Abstract
An emerging theme in cancer biology is that although some malignancies occur through the sequential acquisition of different genetic alterations, certain dominantly acting oncoproteins such as those associated with chromosomal translocations have multiple functions and do not require additional mutations for cell transformation. The ETV6-NTRK3 (EN) chimeric tyrosine kinase, a potent oncoprotein expressed in tumors derived from multiple cell lineages, functions as a constitutively active protein tyrosine kinase. Here, we show that EN suppresses TGF-β signaling by directly binding to the type II TGF-β receptor, thereby preventing it from interacting with the type I TGF-β receptor. This activity requires a functional EN protein tyrosine kinase, and type II TGF-β receptor appears to be a direct target of EN. Our findings provide evidence for a previously undescribed mechanism by which oncogenic tyrosine kinases can block TGF-β tumor suppressor activity.
Keywords: chromosomal translocation, congenital fibrosarcoma, TGF-β receptor
The TGF-β signaling pathway has a complex role in human oncogenesis (1–4). In early or localized lesions, TGF-β functions predominantly as a tumor suppressor protein that inhibits growth of transformed cells. In fact, mutations and transcriptional repression of the type II TGF-β receptor (TβRII) have been described in human tumors (1, 3). However, in advanced malignancies a functional TGF-β pathway appears to be essential for tumor cells to acquire invasive properties and establish distant metastases.
The breakpoints of the t(12;15)(p13;q25) associated with congenital fibrosarcoma (CFS), a pediatric spindle cell malignancy of the soft tissues, were recently cloned (5). This rearrangement generates a gene fusion encoding the sterile α motif domain of the ETV6 (TEL) transcription factor linked to the protein tyrosine kinase (PTK) domain of the neurotrophin-3 receptor NTRK3 (TrkC) (5). The resulting ETV6-NTRK3 (EN) protein functions as a chimeric PTK with potent transforming activity (6, 7). EN expression leads to the constitutive activation of two major effector pathways of wild-type (WT) NTRK3, namely the Ras-mitogen-activated protein kinase (MAPK) mitogenic pathway and the phosphatidyl inositol-3-kinase (PI3K)–AKT pathway mediating cell survival, and both are required for EN transformation (6, 8). ETV6-NTRK3 fusion transcripts also have been identified in a related pediatric tumor, cellular mesoblastic nephroma (CMN) (9, 10), and in a case report of adult acute myeloid leukemia (11). Moreover, we recently demonstrated that human secretory breast carcinoma, a rare subtype of infiltrating ductal carcinoma, also expresses the ETV6-NTRK3 gene fusion (12). EN is therefore unique among known chimeric oncoproteins in that it is expressed in malignancies derived from mesenchymal, hematopoietic, and epithelial cell lineages.
We recently observed by microarray analysis that EN-transformed NIH3T3 fibroblasts show marked up-regulation of TGF-β1 transcripts compared with those expressing a kinase dead form of EN or vector alone, which was confirmed by Northern and Western blotting (C.T., W.J., S.P., S.-J.K., and P.H.B.S., unpublished data). Moreover, immunohistochemistry showed strong TGF-β expression in CFS, CMN, and secretory breast carcinoma primary tumors. Therefore, we hypothesized that EN transformation may be a useful model in which to study how TGF-β signaling may be regulated by dominantly acting oncoproteins in early stage tumors. Here, we show that EN suppresses TGF-β signaling by directly binding to the TβRII, thereby preventing it from recruiting the type I TGF-β receptor (TβRI) and activating downstream TGF-β effector cascades.
Materials and Methods
Cell Culture. NIH3T3, HaCaT, and 293T cells were grown in DMEM (GIBCO) supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin (PSG; Invitrogen).
Transfection and Reporter Assays. NIH3T3 cells were transiently transfected with 3TP-Lux (13), Smad-binding element (SBE) 4-luc (14), pAR3-luc (15), BRE-lux (16), and the internal control pCMV-β-gal in six-well plates by using Lipofectin (Invitrogen) according to the manufacturer's instructions. After 24-h transfection, cells were treated with 5 ng/ml TGF-β1, 50 ng/ml BMP-7, or 50 ng/ml activin for 24 h in medium. Luciferase activity was quantified by using the Enhanced Luciferase Assay Kit (BD Biosciences). Values were normalized with the β-gal activity. All assays were performed in triplicate and represented as mean (±SE) of three independent transfections.
Immunoblotting and Immunoprecipitation. 293T cells were used for the detection of protein–protein interaction in vivo. 293T cells were transiently transfected with the indicated plasmids. After 24-h transfection, cells were switched to 0.2% serum overnight. Cells were lysed in a buffer containing 25 mM Hepes (pH 7.5), 150 mM NaCl, 1% Triton X-100, 10% glycerol, 5 mM EDTA, and protease inhibitor mixture (Complete, Roche, Gipf-Oberfrick, Switzerland). Extracts were separated by SDS/PAGE followed by electrotransfer to poly(vinylidene difluoride) membranes and probed with polyclonal or monoclonal antisera, followed by horseradish peroxidase-conjugated anti-rabbit, anti-mouse IgG, respectively, and visualized by chemiluminescence according to the manufacturer's instructions (Pierce). For immunoprecipitation, the cell lysates were incubated with the appropriate antibody (Ab) for 1 h, followed by incubation with Gamma-bind beads (Amersham Pharmacia Biosciences) for 1 h at 4°C. Beads were washed four times with the buffer used for cell solubilization. Immune complexes then were eluted by boiling for 3 min in 2× Laemmli buffer (pH 6.8), and then extracts were analyzed by immunoblotting as described above.
RT-PCR Analysis and Generation of Stable Cell Lines. For details on RT-PCR and cell line generation, see Supporting Text, which is published as supporting information on the PNAS web site.
Results
EN Suppresses TGF-β Signaling. To examine whether EN modulates TGF-β signaling, NIH3T3 cells were infected with recombinant retroviruses carrying either EN full-length cDNA, EN-K380N kinase dead mutant in which lysine (K) 380 (5), the ATP-binding site, was substituted with asparagine (N), or empty MSCVpac vector as a control. Individual positive transformants were obtained by puromycin selection. Expression of the predicted 73- and 68-kDa EN or EN-K380N mutant protein doublet in transformed NIH3T3 cells was verified by Western blotting using the anti-TrkC (C-14) Ab (Fig. 1a) as described in ref. 6.
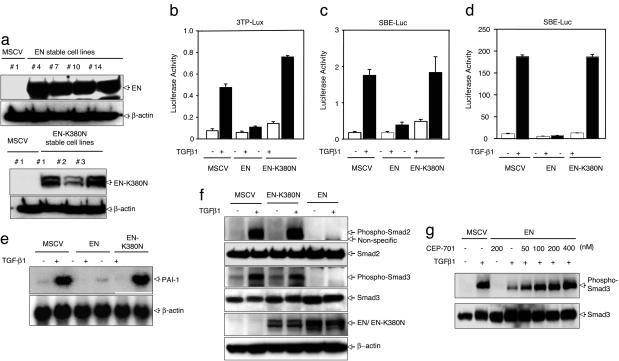
Fig. 1.
Expression of EN represses TGF-β-induced transcriptional activation. (a) To generate NIH3T3 cell lines stably expressing EN or EN–K380N, NIH3T3 cells were infected with retroviruses containing control vector alone (MSCV), EN, or EN-K380N. Stably expressing cell clones were selected, and successful expression of EN or EN-K380N was determined by using anti-TrkC (NTRK3) Ab. (b and c) TGF-β-responsive reporters 3TP-lux (b) or SBE4-luc (c) were transfected into NIH3T3 cell lines expressing EN or EN-K380N as well as control cell (MSCV). Luciferase activity was measured 24 h after TGF-β1 stimulation. (d) Either EN or EN–K380N was cotransfected into NIH3T3 cells with SBE4-luc, and luciferase activity was measured 24 h after TGF-β1 stimulation. (e) Suppression of TGF-β1-induced PAI-1 expression in EN-expressing NIH3T3 cells. Either EN or EN-K380N expressing NIH3T3 cells or vector control cells were incubated in the presence or absence of TGF-β1 for 18 h. Total RNA was isolated from these cell lines and analyzed by Northern blot using 32P-labeled PAI-1 probe. (f) EN suppresses TGF-β1-induced phosphorylation of Smad2 and Smad3. EN inhibits Smad2 and Smad3 phosphorylation induced by 30 min stimulation with TGF-β1(5 ng/ml). (g) EN expressing NIH3T3 cells or vector control cells were pretreated with CEP-701 for 2 h, and Smad3 phosphorylation was examined 30 min after stimulation with TGF-β1 (5 ng/ml). Phosphorylated Smad3 and total Smad3 were blotted with antiphosphorylated Smad3 Ab or anti-Smad3 Ab.
To test whether EN influences TGF-β1-induced transcriptional activation, we transfected these cells with the TGF-β1-responsive 3TP-lux reporter construct (13) or the SBE4-luc reporter construct, which contains four SBE sites in tandem (14). Expression of EN dramatically repressed the TGF-β1-dependent activities of these reporter gene constructs (Fig. 1 b and c), whereas EN-K380N mutant expression had no effect. We also assessed the effects on SBE4-luc activity by cotransfection of EN and SBE4-luc reporters in NIH3T3 cells and observed EN-specific suppression of TGF-β1-induced transcriptional activity of this reporter gene construct (Fig. 1d). Consistent with this suppressive activity of EN, we also observed a reproducible slight reduction of the basal, uninduced transcription level of TGF-β-responsive reporters (Fig. 1d). One of the critical cellular activities of TGF-β is the transcriptional activation of matrix-related genes including those encoding collagen, fibronectin, and the plasminogen activation inhibitor-1 (PAI-1). Because the introduction of EN suppresses TGF-β1-induced transcriptional activation, we speculated that stable expression of EN also may suppress the ability of these cells to up-regulate endogenous matrix genes in response to TGF-β1. Although activation of PAI-1 mRNA expression was markedly enhanced by TGF-β1 in control NIH3T3 cells [murine stem cell virus (MSCV)] induction of PAI-1 transcripts by TGF-β1 was markedly suppressed in EN-expressing NIH3T3 cells (Fig. 1e). This result was not seen in EN-K380N-expressing cells, where PAI-1 induction was similar to that of the control cells. These results suggest that EN can suppress TGF-β1-induced transcription in vivo.
EN Inhibits TGF-β1-Induced Smad2 or Smad3 Phosphorylation. The transcriptional activities of both Smad2 and Smad3 are dependent on their phosphorylation by activated TβRI (1–4). Therefore, we examined whether EN regulates TGF-β1-stimulated Smad2/3 phosphorylation. In comparison with control cells, EN expression significantly suppressed TGF-β1-stimulated endogenous Smad2/3 phosphorylation in NIH3T3 cells (Fig. 1f), whereas the kinase dead EN-K380N mutant completely lacked this suppressive activity. This result suggests that the EN suppressive activity is positioned upstream of Smad2 and Smad3 phosphorylation. We also examined the effect of CEP-701, an inhibitor of Trk (NTRK) tyrosine kinases, to further asses whether the NTRK3 kinase activity of EN is required for its ability to suppress TGF-β1-mediated Smad2/3 phosphorylation. CEP-701 is known to inhibit kinase activity of neurotrophin-specific trk receptors (17). As shown in Fig. 1g, TGF-β1-induced Smad3 phosphorylation was markedly reduced in NIH3T3 cells expressing EN, whereas pretreatment of CEP-701 restored Smad3 phosphoryation in a dose-dependent manner. CEP-701 treatment inhibited EN autophosphorylation, demonstrating that rescue of Smad phosphorylation correlates with inhibition of EN kinase activity (see Fig. 6, which is published as supporting information on the PNAS web site). This result indicates that the NTRK3 kinase activity of EN is required for suppression of TGF-β signaling, as suggested by the inability of the EN-K380N kinase dead mutant to suppress Smad2/3 phosphorylation by TGF-β1 (Fig. 1f). For more details, see Supporting Text.
EN Directly Interacts with the TβRII. Because our results suggested that the actions of EN are positioned upstream of Smad2 and Smad3 phosphorylation, we examined the possibility that EN may interact directly with the surface receptors for TGF-β in vivo. 293T cells were transfected with Flag-tagged Smad2, Smad3, or Myc-tagged Smad4, or hemagglutinin (HA)-tagged TβRI, TβRII, and EN in the presence or absence of TGF-β1 stimulation. Total cell extracts were immunoprecipitated with the anti-Flag, anti-Myc, or anti-HA Abs followed by immunoblotting with the anti-TrkC Ab. As shown in Fig. 2a, EN failed to interact with Smad2, Smad3, Smad4, or TβRI, whereas it interacted strongly with TβRII in a ligand-independent manner. The interaction between EN and TβRII also was confirmed by using HaCaT cells expressing EN (see Fig. 7e, which is published as supporting information on the PNAS web site). We next used V5-tagged EN to examine the interaction between EN and TβRII. After transfection of V5-tagged EN expression plasmid along with either HA-tagged TβRI or TβRII expression constructs into 293T cells, we performed immunoprecipitation experiments using anti-HA Abs followed by immunoblotting with the anti-V5 Ab, confirming the interaction between EN and TβRII (Fig. 2 b and c). The kinase dead EN-K380N mutant, which fails to autophosphorylate and completely lacks transformation activity (6), failed to interact with TβRII (Fig. 2c).
Fig. 2.
EN interacts with TβRII. (a) EN was cotransfected into 293T cells with the Flag-tagged Smad2, Smad3, Myc-tagged Smad4, HA-tagged TβRI, or HA-tagged TβRII constructs. Cells were treated with TGF-β1 for 1 h. Cell extracts were subjected to immunoprecipitation using anti-Flag, anti-Myc, or anti-HA Ab and Gamma-bind beads (Amersham Pharmacia Biosciences), followed by immunoblotting with anti-TrkC (NTRK3) Ab. The expression of TβRI, TβRII, or Smads was monitored as indicated. (b) V5-tagged EN was cotransfected into 293T cells with the HA-tagged TβRI or TβRII constructs. (c) TβRII does not associate with the kinase-dead EN–K380N mutant protein. HA–TβRII was cotransfected into 293T cells with the V5-tagged EN or Myc-tagged EN–K380N, a kinase-inactive EN mutant construct.
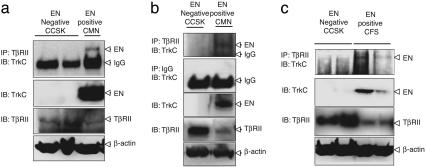
We next wished to confirm that the above findings are relevant to human tumors expressing EN. We therefore screened for the presence of EN-TβRII complexes in human primary tumors positive for the ETV6-NTRK3 gene fusion. These tumors included two CMN cases occurring in 5-month- and 5-week-old infants (Fig. 3 a and b, respectively) and two CFS cases occurring in 15- and 5-month-old infants (Fig. 3c). As controls, we tested two childhood human clear cell sarcomas of the kidney, because this EN fusion negative tumor type occurs in the same age range as CMN and CFS. Tumors were diagnosed by using standard histopathological criteria. All cases were screened for ETV6-NTRK3 fusion transcripts by using established methods (5), but only the CMN and CFS cases were fusion positive (data not shown). We screened 12 primary tumor samples (4 CFSs and 8 CMNs), but only 4 samples were of sufficient integrity for protein studies. Therefore, we examined the interaction between TβRII and EN using these four samples. Expression of TβRII was lower in EN-positive CMN and CFS cases compared with EN-negative human clear cell sarcoma of the kidney cases. This finding is consistent with our unpublished results (W.J., and S.-J.K., unpublished data) showing that EN expression also may suppress TβRII expression transcriptionally or increase its proteasomal degradation. Total tissue extracts were isolated from primary tumors and immunoprecipitated with the anti-TβRII Ab followed by immunoblotting with the anti-TrkC Ab. As shown in Fig. 3, EN-TβRII complexes were found in the CMN and CFS cases, but not in control tumor samples. These results clearly demonstrate that the interaction between EN and TβRII occurs in vivo in human primary cancer tissues expressing ETV6-NTRK3 gene fusions.
Fig. 3.
Identification of EN–TβRII complexes in human primary tumors. Primary tumor tissue extracts from EN negative clear cell sarcomas of the kidney (CCSK1 and -2), as well as EN-positive CMN (a and b) and CFS (c) were subjected to immunoprecipitation using anti-TβRII Ab or IgG as a control, followed by immunoblotting with anti-TrkC (NTRK3) Ab. Whole-tissue lysates from primary tumors also were probed for EN, TβRII, and β-actin as a loading control.
For the results of whether EN interacts with the activin and BMP type II receptors, see Supporting Text and Fig. 8, which is published on the PNAS web site.
EN Interaction Sequences of TβRII. Because the interaction between TβRII and EN potentially represents a previously undescribed mechanism of crosstalk between two distinct signaling pathways, we investigated how these proteins associate. To identify the region(s) of TβRII (18) that interact with EN, several truncated versions of TβRII were generated (Fig. 4a). To facilitate analysis of these mutants, all of the constructs contained an epitope tag of Flag or Myc at the N terminus. Transfection of these constructs into 293T cells confirmed that all of the mutant versions of TβRII were expressed, as evidenced by Western blot analysis (Fig. 4 b and c). Immunoprecipitation with anti-V5 demonstrated that the Flag-TβRII (1–567) and Flag-TβRII (1–400) coprecipitated with V5-EN, whereas Flag-TβRII (1–299) only weakly interacted with EN, and TβRII (1–219) showed no interaction with EN (Fig. 4 b and c). These results suggest that EN interacts with residues 219–400 of the intracellular domain of TβRII.
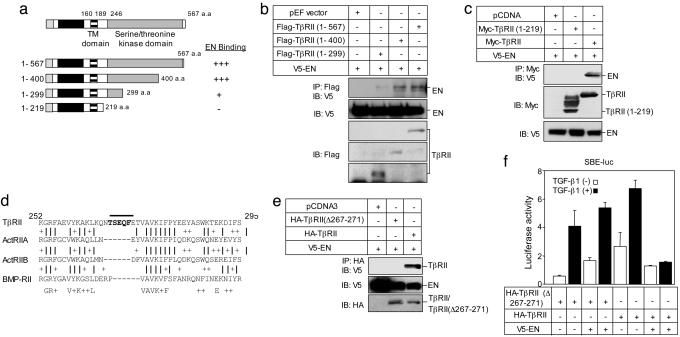
Fig. 4.
Immunoprecipitation analyses of overexpressed EN and its interaction with TβRII proteins in 293T cells. (a) Schematic representation of full-length and truncated TβRII proteins is shown in Upper.(b and c) Immunoprecipitation and immunoblot analyses of Flag-tagged TβRII proteins (b) or Myc-tagged TβRII protein (c) interacting with V5-tagged EN. (d) Comparison of the cytoplasmic segment (residues 244–295) of the TβRII with representative sequences of ActRIIA, ActRIIB, and BMPRII. (e) Identification of residues critical for EN/TβRII. TβRII (TβRII Δ267–271) mutant, which deleted residues 269–271, was generated and examined its ability to interact with EN in 293T cells. (f) EN does not suppress TβRIIΔ267–271-mediated transcriptional activity in 293T cells.
To better define the residues in the intracellular domain of TβRII responsible for EN binding, we compared residues 219–400 of TβRII with corresponding domains of activin R-IIA (ActRIIA), activin R-IIB (ActRIIB), and BMP-RII, related TGF-β type II receptors that do not interact with EN (see Fig. 8a). This comparison highlighted two short sequences, namely residues 234–240 and 267–271 of the TβRII intracellular domain, which are not present in equivalent domains of ActRIIA, ActRIIB, and BMP-RII (Fig. 4d). We focused on residues 267–271, which is localized within the kinase subdomain I of TβRII. We deleted residues 267–271 (TβRII Δ267–271) and tested the ability of this mutant to interact with EN. This mutant failed to interact with EN (Fig. 4e), suggesting that residues 267–271 in the intracellular domain of TβRII are critical for the EN/TβRII interaction. To confirm this hypothesis, we next examined the effect of EN on the transcriptional activity mediated by the non-EN-binding TβRIIΔ267–271 mutant in 293T cells. The TβRIIΔ267–271 mutant was able to confer TGF-β-inducible transcription of SBE, although with slightly lower activity than that of WT TβRII (Fig. 4f). However, EN failed to suppress TβRIIΔ267–271-mediated transcriptional activity (Fig. 4f). We also attempted to overexpress TβRIIΔ267–271 in several EN transformed cells including HaCaT and NIH3T3 cells, but unfortunately we could not generate stable lines expressing the TβRIIΔ267–271 construct. This finding suggests that expression of this mutant may have a negative effect on cell growth and is selected against in transformed cells. Therefore, we examined whether TβRIIΔ267–271 can reverse the suppressive effect of EN on TGF-β1 growth inhibitory activity in a transient transfection system. In HaCaT cells, expression of EN blocked the ability of TGF-β1 to inhibit DNA synthesis. However, transfection of TβRIIΔ267–271 restored TGF-β1-mediated inhibition of DNA synthesis in EN-expressing cells (see Fig. 9, which is published as supporting information on the PNAS web site). These findings not only provide further evidence that EN/TβRII complex formation is required for the inhibition of TGF-β signaling, but also suggest that blocking TGF-β1 signaling may be important for induction of DNA synthesis in EN-transformed cells.
EN/TβRII Interaction Blocks the Ability of TβRII to Recruit and Activate TβRI. Signaling from TGF-β receptors is initiated when the ligand induces the assembly of heteromeric complexes of the type II and I receptor Ser-Thr kinases at the plasma membrane (13). Within this receptor complex, the TβRII kinase phosphorylates the TβRI within a Ser/Thr-rich domain located immediately N-terminal to the kinase domain of the TβRI (the GS box) (13, 19). This phosphorylation results in activation of TβRI with subsequent initiation of downstream signaling pathways. Because EN interacts with TβRII and not TβRI, we hypothesized that EN may exert its effect by blocking the ability of TβRII to recruit and activate TβRI. To test whether EN/TβRII complex formation inhibits the ability of TβRII to phosphorylate TβRI, we immunoprecipitated endogenous TβRI in NIH3T3 cells stably expressing EN or EN-K380N after 30-min stimulation with TGF-β1. The immunoprecipitated type I receptor was immunoblotted with either anti-phosphoserine or anti-TβRI. Ser phosphorylation of TβRI was enhanced by TGF-β1 in control NIH3T3 cells and NIH3T3 cells stably expressing EN-K380N, whereas this phosphorylation was blocked in NIH3T3 cells stably expressing EN (Fig. 5a). This result suggests that EN can suppress Ser phosphorylation of TβRI. We next used a transient transfection system in NIH3T3 cells to further explore this finding. Flag-tagged TβRI was coexpressed along with HA-TβRII with or without V5-EN in the presence or absence of exogenous TGF-β1. The type I receptor was immunoprecipitated by using anti-Flag Ab, followed by immunoblotting with antiphosphoserine Abs. As shown in Fig. 5b, TGF-β1 treatment strongly induced Ser phosphorylation of TβRI, but this phosphorylation was markedly attenuated by coexpression of EN. These findings indicate that EN suppresses the ability of the TβRII kinase to transphosphorylate and hence activate TβRI.
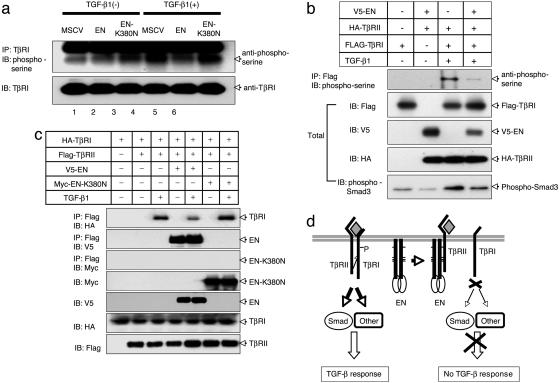
Fig. 5.
EN blocks TβRI and TβRII complex formation. (a) EN suppresses Ser phosphorylation of the TβRI in vivo. NIH3T3 cell lines stably expressing EN and EN–K380N as well as control NIH3T3 cells (MSCV) were treated with TGF-β1(5 ng/ml) for 30 min. Cell extracts were immunoprecipitated using anti-TβRI Ab and Gamma-bind beads (Amersham Pharmacia Biosciences), followed by immunoblotting with anti-phosphoserine Ab. (b) V5-tagged EN was cotransfected into 293T cells with both Flag–TβRI and HA–TβRII. Twenty-four hours after transfection, cells were treated with or without TGF-β1(5 ng/ml) for 30 min. Cell extracts were immunoprecipitated using anti-HA Ab and Gamma-bind beads, followed by immunoblotting with anti-phosphoserine Ab. The expression of EN, TβRI, and TβRII was monitored as indicated. (c) EN blocks TβRI and TβRII complex formation. Flag-tagged TβRII was cotransfected into 293T cells with HA-tagged TβRI along with or without V5-tagged EN or Myc-tagged EN-K380N. Twenty-four hours after transfection, cells were treated with or without TGF-β1(5ng/ml) for 30 min. Cell extracts were immunoprecipitated using anti-Flag Ab and Gamma-bind beads, followed by immunoblotting either with anti-V5 Ab (EN) or anti-HA Ab (TβRI). The expression of EN, EN–K380N, TβRI, and TβRII was monitored as indicated. (d) Model for repression of TGF-β signaling by the EN.
To examine whether EN suppresses the ability of TβRII kinase to activate TβRI by blocking TβRI–TβRII complex formation, Flag-TβRII was coexpressed with V5-EN along with HA-TβRI in 293T cells. Total cell extracts were immunoprecipitated with anti-Flag Ab followed by immunoblotting with anti-V5 Ab to detect V5-EN or anti-HA Ab to detect HA-TβRI. As expected, TβRII-associated TβRI was only observed after stimulation with TGF-β1 (compare lanes 2 and 3, Fig. 5c). However, coexpression of EN significantly reduced the level of TβRI associated with TβRII (compare lanes 3 and 5, Fig. 5c). These findings strongly indicate that EN inhibits TGF-β signaling through its interaction with TβRII, and this association inhibits TβRI–TβRII complex formation (Fig. 5d).
Discussion
There is great interest in understanding how oncogenic tyrosine kinases function in malignant transformation, because these proteins are attractive targets for therapeutic intervention (20). The EN chimeric product functions as a constitutively active protein-tyrosine kinase with potent transforming activity in multiple cell lineages (5). However, the mechanism of EN-mediated oncogenesis remains mostly unknown. In this work, we show that the EN chimeric oncoprotein suppresses TGF-β signaling. EN specifically interacts with TβRII and blocks its ability to activate downstream signaling, including phosphorylation of Smad2 and Smad3 by TβRI. EN–TβRII complexes were also present in human primary cancers expressing the EN gene fusion. Our results indicate a previously undescribed mechanism for inhibition of TGF-β-mediated growth suppression in tumor cells in which EN directly binds to TβRII and blocks its ability to recruit, phosphorylate, and activate TβRI (Fig. 5d). These findings provide evidence for direct crosstalk between two distinct kinase-signaling cascades.
EN transformation requires an intact PTK domain and a functional sterile α motif oligomerization domain (6), and recent studies show that sterile α motif domain-mediated higher-order polymer formation is necessary for sustained PTK activation (21). EN constitutively activates the Ras–MAPK and PI3K–Akt pathways, and both are essential for EN transformation (4). EN expression is associated with constitutive Tyr phosphorylation of insulin receptor substrate-1 (IRS-1), which functions as the adaptor protein linking EN to Ras–MAPK and PI3K–Akt cascades (22, 23). Recently, we demonstrated that EN specifically binds the phospho-Tyr-binding domain of IRS-1 via an interaction at the C terminus of EN, and that this binding is essential for Ras–MAPK and PI3K–Akt activation (23). In the present work, we show that the ability of EN to interact with TβRII requires EN PTK activity because a kinase-dead form of EN fails to bind TβRII and the CEP-701 NTRK inhibitor blocks EN inhibitory effects on TGF-β signaling. However, a kinase-active but nontransforming EN mutant lacking the C-terminal 19 aa essential for IRS-1 binding (23) still interacts with TβRII (data not shown). This finding indicates that the inhibitory effect of EN on TGF-β signaling is most likely distinct from its ability to activate Ras–MAPK and PI3K–Akt. Therefore, EN transformation seems complex, involving not only activation of cell proliferation and survival pathways but also inactivation of cascades such as the TGF-β tumor suppressor pathway. Whether the latter is necessary to block potential proapoptotic functions of TGF-β or whether TGF-β signaling has other antineoplastic roles remains to be determined. Our preliminary results suggest that expression of the non-EN-interacting TβRIIΔ267–271 mutant in EN-positive cells restored TGF-β1-mediated inhibition of DNA synthesis in EN-expressing cells (Fig. 9). These findings suggest that inhibition of TGF-β1 signaling may be important for induction of cell growth in EN-transformed cells. Further studies are needed to rigorously test this possibility and elucidate possible mechanisms.
Although EN PTK activity is required for EN/TβRII complex formation and inhibition of TGF-β signaling, its role in these processes remains unclear. We have observed that TβRII becomes Tyr-phosphorylated by EN (data not shown), but the significance of this phosphorylation requires further evaluation. It is possible that Tyr phosphorylation of TβRII alters its affinity for the TβRI or, alternatively, that Tyr phosphorylation of EN itself changes its conformation such that it can interact with TβRII.
There is remarkable specificity in the regulation of the TGF-β family of type II receptors by EN. This oncoprotein does not interact with activin or BMP type II receptors, but only with TβRII. This interaction appears to be mediated at least in part by a five-amino acid (TSEQF) sequence within the intracellular domain of TβRII, which is not present in these related TGF-β family type II receptors. Because the NTRK3 portion of EN is necessary for its association with TβRII, it is very likely that TβRII also binds to WT NTRK3 (TrkC) (24). The NTRK family of neurotrophin receptors mediates neuronal cell survival and differentiation, but altered NTRK signaling has also been implicated in oncogenesis. TrkC is frequently overexpressed in human cancers including pancreatic and prostate carcinoma, neuroblastoma, and medulloblastoma (25, 26). Moreover, recent mutational analysis of the Tyr kinome in colorectal cancers has shown somatic mutations within the kinase domain of TrkC (27). Overexpression of TrkC confers constitutive activation of its tyrosine kinase activity to induce continuous proliferation of the cell. There is to date no report of crosstalk between Trk receptor tyrosine kinase and TGF-β receptor Ser-Thr kinases, but to our knowledge this topic has not been explored in detail. The biological significance of the potential crosstalk between Trk receptor signaling and TGF-β signaling therefore remains to be determined but may demonstrate novel roles for TGF-β signaling in neuronal cell survival and differentiation.
In summary, we have identified that the EN chimeric oncoprotein suppresses TGF-β signaling specifically through the interaction with TβRII and inhibition of TβRI–TβRII complex formation. The identification of TβRII as a potential target for suppression by EN suggests that the loss of TGF-β signaling may be an important step in tumorigenesis by the EN chimeric tyrosine kinase.
Supplementary Material
Acknowledgments
We thank A. Roberts, K. Baik, and A. Hobbie for the critical reading of the manuscript; J. Massague (Memorial Sloan–Kettering Cancer Center, New York) and S. Kern (Johns Hopkins University, Baltimore) for TGF-β reporter plasmids; and Bruce Ruggeri and Susan Jones-Bolin of Cephalon (West Chester, PA) for providing CEP-701. Tissue samples were provided by the Children's Oncology Group, which is funded by the National Cancer Institute. This work was supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. W.J. was supported by funds from National Cancer Institute Director's Challenge Grant U01 CA88199: Toward a Molecular Classification (to T.J.T. and P.H.B.S.). The work was also funded in part by a Canadian Institutes for Health Research grant (to P.H.B.S.).
Author contributions: P.H.B.S. and S.-J.K. designed research, analyzed data, and wrote the paper; W.J., B.-C.K., C.T., H.-J.L., and S.P. performed research; and C.L.L., J.M.M., and T.J.T. contributed new reagents/analytic tools.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ActRIIA, activin R-IIA; ActRIIB, activin R-IIB; CFS, congenital fibrosarcoma; CMN, cellular mesoblastic nephroma; EN, ETV6-NTRK3; HA, hemagglutinin; MAPK, mitogen-activated protein kinase; MSCV, murine stem cell virus; PAI-1, plasminogen activation inhibitor-1; PI3K, phosphatidyl inositol-3-kinase; PTK, protein tyrosine kinase; SBE, Smad-binding element; TβRI, type I TGF-β receptor; TβRII, type II TGF-β receptor.
References
- 1.de Caestecker, M. P., Piek, E. & Roberts, A. B. (2000) J. Natl. Cancer Inst. 92, 1388-1402. [DOI] [PubMed] [Google Scholar]
- 2.Derynck, R. & Zhang, Y. E. (2003) Nature 425, 577-584. [DOI] [PubMed] [Google Scholar]
- 3.Kim, S.-J. & Letterio, J. (2003) Leukemia 17, 1731-1737. [DOI] [PubMed] [Google Scholar]
- 4.Shi, Y. & Massague, J. (2003) Cell 113, 685-700. [DOI] [PubMed] [Google Scholar]
- 5.Knezevich, S. R., McFadden, D. E., Tao, W., Lim, J. F. & Sorensen, P. H. (1998) Nat. Genet. 18, 184-187. [DOI] [PubMed] [Google Scholar]
- 6.Wai, D. H., Knezevich, S. R., Lucas, T., Jansen, B., Kay, R. J. & Sorensen, P. H. B. (2000) Oncogene 19, 906-915. [DOI] [PubMed] [Google Scholar]
- 7.Liu, Q., Schwaller, J., Kutok, J., Cain, D., Aster, J. C., Williams, I. R. & Gilliland, D. G. (2000) EMBO J. 19, 1827-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tognon, C., Garnett, M., Kenward, E., Kay, R., Morrison, K. & Sorensen, P. H. (2001) Cancer Res. 61, 8909-8916. [PubMed] [Google Scholar]
- 9.Knezevich, S. R., Garnett, M. J., Pysher, T. J., Beckwith, J. B., Grundy, P. E. & Sorensen, P. H. (1998) Cancer Res. 58, 5046-5048. [PubMed] [Google Scholar]
- 10.Rubin, B. P., Chen, C. J., Morgan, T. W., Xiao, S., Grier, H. E., Kozakewich, H. P., Perez-Atayde, A. R. & Fletcher, J. A. (1998) Am. J. Pathol. 153, 1451-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eguchi, M., Eguchi-Ishimae, M., Tojo, A., Morishita, K., Suzuki, K., Sato, Y., Kudoh, S., Tanaka, K., Setoyama, M., Nagamura, F., et al. (1999) Blood 93, 1355-1363. [PubMed] [Google Scholar]
- 12.Tognon, C., Knezevich, S. R., Huntsman, D., Roskelley, C. D., Melnyk, N., Mathers, J. A., Becker, L., Carneiro, F., MacPherson, N., Horsman, D., et al. (2002) Cancer Cell 2, 367-376. [DOI] [PubMed] [Google Scholar]
- 13.Wrana, J. L., Attisano, L., Carcamo, J., Zentella, A., Doody, J., Laiho, M., Wang, X.-F. & Massagué, J. (1992) Cell 71, 1003-1014. [DOI] [PubMed] [Google Scholar]
- 14.Zawel, L., Dai, J. L., Buckhaults, P., Zhou, S., Kinzler, K. W., Vogelstein, B. & Kern, S. E. (1998) Mol. Cell 1, 611-617. [DOI] [PubMed] [Google Scholar]
- 15.Labbé, E., Silvestri, C., Hoodless, P. A., Wrana, J. L. & Attisano, L. (1998) Mol. Cell 2, 109-120. [DOI] [PubMed] [Google Scholar]
- 16.Jonk, L. J., Itoh, S., Heldin, C. H., ten Dijke, P. & Kruijer, W. (1998) J. Biol. Chem. 273, 21145-21152. [DOI] [PubMed] [Google Scholar]
- 17.Miknyoczki, S. J., Chang, H., Klein-Szanto, A., Dionne, C. A. & Ruggeri, B. A. (1999) Clin. Cancer Res. 5, 2205-2212. [PubMed] [Google Scholar]
- 18.Lin, H. Y., Wang, X.-F., Ng-Eaton, E., Weinberg, R. A. & Lodish, H. F. (1992) Cell 68, 775-785. [DOI] [PubMed] [Google Scholar]
- 19.Wieser, R., Wrana, J. L. & Massague, J. (1995) EMBO J. 14, 2199-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shawver, L. K., Slamon, D. & Ullrich, A. (2002) Cancer Cell 1, 117-123. [DOI] [PubMed] [Google Scholar]
- 21.Tognon, C. E., Mackereth, C. D., Somasiri, A. M., McIntosh, L. P. & Sorensen, P. H. (2004) Mol. Cell Biol. 24, 4636-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison, K. B., Tognon, C. E., Garnett, M. J., Deal, C. & Sorensen, P. H. (2002) Oncogene 21, 5684-5695. [DOI] [PubMed] [Google Scholar]
- 23.Lannon, C. L., Martin, M. J., Tognon, C. E., Jin, W., Kim, S.-J. & Sorensen, P. H. (2004) J. Biol. Chem. 279, 6225-6234. [DOI] [PubMed] [Google Scholar]
- 24.Lee, F. S., Rajagopal, R. & Chao, M. V. (2002) Cytokine Growth Factor Rev. 13, 11-17. [DOI] [PubMed] [Google Scholar]
- 25.Guate, J. L., Fernandez, N., Lanzas, J. M., Escaf, S. & Vega, J. A. (1999) BJU Int. 84, 495-502. [DOI] [PubMed] [Google Scholar]
- 26.Segal, R. A., Goumnerova, L. C., Kwon, Y. K., Stiles, C. D. & Pomeroy, S. L. (1994) Proc. Natl. Acad. Sci. USA 91, 12867-12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bardelli, A., Parsons, D. W., Silliman, N., Ptak, J., Szabo, S., Saha, S., Markowitz, S., Willson, J. K., Parmigiani, G., Kinzler, K. W., et al. (2003) Science 300, 949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.