Abstract
Membrane-damaging toxins are thought to be responsible for the explosive clinical course of Bacillus endophthalmitis. This study analyzed the contribution of phosphatidylinositol-specific phospholipase C (PI-PLC) and phosphatidylcholine-specific phospholipase C (PC-PLC) to the pathogenesis of experimental Bacillus endophthalmitis. Isogenic mutants were constructed by insertion of lacZ into Bacillus thuringiensis genes encoding PI-PLC (plcA) and PC-PLC (plcB). Rabbit eyes were injected intravitreally with 2 log10 CFU of strain BT407 (wild type), the PI-PLC mutant (BTplcA::lacZ), or the PC-PLC mutant (BTplcB::lacZ). The rates of decrease in retinal responses of eyes infected with the isogenic mutants were similar to that of wild type, with all infections resulting in elimination of retinal function by 18 h. Strain BT407 caused a significant increase in the latency of retinal responses at 6 h, but strains BTplcA::lacZ and BTplcB::lacZ did not. All strains elicited significant inflammatory cell influx into the anterior chamber by 12 h. Histologically, eyes infected with each strain were indistinguishable throughout the infection course. In this model, neither PI-PLC nor PC-PLC had an effect on the course or severity of experimental Bacillus endophthalmitis. Alterations in retinal responses early in infection may mark the beginnings of specific photoreceptor or glial cell dysfunction.
The most severe form of intraocular bacterial infection (endophthalmitis) is caused by Bacillus spp. Bacillus cereus, in particular, causes a uniquely explosive intraocular infection that can result in significant vision loss, and frequently loss of the eye itself, within 1 to 2 days (10). Bacillus endophthalmitis typically results from penetrating trauma of the globe with a contaminated foreign body but can also result from metastatic spread of organisms to the eye following bacteremia in the immunocompromised. Despite aggressive antibiotic, anti-inflammatory, and surgical intervention, the disease course is frequently rapid and destructive, highlighting the limitations of present therapeutic strategies.
The unique virulence of B. cereus endophthalmitis is typically ascribed to toxins produced by the organism in the eye during infection. Membrane-damaging toxins such as Staphylococcus aureus alpha-toxin (9, 10), Enterococcus faecalis cytolysin (22), and Streptococcus pneumoniae pneumolysin (34) have been identified as virulence factors in ocular infection. B. cereus secretes a number of factors that may contribute to disease pathogenicity, namely, hemolysins, lipases, enterotoxins, and proteases (14). Our analysis of the contribution of one toxin, hemolysin BL, to the course and severity of experimental endophthalmitis demonstrated that this enterotoxin contributed little to the intraocular virulence of B. cereus (11). Both the wild type and an isogenic hemolysin BL-deficient mutant resulted in the destruction of retinal architecture, complete loss of retinal function, and significant intraocular inflammation within 12 h (11).
A number of other B. cereus toxins are potential intraocular virulence factor candidates. Phosphatidylcholine-phospholipase C (PC-PLC) and sphingomyelinase (SPH), which comprise the cytolytic unit cereolysin AB, could be potential virulence factors because of the combined activities of these components against mammalian cells (15). Like cereolysin AB, the alpha-toxin of Clostridium perfringens possesses PC-PLC and SPH activities and is a key virulence factor in both gas gangrene and food-borne illness (2, 39). PC-PLC is necessary for cell-to-cell spread of invasive Listeria monocytogenes infection (41). The SPH activity of staphylococcal beta-toxin contributes only minimally to the virulence of S. aureus endophthalmitis (10) and keratitis (35) but is a known inducer of apoptosis in mammalian cells (17, 45). The intraocular toxicity of B. cereus PC-PLC and SPH has been analyzed. Purified PC-PLC was toxic to retinal buttons in vitro and to the retina in vivo, whereas purified SPH was minimally toxic in vitro (4). A third toxin, phosphotidylinositol-phospholipase C (PI-PLC), has been suggested to be a virulence factor for L. monocytogenes (38, 43) and S. aureus (12, 29) but has been shown to be minimally toxic to retinal buttons in vitro (4).
This study addressed the contribution of PC-PLC and PI-PLC to the pathogenesis of experimental endophthalmitis by using B. thuringiensis as the test organism. B. thuringiensis is a spore-forming soil entomopathogenic organism that is used widely as an organic pesticide and whose genomic and phenotypic profile is very closely related to, and in some cases overlaps, that of B. cereus (18). Because systems for genetic manipulation of B. thuringiensis have been developed (27), this organism was used in the present studies. B. thuringiensis wild type and isogenic PC-PLC or PI-PLC mutants were analyzed in a highly sensitive in vivo model to define their contributions to the evolution of endophthalmitis.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The wild-type B. thuringiensis strain used in these studies (BT407) has been cured of the plasmid encoding the insecticidal crystalline toxin (Cry−) (27) but produces several of the same toxins as B. cereus. Construction of isogenic mutants of strain BT407 specifically deficient in PI-PLC or PC-PLC is described below. The use of E. coli strains SCS110 and TG1 for plasmid construction and cloning, respectively, has been described previously (16). Bacterial strain and plasmid profiles are summarized in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| Strains | ||
| BT407 | Wild-type B. thuringiensis, Cry− | 27 |
| BTplcA::lacZ | BT407 with lacZ insertion into plcA | This work |
| BTplcB::lacZ | BT407 with lacZ insertion into plcB | This work |
| E. coli TG1 | [Δ(lac-proAB) supE thi hdsD5 (F′ traD36 proA+proB+lacIqZΔM15)] | 16 |
| E. coli SCS110 | [rpsL thr leu endA thi-1 lacY galK galT ara tonA tsx dam dcm supE44 Δ(lac-proAB) (F′ traD36 proAB lacIqZΔM15)] | 16 |
| Plasmids | ||
| pHT304-18Z | Source of promotorless lacZ for insertional mutagenesis of BT407 | 1 |
| pRN1501 | Temperature-sensitive vector containing the ori(Ts) and erm of pE194ts and the oriEc, amp, and multicloning region of pBR322 | 28, 44 |
Culture media and reagents.
For mutant strain construction, Escherichia coli and B. thuringiensis were cultured in Luria-Bertani medium with or without antibiotic selection as appropriate. Antibiotics used for transformant selection included erythromycin (25 μg/ml) and ampicillin (100 μg/ml). For phenotypic analysis of bacterial culture supernatants and inoculum preparation, B. thuringiensis was cultured in brain heart infusion (BHI [Difco, Detroit, Mich.]). All reagents used in phenotypic assays were purchased from Sigma (St. Louis, Mo.) unless otherwise specified.
DNA techniques.
Extraction of plasmid DNA from E. coli and chromosomal DNA from B. thuringiensis and DNA fragment digestions, purifications, and ligations were performed essentially as described previously (30). Restriction enzymes and T4 DNA ligase were used as recommended by the manufacturers. Primers used for PCR amplification of plcA and plcB were synthesized by Genset (Paris, France) and Integrated DNA Technologies (Coralville, Iowa). Primers used for PCR amplification of lacZ were synthesized by Integrated DNA Technologies.
Generation of isogenic B. thuringiensis mutants.
The PI-PLC isogenic mutant (designated BTplcA::lacZ) was created by disruption of the PI-PLC plcA gene of strain BT407 with a promoterless lacZ gene into the 5′ part of the coding sequence. A DNA region just upstream from the plcA coding sequence and an internal region of plcA were amplified by PCR using primers PlcA1 and PlcA2 and primers PlcA3 and PlcA4, respectively (Table 2). Primer sequences were selected from the published nucleotide sequence of B. cereus plcA (accession no. M30809 [24]). Amplified DNA fragments were digested with appropriate restriction enzymes and inserted separately into pUC18. The promoterless lacZ gene was purified as a 3.2-kb XbaI-EcoRI DNA fragment from pHT304-18Z (1). The upstream and internal parts of the plcA gene were purified as HindIII-XbaI and EcoRI-BamHI fragments, respectively, and ligated with the lacZ gene between the HindIII and BamHI sites of the thermosensitive plasmid pRN5101 (28, 44). The ligation mixture was then used to transform E. coli to ampicillin resistance. The plasmid isolated from the transformants was verified by restriction mapping. This recombinant plasmid was introduced into strain BT407 by electroporation as previously described (27). Transformants were resistant to erythromycin and had a blue phenotype (Lac+) on Luria-Bertani plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoxide (X-Gal) (40 μg/ml; Sigma). The chromosomal wild-type copy of plcA was replaced with the disrupted copy by homologous recombination as previously described (25). In the resulting B. thuringiensis recombinant strain, the lacZ gene was transcribed from the plcA promoter and was thus controlled by the transcriptional activator PlcR (26).
TABLE 2.
PCR primers used in this study
| Primer | Sequencea | Restriction site |
|---|---|---|
| PlcA1 | 5′-GTAGATACAACTACAGCTGAAC | |
| PlcA2 | 5′-GCTCTAGACACTTTTTCTGTTTTTACATC | XbaI |
| PlcA3 | 5′-GTATGGGGAATGACGCAAG | |
| PlcA4 | 5′-GCGGATCCTTGAATTCCCAGCCTACTC | BamHI |
| PlcB1 | 5′-CCCAAGCTTGTTTAGATAAGCCTTAATAATAGT | HindIII |
| PlcB2 | 5′-CGGAATTCCATTGTCTGGATCATAGAAATG | EcoRI |
| PlcB3 | 5′-CGGAATTCCATTTGCAAAGCAGGCAAAAG | EcoRI |
| PlcB4 | 5′-CGGGATCCGTACGTATCAAACCAAAGC | BamHI |
| LacZ-F | 5′-GGTTAAATTGCCAACGCTTAATTAAC | |
| LacZ-R | 5′-GTTATCTGGAAGATCAGGATATGTGG |
The restriction site is underlined.
The PC-PLC isogenic mutant (designated BTplcB::lacZ) was created by disruption of the PC-PLC plcB gene of strain BT407 with a promoterless lacZ gene, as described above. Regions corresponding to the 5′ and 3′ ends of plcB were amplified by PCR from the chromosomal DNA of strain BT407, using primers PlcB1 and PlcB2 and primers PlcB3 and PlcB4, respectively (Table 2). The sequences of these primers were selected from published sequences of the B. cereus PC-PLC gene (accession no. X12854 [23]). The promoterless lacZ gene was purified as a 3.2-kb EcoRI-EcoRI DNA fragment from pHT304-18Z (1). The 5′ and 3′ regions of plcB were purified as HindIII-EcoRI and EcoRI-BamHI fragments, respectively, and ligated with the lacZ gene between the HindIII and BamIII sites of pRN5101 (28, 44). The orientation of lacZ was confirmed by restriction mapping, and plasmids carrying lacZ transcribed in the same direction as plcB were selected. These recombinant plasmids were introduced into strain BT407 by electroporation, and the chromosomal wild-type copy of plcB was replaced with the disrupted copies by homologous recombination as previously described (25).
PCR amplification of plcA, plcB, and lacZ.
The presence of plcA, plcB, and lacZ in the genomes of B. thuringiensis wild type and isogenic mutants was confirmed by PCR using primers PlcA1 and PlcA4 (plcA), PlcB1 and PlcB4 (plcB), or LacZ-F and LacZ-R (lacZ), respectively (Table 2). B. thuringiensis chromosomal DNA was isolated by phenol-chloroform extraction. PCR mixtures of 10 μl contained 0.25 to 1.0 μg of genomic DNA, 0.25 mM each deoxynucleoside triphosphate, TaKaRa DNA polymerase buffer (10× supplemented with 25 mM MgCl2 [final concentration, 2.5 mM MgCl2]; PanVera Corp., Madison, Wis.), 0.2 to 1.0 mM each primer, depending on the primer set used, and 0.025 U of TaKaRa Taq polymerase (PanVera). PCR fragments were amplified from B. thuringiensis BT407 genomic DNA under the following conditions: 30 cycles of 94°C for 1 min, 62°C (plcA) or 63°C (plcB) for 1 min, and 72°C for 1 min, followed by one 10-min elongation at 72°C. PCR fragments were amplified from the genomic DNA of strains BTplcA::lacZ and BTplcB::lacZ under the following conditions: 30 cycles of 94°C for 1 min, 57°C (plcA) or 63°C (plcB) for 1 min, and 72°C for 3 min 30 s, followed by one 10-min elongation at 72°C. lacZ fragments were amplified from the genomic DNA of all strains under the following conditions: 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, followed by one 10-min elongation at 72°C. Amplified fragments were resolved on a 0.8% agarose gel.
Phenotypic analysis of Bacillus strains. (i) Hemolytic activity.
Hemolytic activity was determined by quantifying hemoglobin release from sheep and rabbit erythrocytes. Briefly, twofold serial dilutions of filtered logarithmic-phase (8-h) and stationary-phase (18-h) culture supernatants were incubated with an equal volume of 4% (vol/vol) sheep or rabbit erythrocytes (Rockland Inc., Gilbertsville, Pa.) in phosphate-buffered saline. Following a 30-min incubation at 37°C, the optical density at 540 nm (OD540) was measured. The hemolytic titer was determined as the dilution of supernatant exhibiting 50% hemolysis. The hemolytic activity of individual colonies was also analyzed on BHI agar supplemented with 5% sheep or rabbit erythrocytes.
(ii) Proteolytic activity.
Proteolytic activity was determined on hide azure powder (Sigma). Briefly, filtered 10-h culture supenatants were incubated with 10 mg of hide azure powder in assay buffer (10 mM Tris HCl, 10 mM CaCl2 [pH 8.0]) for 2 h and the OD562 was measured (37). Proteolytic activity is expressed in units per milliliter. The proteolytic activity of individual colonies was also analyzed on BHI agar supplemented with 2.5% skim milk (Difco).
(iii) PI-PLC activity.
PI-PLC activity in culture supernatants was measured by a colorimetric microtiter assay adapted from that described by Ikezawa and Taguchi (20). B. cereus PI-PLC standards (Sigma), filtered 10-h culture supernatants, and heat-denatured standards and supernatant controls were serially diluted 1:2 in isotonic phosphate buffer (150 mM NaCl in 5 mM sodium phosphate buffer [pH 7.6]). The 50-μl volumes of PI-PLC standards or culture supernatants were added to 950 μl of bovine erythrocyte ghosts (10% [vol/vol] in isotonic phosphate buffer), and the suspensions were incubated for 30 min at 37°C. The suspensions were centrifuged at 16,000 × g to pellet the cell debris. A 25-μl volume of supernatant was transferred to microtiter wells, to which 200 μl of 100 mM sodium phosphate buffer (pH 7.5), 12.5 μl of 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) (10 mM in sodium phosphate buffer), and 12.5 μl of acetylthiocholine (12.5 mM in sodium phosphate buffer) were added. Following a 4-h vacuum incubation at room temperature, the OD405 was determined. PI-PLC concentrations in culture supernatants were extrapolated from the B . cereus PI-PLC standard curve. Each sample and standard were assayed in triplicate.
(iv) PC-PLC activity.
PC-PLC activities in culture supernatants were measured by an egg yolk agar well diffusion assay adapted from that described by Gilmore et al. (15). B. cereus PC-PLC standards and filtered 8-h culture supernatants were serially diluted 1:2 in dilution buffer (0.5 M Tris-HCl, 2 mM ZnCl2). Then 7-mm wells were punched into BHI agar supplemented with 5% egg yolk enrichment (ICN Biomedicals Inc., Aurora, Ohio); a 50-μl standard or supernatant was added to each well. Following a 5-h incubation at 37°C, turbidity zone diameters were measured. PC-PLC concentrations in culture supernatants were extrapolated from the B. cereus PC-PLC standard curve. Each sample and standard was assayed in triplicate.
(v) SPH assay.
SPH activities in culture supernatants were measured by a colorimetric microtiter assay adapted from that described by Gatt et al. (13). B. cereus SPH standards (Sigma) and filtered 4-, 8-, 10-, 12-, and 18-h culture supernatants were diluted with an equal volume of assay buffer (125 mM Tris-Cl, 25 mg of MgCl2 per ml, 0.5% Triton X-100, 50% BHI [pH 7.4]). The trinitrophenylaminolauroyl-sphingomyelin substrate was prepared by drying under N2 gas and rehydrating with assay buffer to a final concentration of 250 μg/ml. SPH standards or culture supernatants (50 μl) were incubated with 150 μl of TNPAL-sphingomyelin at 25°C for 5 min. Reactions were stopped by adding 750 μl of isopropanol-heptane-5 M H2SO4 (40:10:1 [vol/vol/vol]) to each reaction mixture. Heptane (450 μl) and H2O (400 μl) were added to each reaction mixture, and after thorough mixing and centrifugation for 5 min at 2,200 × g, the OD340 of the upper, heptane-rich phase (200 μl) was determined. SPH concentrations in culture supernatants were extrapolated from the B. cereus SPH standard curve. Each sample and standard were assayed in triplicate. The CAMP test was also used to analyze SPH activities of each strain following 18 h of incubation at 37°C (5).
(vi) Motility.
Relative swarming of B. thuringiensis strains was analyzed on motility agar (Difco). Colony diameters were measured after 18 h of incubation at 37°C.
Experimental Bacillus endophthalmitis.
Experimental B. thuringiensis endophthalmitis was induced in New Zealand White rabbits as previously described (8, 11). The animals were maintained in accordance with institutional animal care guidelines and the Association for Research in Vision and Ophthalmology Statement on the Use of Laboratory Animals in Ophthalmic Research (3). The rabbits were anesthetized with a mixture of ketamine (Ketaved [Phoenix Scientific Inc., St. Joseph, Mo.], 35 mg/kg of body weight) and xylazine (Rompun [Bayer Corp., Shawnee Mission, Kans.]; 5 mg/kg of body weight). Topical anesthetic (0.5% proparacaine HCl [Ophthetic Allergan, Hormigueros, Puerto Rico]) was applied before each surgical procedure. A 100-μl volume of aqueous humor was withdrawn prior to each intravitreal injection by paracentesis. Then 100 CFU of B. thuringiensis was delivered by slow infusion into the midvitreous via a 30-gauge needle attached to a 1-ml syringe introduced through the pars plana. The contralateral eye was injected with BHI (surgical control) or was left undisturbed (absolute control). At various times postinjection, infection courses were analyzed by biomicroscopy, electroretinography (ERG), histopathology, and bacterial and inflammatory cell quantification as described below.
Biomicroscopy.
Rabbits were observed with a Topcon SL-5D slit-lamp biomicroscope (Kogaku Kikai K.K., Tokyo, Japan) prior to and at 6, 12, and 18 h following intravitreal injection.
Retinal function analysis.
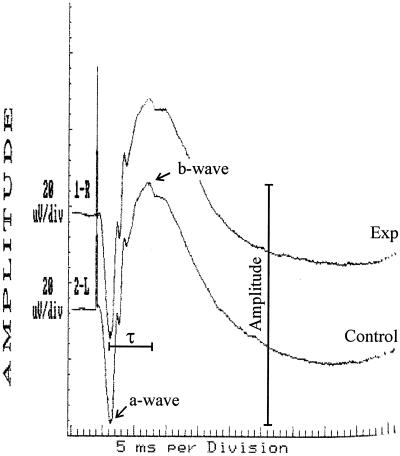
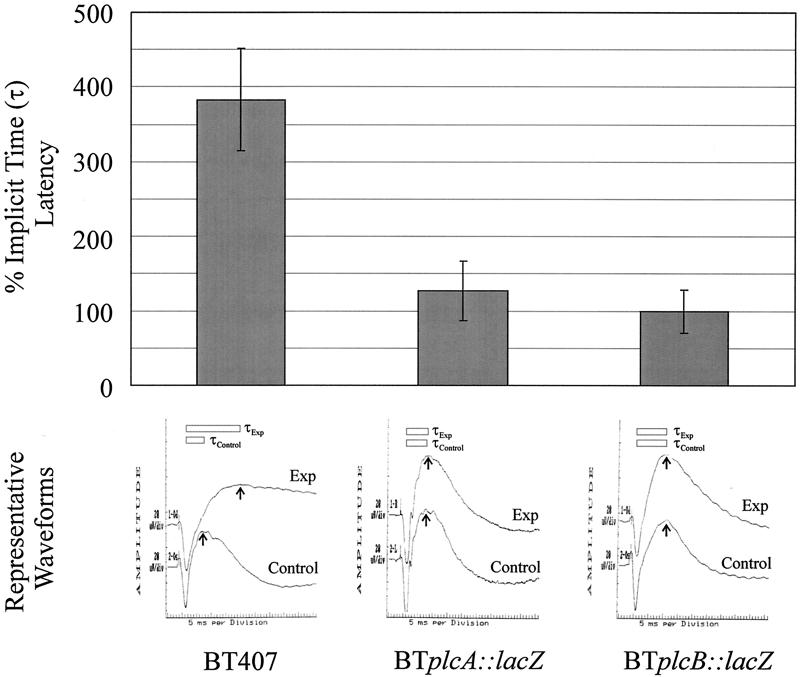
Retinal function was measured by ERG as previously described (8, 11). After dilation and 30 min of dark adaptation, b-wave responses to single light flashes (1/s) were measured (Fig. 1). b-wave amplitudes were recorded for each eye before and at various times postinjection, using scotopic bright-flash ERG (EPIC2000 [LKC Technologies, Inc., Gaithersburg, Md.]). b-wave responses for each time point represented the average of 14 repeated measures. The percent retinal function retained was calculated as 100 − ([1 − (experimental b-wave amplitude/control b-wave amplitude)] × 100) (8, 11). The percent increase in implicit time (τ) from the a-wave valley to the b-wave peak (latency) was calculated as [1 − (τexperimental/τcontrol)] × 100 (Fig. 1).
FIG. 1.
Representative ERG waveform. This waveform was obtained 6 h following intravitreal injection of saline (Exp). The control eye was left undisturbed (Control). Retinal-function analyses included measurement of the amplitude and implicit time (τ) from a-wave to b-wave peaks of each waveform, as indicated.
Bacterial enumeration.
Enumeration of organisms in ocular tissues has been previously described (8, 11). Briefly, globes were enucleated and the vitreous removed and homogenized. Bacteria in the vitreous were quantified by track plating serial 10-fold dilutions onto BHI (21). Retention of mutant phenotypes was confirmed by phenotypic assays of bacteria recovered from the eyes.
Anterior-segment inflammation.
Aqueous humor samples (approximately 100 μl per eye) were recovered by paracentesis. Infiltrating inflammatory cells in 10-μl aliquots were stained with 0.4% trypan blue and enumerated using a hemocytometer.
Thin-section histology.
Globes recovered for histological analysis were fixed in 10% formalin for 24 h. The eyes were sectioned and stained with hematoxylin and eosin by standard procedures (42).
Statistical analysis.
Values for parameters used to analyze progressive infection represented the mean ± standard error of the mean (SEM) for at least four eyes per time point, unless otherwise specified. Wilcoxon's rank sum test was used for statistical comparison between infection groups unless otherwise specified. P ≤ 0.05 was considered significant.
RESULTS
B. thuringiensis isogenic mutants.
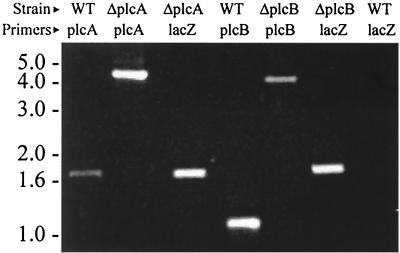
Transformation of strain BT407 with plasmids containing plcA or plcB each disrupted with lacZ yielded isogenic insertional mutants that were Lac+ on X-Gal agar and were erythromycin sensitive, indicating a double-crossover recombination event. PCR analysis of plcA in strain BT407 produced the predicted 1.7-kb fragment. PCR analysis of plcA in strain BTplcA::lacZ produced the predicted 4.7-kb fragment due to the insertion of the 3.2-kb lacZ fragment between plcA fragments of 0.8 kb (PlcA1-PlcA2 product) and 0.7 kb (PlcA3 and PlcA4 product). PCR analysis of plcB in strain BT407 producted the predicted 1.1-kb fragment. PCR analysis of plcB in strain BTplcB::lacZ produced the predicted 4.3-kb fragment due to the insertion of the 3.2-kb lacZ fragment between plcB fragments of 0.6 kb (PlcB1-PlcB2 product) and 0.5 kb (PlcB3-PlcB4 product). PCR analysis using lacZ-specific primers confirmed the presence of the 1.7-kb lacZ fragment in the mutant strains but not in strain BT407 (Fig. 2). Additional verification of the integration of lacZ into plcA or plcB was carried out by PCR using upstream primers external to the plcA and plcB fragments used in strain constructions and a primer located near the 5′ end of lacZ. Fragments of 1.3 kb were detected following amplification of chromosomal DNA from each mutant but not from wild-type strain BT407, as anticipated (data not shown).
FIG. 2.
PCR analysis of B. thuringiensis wild type and isogenic mutants. Chromosomal DNA from wild-type strain BT407 and mutant strains BTplcA::lacZ (ΔplcA), and BTplcB::lacZ (ΔplcB), were used to amplify plcA, plcB, or lacZ, as indicated in the figure. PCR products were of the predicted sizes (indicated by molecular weights [in thousands] on the left).
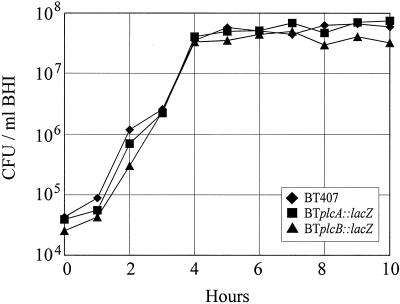
Results of the phenotypic assessment of strains BT407, BTplcA::lacZ and BTplcB::lacZ are summarized in Table 3. Strains BTplcA::lacZ and BTplcB::lacZ differed from wild-type strain BT407 in the disrupted gene product of interest, as measured by chromogenic and agar well diffusion assays specifically for PI-PLC and PC-PLC, respectively. The hemolytic and proteolytic activities of all strains were equivalent throughout 18 h of in vitro growth, and all strains were motile (Table 3). The in vitro growth of all strains over a 10-h period was also similar (Fig. 3).
TABLE 3.
Phenotypic analysis of B. thuringiensis BT407 and its isogenic mutants BTplcA::lacZ and BTplcB::lacZ
| Strain | Hemolytic titera
|
Phenotypic assay
|
|||||
|---|---|---|---|---|---|---|---|
| Log phase | Stationary phase | PI-PLCb (μg/ml) | PC-PLCc (μg/ml) | SPHe | Proteasef (U/ml) | Motilityg (mm) | |
| BT407 | 64 | 128 | 12.9 ± 0.2 | 1.7 ± 0.4 | ND | 6.1 ± 1.6 | 61.5 ± 0.9 |
| BTplcA::lacZ | 64 | 128 | 1.6 ± 0.6 | 2.3 ± 0.2 | ND | 4.2 ± 1.3 | 62.8 ± 0.8 |
| BTplcB::lacZ | 64 | 128 | 13.3 ± 0.1 | NDd | ND | 4.9 ± 2.0 | 76.0 ± 2.2 |
Hemolytic assay of filtered supernatants of 8-h (logarithmic phase) and 18-h (stationary phase) B. thuringiensis cultures. A twofold difference in titer was considered significant.
Chromogenic PI-PLC assay. Values represent mean ± standard deviation in filtered supernatants of 10-h B. thuringiensis cultures. PI-PLC activity in strain BTplcA::lacZ supernatants was significantly lower than in other strains (P ≤ 0.002, Student's t test) but similar to that in heat-denatured controls (P ≥ 0.433, Student's t test).
Egg yolk agar well diffusion assay for PC-PLC activity. Values represent mean ± standard deviation in filtered supernatants of 8-h B. thuringiensis cultures. Values for BT407 and BTplcA::lacZ were not significantly different (P ≥ 0.06, Student's t test).
ND, not detected.
Chromogenic SPH assay and CAMP test. No SPH activity was detected in filtered supernatants of 4-, 8-, 10-, 12-, and 18-h B. thuringiensis cultures. Negative CAMP reactions were also observed.
Hide azure blue protease assay. Values represent mean ± standard deviation in filtered supernatants of 10-h B. thuringiensis cultures. Values were not significantly different (P ≥ 0.08, Student's t test).
Swarming on motility agar. Values represent mean diameter ± standard deviation after an 8-h incubation at 37°C. Diameters of BTplcB::lacZ colonies were significantly larger than that of the other groups at 18 h (P ≤ 0.001, Student's t test).
FIG. 3.
In vitro growth of B. thuringiensis wild type and isogenic mutants. Bacteria were quantified hourly throughout 10 h of growth in BHI. The strains analyzed were wild-type strain BT407, BTplcA::lacZ, and BTplcB::lacZ.
Experimental B. thuringiensis endophthalmitis.
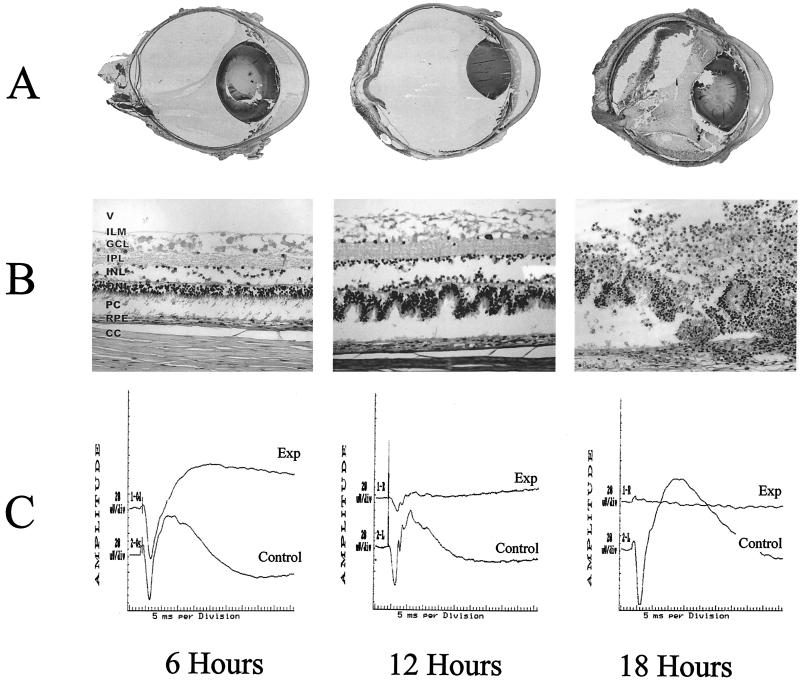
Reproducible B. thuringiensis endophthalmitis was achieved by intravitreal injection of 1.99 ± 0.06 log10 CFU of strain BT407. Whole-organ and retinal histology and representative ERG waveforms of progressive endophthalmitis are depicted in Fig. 4. A mild posterior-segment inflammatory response began as early as 6 h. Mild conjunctival inflammation and 5 to 10 inflammatory cells per microscope field were present in the anterior chamber. At 6 h, all eyes had a normal fundus reflex. At 12 h postinfection, inflammatory changes in the posterior segment and anterior chamber progressed. A haze developed in the vitreous, and the fundus reflex was diminished in all eyes. From 12 to 18 h, inflammatory symptoms were severe in all eyes, with severe iritis, significant inflammatory-cell infiltration into the vitreous and cornea, and no fundus reflex. By 18 h, corneal ring infiltrates were present in at least one-third of eyes in each infection group. Severe inflammation of periorbital tissues was also present in all eyes. Because of the impending panophthalmitis at this time point, infections were not allowed to progress further. No pathologic changes were observed in surgical or absolute controls 6, 12, and 18 h postinfection.
FIG. 4.
Histologic and ERG analysis of progressive experimental B. thuringiensis endophthalmitis. Whole-organ (A) and retinal (B) histology of eyes intravitreally injected with approximately 2 log10 of strain BT407 at 6, 12, and 18 h postinfection are shown. (Panel A reprinted from reference 11a with permission of the publisher.) All representative histologic sections were stained with hematoxylin and eosin. In whole-organ sections, severe inflammation and retinal detachment were observed by 18 h. Photoreceptor folding was observed in retinal sections by 12 h. By 18 h, retinal layers were difficult to differentiate. Abbreviations: V, vitreous; ILM, inner limiting membrane; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; PC, photoreceptor cell layer; RPE, retinal pigment epithelium; CC, choriocapillaris. Magnifications, ×8 (A) and ×160 (B). (C) Representative ERG waveforms obtained at 6, 12, and 18 h following intravitreal injection of strain BT407 (Exp). Control eyes were left undisturbed (Control). Sharp decreases in b-wave amplitude were observed in infected eyes at 12 h. b-wave responses were virtually absent in infected eyes by 18 h.
Experimental PI-PLC- and PC-PLC-deficient B. thuringiensis endophthalmitis.
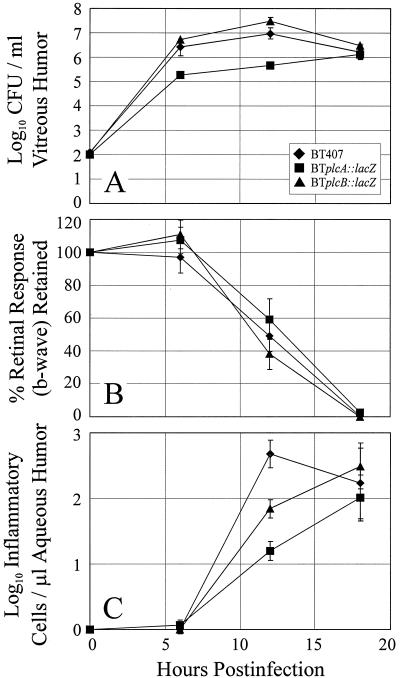
The in vivo growth of B. thuringiensis strains is summarized in Fig. 5A. Intraocular numbers of strain BTplcA::lacZ were significantly smaller at 6 and 12 h than were those of strain BT407 (P ≤ 0.01) but were similar to those of strain BT407 at 18 h (P = 0.79). Intraocular growth patterns of strains BT407 and BTplcB::lacZ were similar at each time point assayed (P ≥ 0.08). In general, each strain grew logarithmically until approximately 6 h, after which a stationary phase of growth was maintained until the termination of the experiment. Gross inflammatory changes observed in eyes infected with strains BTplcA::lacZ or BT plcB::lacZ were similar to those observed in eyes infected with wild-type strain BT407 at all time points throughout the infection courses.
FIG. 5.
Comparison of experimental B. thuringiensis endophthalmitis initiated by wild-type and isogenic mutant strains. Inocula of approximately 2 log10 of either strain BT407, BTplcA::lacZ, or BTplcB::lacZ were injected intravitreally, and infectious were analyzed by bacterial enumeration (A), ERG (B), and inflammatory cell quantitation (C). All values represent the mean and SEM for at least four eyes per group.
Retinal function analyses.
Retinal function analyses of eyes infected with B. thuringiensis wild-type and mutant strains are summarized in Fig. 5B. The b-wave amplitudes of surgical and absolute control eyes remained at preoperative levels throughout the duration of the experiment (data not shown). The b-wave amplitudes of eyes infected with strain BT407 were similar to preoperative levels and to control eyes at 6 h (P ≥ 0.88) but retained only 48.9% ± 9.2% responsiveness by 12 h. At 18 h, retinal responses were nearly absent (retinal function retained = 0.41% ± 0.33%).
The b-wave amplitudes of eyes injected with strains BTplcA::lacZ or BTplcB::lacZ were similar to preoperative levels and to those of controls and strain BT407 at 6 h (P ≥ 0.33). At 12 and 18 h, b-wave amplitudes in eyes infected with each mutant were similar to that of strain BT407 (P ≥ 0.19). By 18 h, b-wave responses in eyes infected with each mutant strain were essentially absent (Fig. 5B).
Representative waveforms showing implicit time values (τ) from ERG responses are shown in Fig. 6. τ values for eyes infected with strain BT407 were increased by 383% ± 68% compared to controls at 6 h (P = 0.0001). τ values for eyes infected with strains BTplcA::lacZ or BTplcB::lacZ were not increased above that of controls at 6 h (P ≥ 0.85). τ values for all infection groups were similar to those of controls at 12 and 18 h (P ≥ 0.25, data not shown).
FIG. 6.
Analysis of latent b-wave responses in experimental B. thuringiensis endophthalmitis. The percent implicit time (τ) from a-wave valleys to b-wave peaks and representative waveforms from eyes intravitreally injected with strains BT407, BTplcA::lacZ, and BTplcB::lacZ at 6 h postinfection are shown. All values represent the mean and SEM for at least four eyes per group.
Anterior-segment inflammation.
Enumeration of inflammatory cells in aspirated aqueous humor samples is summarized in Fig. 5C. Few inflammatory cells were recovered from infected eyes at 6 h. By 12 h, significantly larger numbers of inflammatory cells were recovered from strain BT407-infected eyes than from eyes infected with each mutant strain (P ≤ 0.03). By 18 h, the numbers of inflammatory cells recovered from eyes infected with each mutant strain increased and were similar to those recovered from eyes infected with BT407 (P ≥ 0.73).
Histology.
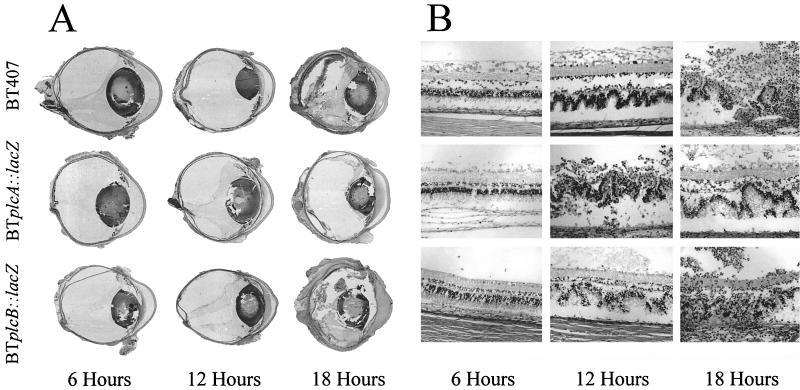
Whole-organ and retinal histologic analysis of progressive B. thuringiensis endophthalmitis is summarized in Fig. 7. Immediately following intravitreal injection, eyes from all infection groups possessed intact retinal architecture, no observable inflammation, and few bacilli in the vitreous (data not shown). At 6 h, eyes injected with wild-type strain BT407 and each mutant exhibited a mild inflammatory response in the posterior segment that originated from the optic nerve head and the ciliary body. The retinal architecture of all infected eyes was intact, and bacilli were observed throughout the vitreous, primarily within vitreous structures. Few inflammatory cells were observed in the anterior segment, and the corneas appeared normal. By 12 h, significant inflammation was observed in all eyes, with large numbers of inflammatory cells and fibrin seen in the posterior and anterior segments. Distinct folding of retinal layers and partial retinal detachments were also observed. Bacilli could be seen in both the posterior and anterior segments of the majority of infected eyes at 12 h. By 18 h, complete retinal tissue dissolution, significant edema, and chemosis of all ocular and surrounding periocular tissues and inflammatory cells in all parts of the eye and surrounding tissues were observed. Histologic specimens from eyes infected with strain BT407 or each mutant were indistinguishable at each time point throughout infection (Fig. 7).
FIG. 7.
Histologic analysis of experimental B. thuringiensis endophthalmitis. Representative whole-organ (A) and retinal (B) histology of eyes intravitreally injected with strains BT407, BTplcA::lacZ, and BTplcB::lacZ at 6, 12, and 18 h postinfection are shown. All histologic sections were stained with hematoxylin and eosin. In whole-organ sections, severe inflammation and retinal detachment were observed by 18 h. Photoreceptor folding and detachment were observed in retinal sections by 12 h. By 18 h, retinal layers were virtually indistinguishable. Gross pathologic changes observed in all infection groups were similar at all time points. Magnifications, ×9 (A) and ×180 (B). (Top row of panel A is reprinted from reference 11a with permission of the publisher.)
DISCUSSION
B. cereus, B. thuringiensis, and B. anthracis are members of the B. cereus group, a taxonomic group demonstrating similar genetic profiles, as demonstrated by multilocus enzyme electrophoresis comparing a number of chromosomal genes in these strains (18). The key difference between B. thuringiensis and B. cereus is the presence of genes coding for insectidical (cry) toxins in B. thuringiensis. If these cry plasmids are lost, as is the case with the strains used in these studies, B. thuringiensis and B. cereus are essentially indistinguishable. Both species are motile and produce a plethora of hemolysins, enterotoxins, and proteases, all of which are possible virulence factors that may contribute to the pathogenesis of disease.
Because of the genetic and phenotypic similarities between B. cereus and B. thuringiensis, we used a well-characterized strain of B. thuringiensis in our model of experimental endophthalmitis (8, 11). Experimental B. thuringiensis endophthalmitis resulted in extensive inflammation and loss of retinal function in a manner similar to that of experimental B. cereus endophthalmitis but at a slightly lower rate compared to a clinical isolate of B. cereus that caused a highly destructive endophthalmitis (8, 11). We previously reported that experimental B. cereus endophthalmitis abolished retinal function by 12 h (8). Extensive inflammation, complete loss of retinal function, and a severe panophthalmitis occurred with either organism within 1 day of infection with as few as 100 organisms.
The contributions of PI-PLC and PC-PLC to endophthalmitis pathogenesis were analyzed using isogenic mutants with lacZ insertions disrupting each gene of interest. Endophthalmitis caused by these mutant strains resulted in complete or nearly complete destruction of retinal function by 18 h, irrespective of the mutation present. Comparable levels of inflammation and retinal photoreceptor layer detachment, folding, and dissolution occurred regardless of the strain used. As in our previous finding of the limited role of hemolysin BL in B. cereus endophthalmitis, our results demonstrated that neither PI-PLC nor PC-PLC contributed to B. thuringiensis endophthalmitis pathogenesis.
An unexpected finding was the increased latency of b-wave response without loss of b-wave amplitude at 6 h in eyes infected with wild-type strain BT407 but not in eyes infected with BTplcA::lacZ or BTplcB::lacZ strains. Latent b-wave responses mark the initial events in retinal dysfunction and have been shown to occur in the early stages of degenerative retinal syndromes including diabetic retinopathy (7, 40), retinitis pigmentosa (19), and retinal ischemia (6). b-wave function results from the flow of current from the photoreceptor cell layers of the retina into the vitreous via the Müller cell in response to a visual stimulus (33). The Müller cell, which spans the length of the retina, provides architectural support and regulates neurotransmitter levels in the retina by degrading or removing these substances during neuronal activity (36). Müller cells also maintain homeostasis of potassium (K+) levels in the retina by removing K+ from the outer retina (31). K+ siphoning from the photoreceptor cell layers of the retina into the vitreous during light stimulation is, in large part, the basis for the b-wave response (32). Therefore, a delayed b-wave response in this case may mark the beginnings of Müller cell dysfunction in response to infection. In endophthalmitis, retinal layers are irreversibly damaged and ultimately disrupted, but the exact mechanisms of damage to Müller cells or other retinal cell types are not known. Further analysis of the early stages of infection is needed to determine when these effects begin, what cell types are affected, and what toxins or inflammatory mediators are involved in this initial damage.
Acknowledgments
We thank Sonia Senesi, Emilia Ghelardi (Dipartimento di Patologia Sperimentale, Universita di Pisa, Pisa, Italy), and Doug Beecher (Hazardous Materials Response Unit, FBI Academy, Quantico, Va.) for sharing BT407 and BTplcA::lacZ strains, and we thank Wei Cao (University of Oklahoma Health Sciences Center, Oklahoma City, Okla.) for stimulating discussions. The technical assistance of Mark Dittmar (DMEI Animal Facility, Oklahoma City, Okla.) and Paula Pierce (DMEI Pathology Laboratory, Oklahoma City, Okla.) is also greatly appreciated.
This work was supported by the National Institutes of Health (EY12985), by Fight for Sight/Prevent Blindness America, and by an unrestricted Career Development Award from Research to Prevent Blindness, Inc. (M.C.C.).
Editor: E. I. Tuomanen
REFERENCES
- 1.Agaisse, H., and D. Lereclus. 1994. Structural and functional analysis of the promoter region involved in full expression of the cryIIIA toxin gene of Bacillus thuringiensis. Mol. Microbiol. 13:97-107. [DOI] [PubMed] [Google Scholar]
- 2.Alape-Giron, A., M. Flores-Diaz, I. Guillouard, C. E. Naylor, R. W. Titball, A. Rucavado, B. Lomonte, A. K. Basak, J. M. Gutierrez, S. T. Cole, and M. Thelestam. 2000. Identification of residues critical for toxicity in Clostridium perfringens phospholipase C, the key toxin in gas gangrene. Eur. J. Biochem. 267:5191-5197. [DOI] [PubMed] [Google Scholar]
- 3.Association for Research in Vision and Ophthalmology. 2000. Statement for the use of animals in ophthalmic and visual research. [Online.] http://www.arvo.org/animalst.htm. Association for Research in Vision and Ophthalmology, Hagerstown, Md.
- 4.Beecher, D. J., T. W. Olsen, E. B. Somers, and A. C. Wong. 2000. Evidence for contribution of tripartite hemolysin BL, phosphatidylcholine-preferring phospholipase C, and collagenase to virulence of Bacillus cereus endophthalmitis. Infect. Immun. 68:5269-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernheimer, A. W., R. Linder, and L. S. Avigad. 1979. Nature and mechanism of action of the CAMP protein of group B streptococci. Infect. Immun. 3:838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block, F., and M. Schwarz. 1998. The b-wave of the electroretinogram as an index of retinal ischemia. Gen. Pharmacol. 30:281-287. [DOI] [PubMed] [Google Scholar]
- 7.Bresnick, G. H., and M. Palta. 1987. Temporal aspects of the electroretinogram in diabetic retinopathy. Arch. Ophthalmol. 105:660-664. [DOI] [PubMed] [Google Scholar]
- 8.Callegan, M. C., M. C. Booth, B. D. Jett, and M. S. Gilmore. 1999. Pathogenesis of gram-positive bacterial endophthalmitis. Infect. Immun. 67:3348-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callegan, M. C., L. S. Engel, J. M. Hill, and R. J. O'Callaghan. 1994. Corneal virulence of Staphylococcus aureus: roles of alpha-toxin and protein A in pathogenesis. Infect. Immun. 62:2478-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callegan, M. C., M. Engelbert, D. W. Parke, and B. D. Jett, M. S. Gilmore. 2002. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin. Microbiol. Rev. 15:111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callegan, M. C., B. D. Jett, L. E. Hancock, and M. S. Gilmore. 1999. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection as assessed using an endophthalmitis model. Infect. Immun. 67:3357-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Callegan, M. C., S. T. Kane, D. C. Cochran, and M. S. Gilmore. 2002. Molecular mechanisms of Bacillus endophthalmitis pathogenesis. DNA Cell Biol. 21:367-373. [DOI] [PubMed] [Google Scholar]
- 12.Daugherty, S., and M. G. Low. 1993. Cloning, expression, and mutagenesis of phosphatidylinositol-specific phospholipase C from Staphylococcus aureus: a potential staphylococcal virulence factor. Infect. Immun. 61:5078-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatt S., T. Dinur, and Y. Barenholz. 1978. A spectrophotometric method for determination of sphingomyelinase. Biochim. Biophys. Acta 530:503-507. [DOI] [PubMed] [Google Scholar]
- 14.Gilmore, M. S., M. C. Callegan, and B. D. Jett. 1999. Multicomponent cytolysins of Enterococcus faecalis and Bacillus cereus, p. 419-434. In J. E. Alouf and J. H. Freer (ed.), Comprehensive sourcebook: bacterial protein toxins. Academic Press, Ltd., London, United Kingdom.
- 15.Gilmore M. S., A. M. Cruz-Rodz, M. Leimeister-Wächter, J. Kreft, and W. Goebel. 1989. A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: nucleotide sequence and genetic linkage. J. Bacteriol. 171:744-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gominet, M., L. Slamti, N. Gilois, M. Rose, and D. Lereclus. 2001. Oligopeptide permease is required for expression of the Bacillus thuringiensis plcR regulon and for virulence. Mol. Microbiol. 40:963-975. [DOI] [PubMed] [Google Scholar]
- 17.Grassme, H., V. Jendrossek, and E. Gulbins. 2001. Molecular mechanisms of bacteria induced apoptosis. Apoptosis 6:441-445. [DOI] [PubMed] [Google Scholar]
- 18.Helgason, E., O. A. Okstad, D. A. Caugant, H. A. Johansen, A. Fouet, M. Mock, I. Hegna, and A. B. Kolsto. 2000. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis: one species on the basis of genetic evidence. Appl. Environ. Microbiol. 66:2627-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iijima H., S. Yamaguchi, and O. Hosaka. 1993. Photopic electroretinogram implicit time in retinitis pigmentosa. Jpn. J. Ophthalmol. 37:130-135. [PubMed] [Google Scholar]
- 20.Ikezawa, H., and R. Taguchi. 1981. Phosphatidylinositol-specific phospholipase C from Bacillus cereus and Bacillus thuringiensis. Methods Enzymol. 71:731-741. [Google Scholar]
- 21.Jett, B. D., K. L. Hatter, M. M. Huycke, and M. S. Gilmore. 1997. Simplified agar plate method for quantifying viable bacteria. BioTechniques 23:648-650. [DOI] [PubMed] [Google Scholar]
- 22.Jett, B. D., H. G. Jensen, R. E. Nordquist, and M. S. Gilmore. 1992. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect. Immun. 60:2445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen, T., T. Holm, P. H. Guddal, K. Sletten, F. B. Haugli, and C. Little. 1988. Cloning and sequencing of the gene encoding the phosphatidylcholine-preferring phospholipase C of Bacillus cereus. Gene 65:293-304. [DOI] [PubMed] [Google Scholar]
- 24.Kuppe, A., L. M. Evans, D. A. McMillan, and O. H. Griffith. 1989. Phosphatidylinositol-specific phospholipase C of Bacillus cereus: cloning, sequencing, and relationship to other phospholipases. J. Bacteriol. 171:6077-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lereclus, D., H. Agaisse, M. Gominet, and J. Chaufaux. 1995. Overproduction of encapsulated insecticidal crystal proteins in a Bacillus thuringiensis spo0A mutant. Bio/Technology 13:67-71. [DOI] [PubMed] [Google Scholar]
- 26.Lereclus, D., H. Agaisse, M. Gominet, S. Salamitou, and V. Sanchis. 1996. Identification of a Bacillus thuringiensis gene that positively regulates transcription of the phosphatidylinositol-specific phospholipase C gene at the onset of the stationary phase. J. Bacteriol. 178:2749-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lereclus, D., O. Arantes, J. Chaufaux, and M. M. Lecadet. 1989. Transformation and expression of a cloned δ-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol. Lett. 60:211-217. [DOI] [PubMed] [Google Scholar]
- 28.Lereclus, D., M. Vallade, J. Chaufaux, O. Arantes, and S. Rambaud. 1992. Expansion of insecticidal host range of Bacillus thuringiensis by in vivo genetic recombination. Bio/Technology 10:418-421. [DOI] [PubMed] [Google Scholar]
- 29.Marques, M. B., P. F. Weller, J. Parsonnet, B. J. Ransil, and A. Nicholson-Weller. 1989. Phosphatidylinositol-specific phospholipase C, a possible virulence factor of Staphylococcus aureus. Infect. Immun. 27:2451-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Msadek, T., F. Kunst, D. Henner, A. Klier, G. Rapoport, and R. Dedonder. 1990. Signal transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J. Bacteriol. 172:824-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman, E. A. 1985. Regulation of extracellular potassium by glial cells in the retina. Trends Neurosci. 8:156-159. [Google Scholar]
- 32.Newman, E. A., D. A. Frambach, and L. L. Odette. 1984. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science 225:1174-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman, E. A., and L. L. Odette. 1984. Model of electroretinogram b-wave generation: a test of the K+ hypothesis. J. Neurophysiol. 51:164-182. [DOI] [PubMed] [Google Scholar]
- 34.Ng, E. W., N. Samiy, J. B. Rubins, F. V. Cousins, K. L. Ruoff, A. S. Baker, and D. J. D'Amico. 1997. Implication of pneumolysin as a virulence factor in Streptococcus pneumoniae endophthalmitis. Retina 17:521-529. [PubMed] [Google Scholar]
- 35.O'Callaghan, R. J., M. C. Callegan, J. M. Moreau, L. C. Green, T. J. Foster, O. M. Hartford, L. S. Engel, and J. M. Hill. 1997. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect. Immun. 65:1571-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reichenbach A., and S. R. Robinson. 1995. The involvement of Müller cells in the outer retina, p. 395-416. In M. B. A. Djamgoz, S. N. Archer, and S. Vallerga (ed.), Neurobiology and Clinical aspects of the outer retina. Chapman & Hall, London, United Kingdom.
- 37.Rinderknecht, H., M. C. Geokas, P. Silverman, and B. J. Haverback. 1968. A new ultrasensitive method for the determination of proteolytic activity. Clin. Chim. Acta 21:197-203. [DOI] [PubMed] [Google Scholar]
- 38.Rose, F., S. A. Zeller, T. Chakraborty, E. Domann, T. Machleidt, M. Kronke, W. Seeger, F. Grimminger, and U. Sibelius. 2001. Human endothelial cell activation and mediator release in response to Listeria monocytogenes virulence factors. Infect. Immun. 69:897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saint-Joanis, B., T. Garnier, and S. T. Cole. 1989. Gene cloning shows the alpha-toxin of Clostridium perfringens to contain both sphingomyelinase and lecithinase activities. Mol. Gen. Genet. 219:453-460. [DOI] [PubMed] [Google Scholar]
- 40.Satoh, S., H. Iijima, M. Imai, K. Abe, and T. Shibuya. 1994. Photopic electroretinogram implicit time in diabetic retinopathy. Jpn. J. Ophthalmol. 38:178-184. [PubMed] [Google Scholar]
- 41.Schluter, D., E. Domann, C. Buck, T. Hain, H. Hof, T. Chakraborty, and M. Deckert-Schluter. 1998. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect. Immun. 66:5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheehan, D. C., and B. B. Hrapchak. 1987. Theory and practice of histotechnology. Batelle Press, Columbus, Ohio.
- 43.Sibelius, U., E. C. Schulz, F. Rose, K. Hattar, T. Jacobs, S. Weiss, T. Chakraborty, W. Seeger, and F. Grimminger. 1999. Role of Listeria monocytogenes exotoxins listeriolysin and phosphatidylinositol-specific phospholipase C in activation of human neutrophils. Infect. Immun. 67:1125-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villafane, R., D. H. Bechhofer C. S. Narayanan, and D. Dubnau. 1987. Replication control genes of plasmid pE194. J. Bacteriol. 169:4822-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, P., B. Liu, G. M. Jenkins, Y. A. Hannun, and L. M. Obeid. 1997. Expression of neutral sphingomyelinase identifies a distinct pool of sphingomyelin involved in apoptosis. J. Biol. Chem. 272:9609-9612. [DOI] [PubMed] [Google Scholar]