizemann, T. M., J. H. Heinrichs, J. E. Adamou, A. L. Erwin, C. Kunsch, G. H. Choi, S. C. Barash, C. A. Rosen, H. R. Masure, E. Tuomanen, A. Gayle, Y. A. Brewah, W. Walsh, P. Barren, R. Lathigra, M. Hanson, S. Langermann, S. Johnson, and S. Koenig. 2001. Use of a whole-genome approach to identify vaccine molecules affording protection against Streptococcus pneumoniae infection. Infect. Immun. 69:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wortham, C., L. Grinberg, D. C. Kaslow, D. E. Briles, L. S. McDaniel, A. Lees, M. Flora, C. M. Snapper, and J. J. Mond. 1998. Enhanced protective antibody responses to PspA after intranasal or subcutaneous injections of PspA genetically fused to granulocyte-macrophage colony-stimulating factor or interleukin-2. Infect. Immun. 66:1513-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yip, H. C., A. Y. Karulin, M. Tary-Lehmann, M. D. Hesse, H. Radeke, P. S. Heeger, R. P. Trezza, F. P. Heinzel, T. Forsthuber, and P. V. Lehmann. 1999. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J. Immunol. 162:3942-3949. [PubMed] [Google Scholar]