Abstract
We report that enteroinvasive Escherichia coli (EIEC) serotypes considered to be nonmotile produce an unusually large (77 kDa) flagellin that is assembled into functional flagellum filaments that allow the bacteria to swim in modified motility agar. The EIEC flagellin showed N-terminal identity to most common enterobacterial flagellins, especially those of the E. coli H7 serotype. These data are important in terms of the epidemiology, evolution, and biology of EIEC.
Shigella species and enteroinvasive Escherichia coli (EIEC) strains cause bacillary dysentery in humans by invading and multiplying within epithelial cells of the colonic mucosa. This remarkable tropism results in an intense inflammatory response characterized by abscesses and ulcerations that damage the integrity of the epithelial cell lining of the colon, producing the notable symptoms of dysentery (12). E. coli strains isolated from patients with dysentery were also able to cause experimental keratoconjunctivitis in guinea pigs (22, 24, 30). The entry of Shigella and EIEC bacteria into susceptible host target cells requires the coordinated expression of numerous genes that are activated in response to the microenvironment signals. The entire repertoire of genes required for entry into host cells is clustered in a 230-kb virulence-associated invasion plasmid present both in Shigella and EIEC strains (13, 23, 26). However, most of the genetic and structure-function studies regarding the biological inter action of these invasive bacteria with eukaryotic cells have been centered on Shigella rather than EIEC. Most of the EIEC serotypes described so far share antigenic, biochemical, genetic, and pathogenetic properties with Shigella spp. (6). Like Shigella, EIEC strains are consistently lysine decarboxylase negative, usually nonmotile, and frequently lactose negative (25).
The incidence of EIEC in developing countries is thought to be low, but this could be attributed to misidentification with nonpathogenic E. coli strains (4, 5). EIEC is mostly associated with occasional food-borne outbreaks (19), and some studies indicate that EIEC can be isolated with relatively high frequency depending on the population investigated (28).
The lack of flagella and motility constitute important taxonomic and diagnostic criteria that differentiate Shigella and Klebsiella species from other members of the Enterobacteriaceae (6). Genetic analysis of the four Shigella species showed that all of them contain the genetic elements necessary to assemble flagella, at least when cloned in E. coli K-12 (29). Further ultrastructural studies of Shigella strains propagated in different growth media demonstrated that these organisms do produce flagella albeit to a lesser extent than E. coli and Salmonella enterica (10). It is not known what biological relevance the production of flagella represents for Shigella spp. or for EIEC, for that matter. Except for strains belonging to serotype O124:H30, EIEC strains are nonmotile in standard motility assays and typically do not produce a detectable flagellar antigen. In this study, we analyzed a collection of well-defined EIEC nonmotile strains for the expression of flagella and motility (Table 1). We studied 28 EIEC lysine decarboxylase-negative strains belonging to 11 serotypes that were isolated from patients with acute diarrhea (2, 17). This collection includes three isolates (FBC112/2, FBC112/3, and FBC112/4) obtained from the Centers for Disease Control and Prevention and strains isolated in different areas of Brazil from 1965 to 1986. Except for isolates FBC29/9, FBC29/10, FBC112/3, FBC136/6, and FBC164/1, all of the EIEC strains were positive on the Sereny test (17, 24). To determine which culture media and temperature were more appropriate for stimulating expression of flagella, nonmotile strain FBC28/45 was grown in several solid culture media, including MacConkey agar (Oxoid), brain heart infusion (Difco), 0.1% tryptone, Brucella, casetone, Trypticase soy agar (Oxoid) plus 5% sheep blood, and Trypticase soy broth (Oxoid), for 24 h at 25, 37, and 42°C.
TABLE 1.
Production of flagella and motility by EIEC strains and distribution of the flagellin gene
| Serotype | Strain | Result fora
|
|||
|---|---|---|---|---|---|
| Flagella | Flagellin | Motility | PCR | ||
| O28:NM | FBC28/1 | + | + | + | + |
| FBC28/45 | + | + | − | + | |
| O29:NM | FBC29/7 | + | + | + | + |
| FBC29/8 | + | + | + | + | |
| FBC29/9 | + | + | + | + | |
| O112:NM | FBC112/2 | + | + | − | + |
| FBC112/4 | + | + | − | + | |
| FBC112/3 | + | + | − | + | |
| O124:NM | FBC124/13 | + | + | + | + |
| O124:H30 | FBC124/4 | + | + | + | + |
| O136:NM | FBC136/1 | + | + | + | + |
| FBC136/6 | + | + | + | + | |
| FBC136/11 | + | + | − | + | |
| O143:NM | FBC143/2 | + | + | + | + |
| FBC143/10 | + | + | + | + | |
| FBC143/14 | + | + | + | + | |
| O144:NM | FBC144/2 | + | + | + | + |
| FBC144/7 | + | + | + | + | |
| FBC144/8 | + | + | + | + | |
| O152:NM | FBC152/5 | + | + | + | + |
| FBC152/10 | + | + | + | + | |
| FBC152/26 | + | + | + | + | |
| O164:NM | FBC164/1 | + | + | + | + |
| FBC164/6 | + | + | − | + | |
| FBC164/4 | + | + | + | + | |
| O167:NM | FBC167/1 | + | + | + | + |
| FBC167/5 | + | + | + | + | |
| FBC167/8 | + | + | − | + | |
Flagella were observed by electron microscopy, and motility was recorded after incubation for 24 to 72 h at 37°C in 0.17% motility agar. Flagellin was demonstrated by immunoblotting as described in the text. PCR was performed with primers derived from the E. coli K-12 fliC sequence (14).
To determine production of flagella, bacterial colonies growing on the different bacteriological media were resuspended in phosphate-buffered saline and then analyzed by electron microscopy after negative staining with 1% phosphotungstic acid (pH 7.4) on carbon-Formvar copper grids (10). For immunogold labeling, rabbit anti-flagellum antiserum and goat anti-rabbit immunoglobulin G labeled with 10-nm-diameter gold particles (Amersham-Pharmacia) were employed (11). MacConkey agar appeared to be the best medium to induce flagellation in FBC28/45. We then propagated 27 H− EIEC strains belonging to different serotypes on MacConkey agar and found that all of them produced flagella to different extents (Table 1). The number of flagella expressed on the surface of the bacteria varied between strains and between serotypes. Generally, one to five flagellar filaments could be observed in a considerably small subset of the bacterial population studied (Fig. 1). Examples of these observations are shown in Fig. 1. This suggests the presence of a strict regulatory system for flagellum production. The level of expression of flagella in EIEC is lower than that in Salmonella or E. coli but slightly higher than that observed previously with Shigella spp. in the sense that only a few of the organisms (∼10%) in a given culture expressed flagella (10). Flagella were observed regardless of the temperature of growth (25, 37, and 42°C), although growth at 37°C appeared to be the most favorable for flagellum production (data not shown).
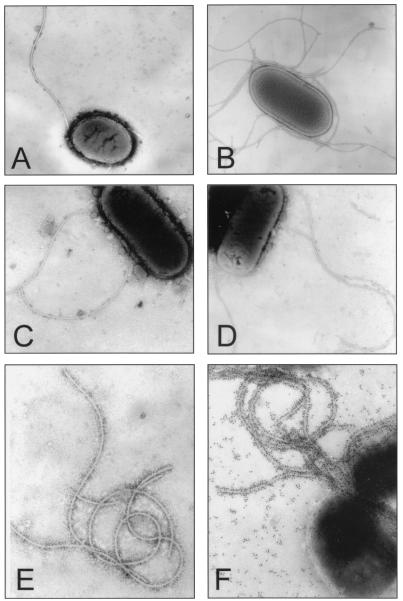
FIG. 1.
Expression of flagella by nonmotile EIEC. (A) FBC167/8 (O167:NM). (B) FBC144/7 (O164:NM). (C) FBC28/45 (O28:NM). (D) FBC124/4 (O124:H30), used as a control. (E) Immunogold labeling of flagella obtained from FBC28/45. (F) Immunogold labeling of FBC28/45 bacteria expressing flagella.
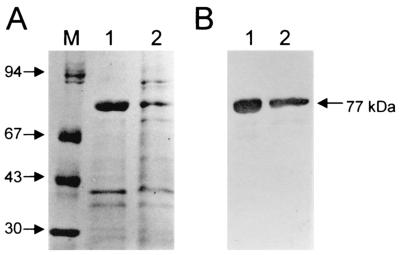
Since expression of flagella was suboptimal under the conditions tested, in order to study the biochemical and antigenic properties of EIEC flagella and flagellin (FliC) in nonmotile FBC28/45, it was necessary to inoculate as many as 100 MacConkey agar plates. Dissociation of flagella from these bacteria was achieved by mechanical shearing and differential centrifugation (10, 11). The partially purified preparations contained flagella as demonstrated by electron microscopy and immunogold labeling with anti-FBC28/45 flagellum antibodies (Fig. 1E). Further, the flagellum filaments expressed on the bacterial surface were also detected with these antibodies and gold labeling (Fig. 1F). The monomeric subunits were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing conditions (15) and showed a major 77-kDa band that reacted with antiserum against the EIEC flagellin obtained in New Zealand White rabbits (Fig. 2) (10). This is particularly interesting since most enterobacterial flagellins are equal to or smaller than 60 kDa.
FIG. 2.
Characterization of purified EIEC flagella. (A) SDS-PAGE and Coomassie blue staining of purified EIEC flagella. (B) Western blot of EIEC flagellins reacted with anti-EIEC flagellum antibodies. Lanes: M, mass standards; 1, flagella obtained from FBC124 (H30); 2, flagella obtained from nonmotile FBC28/45.
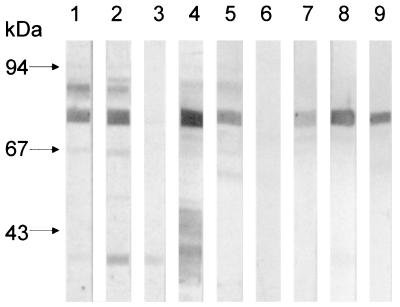
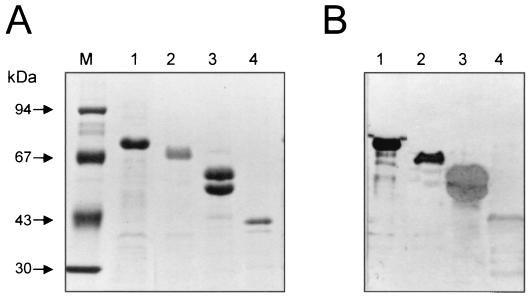
The 77-kDa flagellin protein of FBC28/45 was subjected to N-terminal amino acid analysis with an Applied Biosystems Protein Sequencer model 491. Analysis of this 77-kDa band showed identity with flagellins of several enteric bacteria, such as E. coli and Shigella flexneri (93%), Salmonella enterica serovar Typhimurium, Proteus mirabilis, Yersinia spp., and Serratia spp. (81%) (Fig. 3). The antigenic relatedness between all of these flagellins was assayed by immunoblotting with species-specific anti-flagellum antibodies (namely against flagella of P. mirabilis, Shigella sonnei, S. flexneri, Shigella dysenteriae, S. enterica serovar Typhi, and E. coli H2, H7, H21, and H34) and monoclonal antibody 15D8, which recognizes a common epitope on enterobacterial flagellins (7). Except for antisera against flagellins of E. coli H34 and S. dysenteriae, the rest of the antisera reacted with EIEC FliC (Fig. 4). Conversely, we showed that the flagellins of S. enterica serovar Typhimurium, E. coli, and P. mirabilis shared antigenic determinants with the EIEC flagellin (Fig. 5). It is obvious that these molecules have diverged, yielding proteins with different antigenic but probably similar functional properties. Genetic studies and analysis of amino acid sequences of enterobacterial flagellins show that these flagellins share extended amino- and carboxy-terminal homologies, with considerable divergence existing within the middle regions of the proteins (16, 21). The reactivity of the EIEC flagellin shown here with heterologous antibodies may then be explained by the homology of the end regions between these molecules. To confirm the electron microscopy data, we then assayed the remainder of the nonmotile strains (adjusted to the same bacterial concentration) for flagellin production by immunoblotting with anti-EIEC flagellum antibodies. As shown in Fig. 6 and Table 1, all of the nonmotile strains were able to synthesize flagellin upon growth on MacConkey agar, albeit to different levels of expression.
FIG. 3.
Comparison of N-terminal amino acid sequences of EIEC FBC28/45 and other enterobacterial flagellins. Bold residues indicate nonidentical residues.
FIG. 4.
Immunoblotting of flagellin obtained from EIEC FBC24/45 (O28ac:NM) and reacted with antibodies against heterologous flagellins. Lanes: 1, S. sonnei; 2, S. flexneri; 3, S. dysenteriae; 4, E. coli H30; 5, E. coli H7; 6, E. coli H34; 7, S. enterica serovar Typhi; 8, P. mirabilis; 9, EIEC strain FBC28/45.
FIG. 5.
Cross-reactivity of enterobacterial flagellins. (A) Flagellar preparations obtained from FBC28/45 (O28:NM) (lane 1), E. coli H6 (lane 2), S. enterica serovar Typhimurium (lane 3), and P. mirabilis (lane 4) were electrophoresed in 10% acrylamide gels and stained with Coomassie blue. Lane M, molecular mass standards. (B) Western blot of flagellar preparations in panel A reacted with antisera against EIEC flagella. Lanes are the same as for panel A.
FIG. 6.
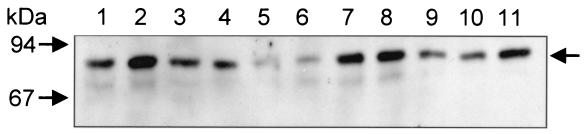
Detection of flagellin in nonmotile EIEC serotypes. Equal numbers of bacteria grown on MacConkey agar were subjected to SDS-PAGE and then reacted with anti-EIEC flagellum antibodies. Note the different levels of flagellin production among the strains. Lanes: 1, FBC28/45; 2, FBC29/7; 3, FBC152/26; 4, FBC124/13; 5, FBC136/11; 6, FBC112/2; 7, FBC164/4; 8, FBC167/8; 9, FBC136/1; 10, FBC152/5; 11, FBC028/1.
We then tested these strains for motility in glass vials containing 5 ml of Trypticase soy broth with 0.175, 0.2, 0.3, or 0.4% agar for 24, 48, and 72 h at 25, 37, and 42°C (10). Each strain was inoculated with a straight needle and tested at least three times. Enteropathogenic E. coli (O55:H6) and Klebsiella pneumoniae ATCC 13883 were used as positive and negative controls, respectively. The majority of the EIEC strains were able to swim when tested in low-percent-agar media (0.175 to 0.2%) after 24 h of incubation (Fig. 7 and Table 1). Other strains were motile only after incubation for 48 to 72 h. In general, motility was better achieved at 37 and 42°C than at room temperature (data not shown). Flagellated EIEC strains which did not show motility were repeatedly subcultured in an effort to induce motility. The failure to detect motility in these particular strains may be explained by the deficiency of one or more of the genes involved in biosynthesis or regulation of flagella or chemotaxis (16).
FIG. 7.
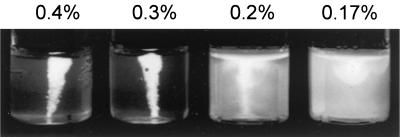
Motility of EIEC. FBC29/9 (O29:NM) was inoculated in 0.4, 0.3, 0.2, and 0.17% motility agars at 37°C for 24 h. The bacteria are unable to swim in 0.4 and 0.3% motility agars but display motility in 0.17 and 0.2% agars.
The N- and C-terminal portions of flagellin are highly conserved among the Enterobacteriaceae, and thus, fliC genes are ideal candidates for DNA amplification because of their conserved nature. This feature has led to the development of a number of diagnostic applications for specific bacteria (3, 8, 32). We then performed PCRs to confirm the presence of the fliC gene in the EIEC strains studied with primers KF1 (5′-GCACAAGTCATTAATACCAACAGCCTC-3′) andKR2 (5′-CCCTGCAGCAGAGACAGAACCTGCTGC-3′), which were derived from the fliC sequence of E. coli K-12 (14) as previously described (11). In all of the cases, a specific fliC amplicon that varied in size (from 1.1 to 2.3 kb) was obtained, suggesting that heterogeneity among these genes and that the failure in the past to observe flagella in most EIEC strains is not due to the lack of the fliC gene (data not shown). Thus, in some of the isolates there is agreement between the size of the FliC protein and the size of the amplified fliC. Since the primers employed are specific for E. coli K-12 fliC, it is possible that, in those isolates showing smaller amplicons, annealing has occurred before the end of the actual gene, rendering truncated PCR products. The 2.1- and 1.7-kb amplified fragments obtained from FBC28/19 and FBC28/45, respectively, were subjected to nucleotide sequencing and compared to the fliC sequences available in GenBank (data not shown). This preliminary analysis revealed high identities with the E. coli O55:H7 and O157:H7 fliC DNA sequences and confirmed that the amplified fragments are indeed flagellin genes.
Several new studies have shown the importance of flagellation and motility in bacterial pathogenicity (1, 9, 11, 18, 20, 27, 31). In some pathogenic E. coli strains, the production of flagella is triggered by the presence of eukaryotic cells and likely by a secreted signal of eukaryotic origin (11). The fact that flagella have not been observed on nonmotile EIEC strains may be attributed to the presence of flagellar genes that are under the influence of a highly regulatory control system, to the lack of chemotaxis or flagellar genes involved in synthesis and assembly, or to the requirement of strict environmental and nutritional factors. Like Shigella species, nonmotile EIEC strains were shown here to display motility only under modified motility assays with reduced agar concentrations. Our data serve as a starting point for future investigations regarding the role of flagella in the invasion process of EIEC and survival within a host. The data presented here are compelling evidence that EIEC is able to assemble flagella. The confirmation of flagella in the nonmotile serotypes of EIEC may provide new insights about the biology of this organism and could be useful for diagnostics, provided that new or existing H types among these strains are serologically demonstrated. The data are certainly interesting in terms of the evolution of EIEC flagellar genes with respect to other enterobacterial species.
Acknowledgments
This study was supported by grants from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP 97/11149-0 and 00/05024-5) and Coordenação e Aperfeiçoamento de Pessoal de Nível Superior (CAPES; to A.A.). Support for J.A.G. was from Conacyt grant 3777-M.
Editor: B. B. Finlay
REFERENCES
- 1.Allen-Vercoe, E., and M. J. Woodward. 1999. The role of flagella, but not fimbriae, in the adherence of Salmonella enterica serotype Enteritidis to chick gut explant. J. Med. Microbiol. 48:771-780. [DOI] [PubMed] [Google Scholar]
- 2.Bando, S. Y., G. R. F. Valle, M. B. Martinez, L. R. Trabulsi, and C. A. Moreira-Filho. 1998. Characterization of enteroinvasive Escherichia coli and Shigella strains by RAPD analysis. FEMS Microbiol. Lett. 165:159-165. [DOI] [PubMed] [Google Scholar]
- 3.Coimbra, R. S., M. Lefevre, F. Grimont, and P. A. D. Grimont. 2001. Clonal relationships among Shigella serotypes suggested by cryptic flagellin gene polymorphism. J. Clin. Microbiol. 39:670-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupont, H. L., S. B. Formal, R. B. Hornick, M. J. Snyder, J. P. Libonati, D. G. Shehan, E. H. Labrec, and J. P. Kalas. 1971. Pathogenesis of Escherichia coli diarrhea. N. Engl. J. Med. 285:1-9. [DOI] [PubMed] [Google Scholar]
- 5.Echeverria, P., O. Sethabutr, O. Serichantalergs, U. Lexomboon, and K. Tamura. 1992. Shigella and enteroinvasive Escherichia coli infections in households of children with dysentery in Bangkok. J. Infect. Dis. 165:144-147. [DOI] [PubMed] [Google Scholar]
- 6.Ewing, W. H. 1986. Edwards and Ewing's identification of Enterobacteriaceae, 4th ed. Elsevier, New York, N.Y.
- 7.Feng, P., R. J. Sugasawara, and A. Schantz. 1990. Identification of a common enterobacterial flagellin epitope with a monoclonal antibody. J. Gen. Microbiol. 136:337-342. [DOI] [PubMed] [Google Scholar]
- 8.Fields, P. I., K. Blom, H. J. Hughes, L. O. Helsel, P. Feng, and B. Swaminathan. 1997. Molecular characterization of the gene encoding H antigen in Escherichia coli and development of a PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J. Clin. Microbiol. 35:1066-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gewirtz, A. T., P. O. Simon, C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girón, J. A. 1995. Expression of flagella and motility by Shigella. Mol. Microbiol. 18:63-75. [DOI] [PubMed] [Google Scholar]
- 11.Girón, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 12.Hale, T. L. 1998. Bacillary dysentery, p. 479-493. In W. J. Hansler and M. Shuman (ed.), Topley and Wilson's microbiology and microbial infections, vol. 3. Arnold, London, England. [Google Scholar]
- 13.Harris, J. R., I. K. Wachsmuth, B. R. Davis, and M. L. Cohen. 1982. High-molecular-weight plasmid correlates with Escherichia coli enteroinvasiveness. Infect. Immun. 37:1295-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuwajima, G., J. I. Aska, T. Fujiwara, K. Node, and K. Kondo. 1986. Nucleotide sequence of the fliC gene encoding flagellin of Escherichia coli. J. Bacteriol. 168:1470-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.MacNab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 17.Martinez, B. M., T. S. Whittam, E. A. McGrow, J. Rodrigues, and L. R. Trabulsi. 1999. Clonal relationship among enteroinvasive Escherichia coli. FEMS Microbiol. Lett. 172:145-151. [DOI] [PubMed] [Google Scholar]
- 18.Mobley, H. T., B. Belas, V. Lockatell, G. Chippendale, A. L. Trifillis, D. E. Johnson, and J. W. Warren. 1996. Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infections. Infect. Immun. 64:5332-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Postnova, T., O. G. Gómez-Duarte, and K. Richardson. 1996. Motility mutants of Vibrio cholerae O1 have reduced adherence in vitro to human small intestinal epithelial cells as demonstrated by ELISA. Microbiology 142:2767-2776. [DOI] [PubMed] [Google Scholar]
- 21.Reid, S. D., R. K. Selander, and T. S. Whittam. 1999. Sequence diversity of flagellin (fliC) alleles in pathogenic Escherichia coli. J. Bacteriol. 181:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakazaki, R., K. Tamura, and M. Saito. 1967. Enteropathogenic Escherichia coli associated with diarrhea in children and adults. Jpn. J. Med. Sci. Biol. 20:387-399. [DOI] [PubMed] [Google Scholar]
- 23.Sansonetti, P. J., D. Kopecko, and S. B. Formal. 1982. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect. Immun. 35:852-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Séreny, B. 1963. Biochemical reactions and virulence of Escherichia coli O124, K72 (B17). Acta Microbiol. Acad. Sci. Hung. 10:11-18. [PubMed] [Google Scholar]
- 25.Silva, R. M., M. R. F. Toledo, and L. R. Trabulsi. 1980. Biochemical and cultural characteristics of invasive Escherichia coli. J. Clin. Microbiol. 11:441-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva, R. M., M. R. F. Toledo, and L. R. Trabulsi. 1982. Correlation of invasiveness with plasmid in enteroinvasive strains of Escherichia coli. J. Infect. Dis. 146:706-710. [DOI] [PubMed] [Google Scholar]
- 27.Tasteyre, A., M. C. Barc, A. Collignon, H. Boureau, and T. Karjalainen. 2001. Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect. Immun. 69:7937-7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toledo, M. R. F., and L. R. Trabulsi. 1990. Frequency of enteroinvasive Escherichia coli in children with diarrhea and healthy controls, in São Paulo, Brazil. Rev. Microbiol. 21:1-4. [Google Scholar]
- 29.Tominaga, A., M. A. H. Mahmoud, T. Mukaiara, and M. Enomoto. 1994. Molecular characterization of intact but cryptic, flagellin genes in the genus Shigella. Mol. Microbiol. 12:277-285. [DOI] [PubMed] [Google Scholar]
- 30.Trabulsi, L. R., M. R. Fernandes, and M. E. Zuliani. 1967. Novas bactérias patogênicas para o intestino do homem. Rev. Inst. Med. Trop. 9:31-39. [PubMed] [Google Scholar]
- 31.Young, G. M., J. L. Badger, and V. L. Miller. 2000. Motility is required to initiate host cell invasion by Yersinia enterocolitica. Infect. Immun. 68:4323-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, W.-L., M. Bielaszewska, J. Bockemühl, H. Schmidt, F. Scheutz, and H. Karch. 2000. Molecular analysis of H antigens reveals that human diarrheagenic Escherichia coli O26 strains belong to the H11 clonal complex. J. Clin. Microbiol. 38:2989-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]