Abstract
Priming with heat-killed Propionibacterium acnes enhances the sensitivity of mice to lipopolysaccharide (LPS) and other biologically active bacterial components. We show that P. acnes priming has protective and deleterious effects on a subsequent serovar Typhimurium infection. It may result in a complete protection or prolonged survival, or it may accelerate mortality of the infected mice, depending on the number of serovar Typhimurium bacteria administered and on the degree of LPS hypersensitivity at the time of infection. Both effects of P. acnes-induced hypersensitivity are mediated by gamma interferon (IFN-γ) and are based on a differential activation of the innate immune mechanisms which recognize and react against the LPS present in infecting bacteria. In P. acnes-primed mice null for LPS-binding protein (LBP−/− mice), the impaired LPS recognition, due to the absence of LBP, resulted in a higher resistance to serovar Typhimurium infection. A similar P. acnes priming of mice had a protective, but no deleterious effect on a subsequent L. monocytogenes infection. This effect was IFN-γ dependent but independent of LBP.
Gram-negative and gram-positive bacteria contain a number of highly conserved components, which are recognized by the innate immune system (28). This recognition and a subsequent activation of the innate immune mechanisms are prerequisites for a successful antimicrobial defense. Lipopolysaccharide (LPS), a major constituent of the outer membrane of gram-negative bacteria, is a primary recognition marker in these microorganisms (21). Experiments with animals and human volunteers have demonstrated that LPS elicits numerous beneficial and harmful biological activities in the host, including those responsible for the clinical manifestations of infection. The latter are primarily the result of LPS-induced endogenous mediators, such as proinflammatory cytokines (22). Of these cytokines, tumor necrosis factor alpha (TNF-α), produced by monocytes/macrophages, is a primary mediator of LPS effects (32, 44). The activity of LPS is expressed optimally in the presence of LPS-binding protein (LBP), an acute-phase protein which catalyzes the transfer of LPS to other acceptors, including the LPS receptor complex on target cells (41). Membrane CD14 is the principal recognition site for LPS in the receptor complex (20, 45), but due to the lack of a transmembrane domain, it is unable to transduce LPS signaling. The transduction of the LPS signal across the cell membrane is a function of the Toll-like-receptor 4 (TLR4) (4, 5), which in association with the adapter protein MD-2 also participates in the recognition of LPS (2). Importantly, all known LPS nonresponder mice strains were shown to carry natural mutations in theTlr4 gene (3, 35, 36).
Susceptibility to LPS varies considerably in different animals and is characteristic for a given mammalian species. The reason for these differences is unknown. Furthermore, animal susceptibility to LPS may be increased under a variety of experimental conditions (12, 13). These include prior treatment with microorganisms such as protozoa, viruses, and bacteria (6, 29, 33, 40). Sensitization may arise from infection with gram-negative or gram-positive bacteria, or following administration of appropriate amounts of the respective killed microorganisms. Importantly, also latent, subclinical infections may induce a strong LPS hypersensitivity. After an initial microbial treatment, a state of enhanced LPS sensitivity becomes detectable 2 to 3 days later, reaches maximum levels after 7 to 14 days, and gradually disappears as the sensitizing agent is eliminated from host. Upon LPS challenge, hypersensitive mice produce levels of proinflammatory cytokines such as TNF-α, interleukin 1, or gamma interferon (IFN-γ) up to 1,000 times higher than those produced by mice that are not hypersensitive, and these mice are highly susceptible to the lethal effects of LPS. Furthermore, they become hypersensitive to the lethal and other effects of endogenously produced or exogenously administered TNF-α (17).
Of the different type of bacteria known to induce LPS hypersensitivity, killed Propionibacterium acnes is well-established model and has been used in this study. As with other microorganisms, the induction of hypersensitivity to LPS and TNF-α by P. acnes is mediated by IFN-γ and does not proceed in mice with an impaired IFN-γ production or function (8, 9, 26). CD14 and TLR4 are not involved in the actual process of P. acnes sensitization (31). The presence of LBP is required for the induction of the LPS enhanced effects (7).
In addition to LPS, other bacterial components, including lipopeptide, peptidoglycan, lipoteichoic acid and DNA, have been shown to be recognition targets of the innate immune system and potential inducers of inflammation. The recognition and signal transduction by these components is a function of members of the toll receptor family, distinct from TLR4 (28). It has been shown that the IFN-γ-dependent sensitization to bacterial lipopeptides may also occur during infection. IFN-γ-dependent hypersensitivity to macrophage-activating lipopeptide 2, which is a ligand for TLR2 in combination with TLR6 (43), has been detected in P. acnes-primed mice (14). It is therefore obvious that hypersensitivity affects at least two signaling pathways, TLR4- and TLR2/TLR6-dependent. Furthermore, P. acnes-primed mice exhibit TNF-α hyper-responses to numerous gram-negative bacteria and to gram-positive Listeria monocytogenes, but not to Staphylococcus aureus, P. acnes, or Streptococcus thermophilus (30).
The vital role of LPS recognition in the resistance to gram-negative bacteria has been documented in the past. It has been shown that LPS nonresponder mouse strains are significantly more susceptible to either serovar Typhimurium (9, 11) or encapsulated Escherichia coli (17) infections than are closely related strains of LPS responder mice. In addition, LBP−/− mice were shown to be more susceptible to intraperitoneal infection with serovar Typhimurium than are their LBP+/− littermates (25). This was attributed to an impaired susceptibility to low LPS doses in the absence of LBP and is in agreement with the concept that the recognition of gram-negative bacteria by the innate immune system is the result of its interaction with LPS present in these microorganisms.
During their usual life span, animals and humans are confronted with the normal microbial flora in the gut and also with pathogenic microorganisms. It may, therefore, be safely assumed that susceptibility to LPS and other bacterial components is under constant fluctuation. Therefore, the question arises as to whether alterations in susceptibility to bacterial components may also affect susceptibility to bacterial infections. To address this question, we have studied the effects of prior sensitization with heat-killed P. acnes (26) on subsequent infection with serovar Typhimurium or L. monocytogenes. We demonstrated that sensitization may have a positive or negative effect on the serovar Typhimurium infection, depending on the size of the infective dose and on the degree of sensitization to LPS. By contrast, only positive effects of sensitization, i.e., higher resistance to infection, were observed in mice infected with L. monocytogenes. The above-mentioned effects of P. acnes priming were caused by IFN-γ-dependent sensitization to components present in the two pathogens.
MATERIALS AND METHODS
Animals.
129Sv/Pas, LBP−/− mice (backcrossed for six generations into a 129Sv/Pas background) and mice null for the IFN-γ receptor (IFN-γR−/− mice) (129Sv/Ev) were bred under specific-pathogen-free conditions in the animal facilities of our institute. Mice of both sexes were used at an age of 6 to 12 weeks.
Materials.
The uniform triethylamine salt of S. enterica serovar Abortus equi LPS used in the present study was prepared as described earlier (15). Murine recombinant LBP (18) was kindly provided by B. Jack and C. Schütt, Ernst-Moritz-Arndt University, Greifswald, Germany. For injection, recombinant LBP was diluted in pyrogen-free serum of LBP−/− mice. Alternatively, as a source of LBP, serum of P. acnes-primed BALB/c mice was used. It contained 4.04 μg of LBP/ml according to a specific enzyme-linked immunosorbent assay (25) and is referred to in this study as LBP serum.
Preparation of live bacteria and infection of mice.
A highly virulent strain of serovar Typhimurium C5 was grown overnight at 37°C on a Luria-Bertani (LB) agar medium (Difco Laboratories, Detroit, Mich.). Bacteria from a single colony were suspended in phosphate-buffered saline (PBS), pH 7.2, and the desired concentration was adjusted turbidometrically. The exact numbers of bacteria were determined by plating the bacterial suspension on LB agar plates and counting the CFU after overnight culture. Virulent L. monocytogenes (EGD strain) was grown in tryptose soy broth (Difco). Aliquots of log-phase growing cultures (4 × 108 CFU/ml) were stored at −80°C until use. For each experiment, a vial was thawed and washed once with saline, and its contents were diluted to the desired concentration in endotoxin-free PBS before injection. The exact numbers of injected bacteria were determined by plating the bacterial suspension on Trypticase soy agar plates and counting the CFU after overnight culture. For infection, different doses of bacteria were administered intravenously (i.v.) in 0.2 ml of PBS into the lateral tail vein.
Preparation of killed bacteria.
Heat-killed P. acnes was prepared as described earlier (26). Overnight cultures of serovar Typhimurium C5 and L. monocytogenes were washed with PBS, adjusted to 5 × 1010 and 1 × 1010 CFU/ml, respectively, and heat killed at 60°C for 1 h. They were stored in aliquots at −80°C until use. The total LPS content of serovar Typhimurium was estimated as already described (10). The Limulus amebocyte lysate test, carried out according to the instructions of the manufacturer (Pyrotell), revealed no detectable amounts of LPS in the L. monocytogenes preparation. All killed bacteria were suspended in pyrogen-free PBS and 0.2 ml of this suspension/25 g of body was weight administered intravenously into the lateral tail vein.
P. acnes sensitization of mice and lethality tests.
If not otherwise stated, mice received P. acnes (25 μg per g) in 0.2 ml of PBS/25 g i.v. Seven days later, groups of untreated (control) and P. acnes-sensitized mice received different amounts of killed or live bacteria (serovar Typhimurium or L. monocytogenes) or LPS, i.v. Lethality was recorded during an observation period of several weeks.
Determination of viable bacteria in mice infected with serovar Typhimurium.
Infected mice were exsanguinated under isoflurane anesthesia, and the liver of each mouse was removed and homogenized in PBS containing 1% saponin. Serial 10-fold dilutions of liver homogenate were transferred to LB agar plates, and bacterial colonies were counted after incubation at 37°C for 16 h.
TNF-α induction.
P. acnes-sensitized and control mice received an i.v. injection of LPS or heat-killed bacteria. One hour after challenge the animals were exsanguinated under ether anesthesia. The heparinized blood was centrifuged at 4°C, and the resulting plasma was aliquoted and stored at −80°C.
TNF-α bioassay.
TNF-α in plasma was measured in a cytotoxicity test using a TNF-sensitive L929 cell line in the presence of actinomycin D as described previously (1). The detection limit of the assay was 32 pg of TNF-α/ml of plasma. Rabbit anti-mouse TNF-α (Genzyme, Boston, Mass.) was used as an inhibitor to test the specificity of the assay.
RESULTS
The effect of P. acnes pretreatment on the susceptibility of mice to serovar Typhimurium infection is dependent on the size of the infective dose.
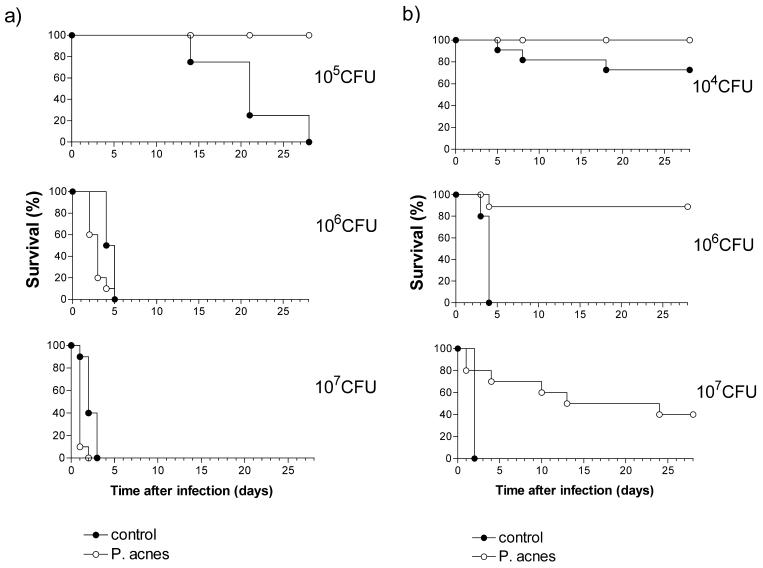
Groups of P. acnes-sensitized 129Sv/Pas mice and unsensitized controls received graded doses of serovar Typhimurium C5 (102 to 107 CFU), and the outcome of infection was monitored over a period of 28 days. All control and sensitized mice survived the infective doses up to 104 CFU (data not shown). As shown in Fig. 1a, further increases of the infective doses caused lethality in the control group. Only 60% of control mice survived doses of 105 CFU, and no mice survived doses of 106 or 107 CFU. There was an obvious correlation between the size of the infective dose and the kinetics of lethality.
FIG. 1.
Time course of lethality in control and P. acnes-sensitized 129Sv/Pas (a) and LBP−/− 129Sv/Pas (b) mice, infected with different doses of serovar Typhimurium. Mice received killed P. acnes (25 μg/g) administered i.v. or remained untreated (controls). One week later groups of 10 to 20 animals were infected with different amounts (104, 105, 106, and 107 CFU) of serovar Typhimurium i.v. Lethality was recorded daily over a period of 28 days.
Prior sensitization with P. acnes had both positive and negative influences on the outcome of infection depending on the magnitude of the serovar Typhimurium inoculum used (Fig. 1a). Thus, in mice infected with 105 CFU, the sensitization was completely protective. In contrast, the sensitization enhanced the infection lethality in the groups of animals infected with 106 CFU or more. This was particularly evident in the group infected with 107 CFU, in which P. acnes-sensitized mice became sick within 1 to 2 h after infection and 90% of the animals died during the first 24 h (Fig. 1a). Similar toxic effects were observed when instead of live bacteria, equivalent numbers of heat-killed bacteria were used. Administration of 106 or 107 CFU of heat-killed serovar Typhimurium to P. acnes-treated mice induced a rapid mortality of 40 and 100%, respectively, while all unsensitized control mice survived the injection of up to 108 CFU killed serovar Typhimurium without any visible symptoms of illness.
The effect of P. acnes pretreatment on the susceptibility of mice to serovar Typhimurium infection is dependent on the degree of LPS hypersensitivity.
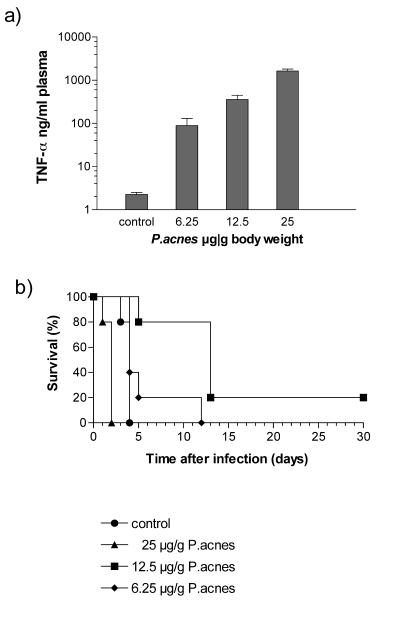
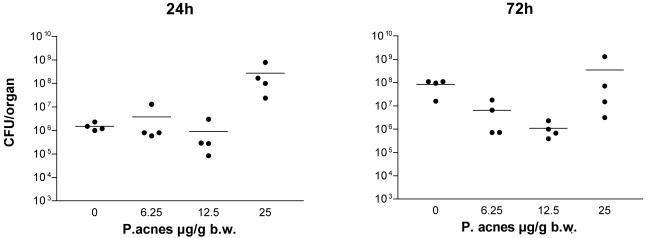
In the above experiment we demonstrated that the number of invading serovar Typhimurium bacteria determines whether the effects of LPS sensitization in mice are protective or deleterious. We next investigated how far the level of sensitization influences the final outcome of a serovar Typhimurium infection. As shown in Fig. 2a, mice were sensitized to a different degree (measured by the magnitude of the LPS-induced TNF-α response) by priming with graded doses of P. acnes (6.25, 12.5, and 25 μg/g). The sensitivity of such mice to serovar Typhimurium is shown in Fig. 2b. Priming mice with the highest, standard dose of P. acnes (25 μg/g) enhanced infection lethality, with the treated mice dying faster than the controls. Priming mice with the medium dose (12.5 μg/g) had the opposite effect. This dose significantly prolonged survival, and 20% of the animals survived the otherwise lethal infection. The lowest dose of P. acnes (6.25 μg/g) caused only a marginal prolongation of survival (Fig. 2b). Priming with graded doses of P. acnes also had a differential effect on the numbers of bacteria detectable in the liver of serovar Typhimurium-infected (2 × 106 CFU) animals. Thus, the group of mice primed with the highest dose of P. acnes exhibited the highest number of bacteria in the liver, which was particularly apparent 24 h after infection. In contrast, a protective effect was observed in two groups, primed with lower amounts of P. acnes, in which, compared to the unprimed controls, the number of bacteria in the liver did not increase between 24 and 72 h (Fig. 3). Thus, the effect of prior sensitization on the outcome of a serovar Typhimurium infection was dependent not only on the number of invading bacteria but also on the degree of LPS hypersensitivity of the animals.
FIG. 2.
Effect of sensitization induced by different doses of P. acnes on the TNF-α response to LPS (a) and the outcome of serovar Typhimurium infection in 129Sv/Pas mice (b). (a) For sensitization mice received killed P. acnes (6.25, 12.5, or 25 μg/g) administered i.v. or remained untreated (controls). One week later groups of 5 to 10 animals were challenge with 1 μg of LPS i.v., and the level of TNF-α in plasma collected 1 h after challenge was determined as described in Materials and Methods. Bars indicate standard deviations. (b) Alternatively similar groups of sensitized mice and controls were infected with 107 CFU of serovar Typhimurium i.v., and lethality was recorded daily over a period of 30 days.
FIG. 3.
Effect of P. acnes sensitization on the growth of serovar Typhimurium in livers of infected 129Sv/Pas mice. For sensitization mice received killed P. acnes (6.25, 12.5, or 25 μg/g) administered i.v. or remained untreated (controls). One week later groups of four animals were infected with 106 CFU of serovar Typhimurium and killed after 24 or 72 h of infection. Numbers of serovar Typhimurium organisms in the livers of infected mice were determined as described in Materials and Methods.
The effect of P. acnes sensitization on the susceptibility to infection is IFN-γ dependent.
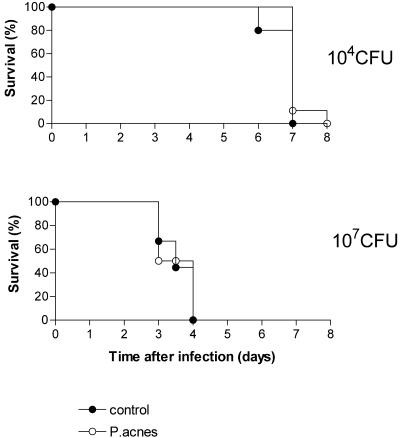
IFN-γ is an important cytokine of the antimicrobial defense (19, 23) and the key mediator of sensitization to LPS by P. acnes (26). To determine if P. acnes-induced effects on the outcome of infection are related to P. acnes-enhanced LPS sensitivity, we investigated whether these effects are IFN-γ dependent. For this purpose, we infected untreated and P. acnes-treated IFN-γR−/− mice with a low (104 CFU) and a high (107 CFU) dose of serovar Typhimurium and monitored mortality up to day 28 after infection. The results confirm the important role of IFN-γ in the defense against serovar Typhimurium (19) since all untreated IFN-γR−/− mice, including those infected with an otherwise nonlethal dose (104 CFU), died (Fig. 4). Moreover in the absence of IFN-γ function, the lethality curves of untreated and pretreated knockout animals in both experimental groups were essentially identical (Fig. 4). We therefore conclude that P. acnes-induced IFN-γ and subsequent LPS sensitization are responsible for both the beneficial and the deleterious effects in infected mice.
FIG. 4.
Time course of lethality in control and P. acnes-sensitized IFN-γR−/− mice infected with serovar Typhimurium. Mice received killed P. acnes (25 μg/g) administered i.v. or remained untreated (controls). One week later groups of 5 to 10 animals were infected with 104 or 107CFU of serovar Typhimurium i.v., and lethality was recorded daily.
Effect of P. acnes treatment on the susceptibility to LPS and to serovar Typhimurium infection in mice lacking LBP.
LBP facilitates LPS responses and plays a protective role in murine salmonellosis (25). In this study, we investigated the role of LBP in the resistance to serovar Typhimurium infection in LPS hypersensitive mice. Groups of P. acnes-treated LBP−/− mice and unsensitized controls were infected with different numbers (104 to 107 CFU) of serovar Typhimurium bacteria, and the animals were monitored for a period of 28 days. In agreement with previous findings (25), the unsensitized LBP−/− mice were more susceptible to the infection than the respective wild-type controls. Of the knockout mice, 27% succumbed to the otherwise-nonlethal dose of 104 CFU (Fig. 1b), and those infected with either 106 or 107 CFU died faster than wild-type mice (Fig. 1). Surprisingly, however, after sensitization with P. acnes, LBP−/− mice were more resistant to serovar Typhimurium infection than wild-type mice. Just as with the latter, all sensitized LBP−/− mice survived the 105 CFU dose (not shown). In addition, knockout mice showed a survival rate of 90% with 106 CFU and 40% with 107 CFU (Fig. 1b), doses that were 100% lethal for sensitized wild-type mice. Furthermore, a significant prolongation of survival was noted in LBP−/− mice ultimately succumbing to infection.
Effect of the absence of LBP on the susceptibility of P. acnes-treated mice to LPS and to whole serovar Typhimurium.
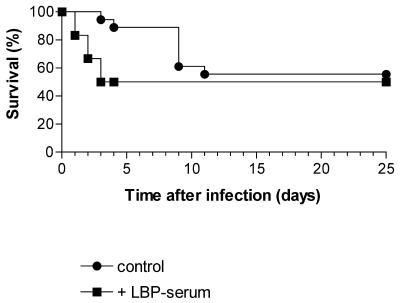
Enhanced LPS toxicity is the most probable reason for the accelerated deaths of P. acnes-primed wild-type mice infected with more than 105 CFU of serovar Typhimurium. We hypothesized that this toxicity might be largely absent in LBP−/− mice. This would explain the unusually high resistance of sensitized LBP−/− mice to serovar Typhimurium infection. We therefore compared the susceptibility of P. acnes-treated LBP−/− and wild-type mice to the toxic effects of LPS and whole heat-killed serovar Typhimurium. As shown in Table 1, P. acnes-treated LBP−/− mice were approximately 300 times less susceptible to the toxic effects of LPS than similarly treated wild-type mice. In comparison, the 50% lethal dose of LPS for unsensitized control mice of both types did not differ and was approximately 500 μg/mouse. A very similar result was also obtained when instead of LPS, killed serovar Typhimurium was administered. P. acnes-treated LBP−/− mice showed no symptoms of illness and exhibited no lethality after administration of high amounts of serovar Typhimurium (107 and 108 CFU), while P. acnes-treated wild-type mice succumbed to the lethal effect of as little as 106 killed bacteria (Table 1). In comparison, 108 CFU of bacteria was nontoxic in both types of unsensitized mice. The above results support the idea that during infection with serovar Typhimurium, the sensitized LBP−/− mice profit from a lower susceptibility to the enhanced toxic effects of LPS. In agreement, administration of LBP-containing serum or recombinant LBP (1 μg/mouse) to P. acnes-treated LBP−/− mice at the time of infection (107 CFU) abolished the protective effect of pretreatment (Fig. 5).
TABLE 1.
Susceptibility of P. acnes-sensitized LBP−/− and wild-type mice to LPS or killed S. enterica serovar Typhimurium
| Inoculum and size | Lethality for mice (no. dead/total no. inoculated)
|
|
|---|---|---|
| LBP−/− | Wild type | |
| LPS of serovar Abortus equi (μg/g) | ||
| 0.001 | 0/5 | |
| 0.01 | 6/6 | |
| 1 | 0/5 | |
| 3 | 2/9 | |
| 10 | 6/6 | |
| Heat-killed serovar Typhimurium (CFU) | ||
| 3 × 106 | 3/7 | |
| 1 × 107 | 0/4 | 4/4 |
| 1 × 108 | 0/6 | |
| 1 × 109 | 4/4 | |
FIG. 5.
Deleterious effect of exogenously administered LBP on the outcome of serovar Typhimurium infection in P. acnes-sensitized LBP−/− mice. Two groups (six mice each) of P. acnes-pretreated LBP−/− (129Sv/Pas) mice received 107 CFU of serovar Typhimurium administered in 0.1 ml of PBS. One group received additionally LBP (0.8 μg of LBP/0.2 ml of serum), and a second group served as the control. Lethality in the two groups was recorded daily over a period of 25 days.
Effect of P. acnes treatment on the susceptibility to L. monocytogenes infection in LBP−/−, IFN-γR−/−, and wild-type mice.
We recently showed that priming of mice with P. acnes enhances responses not only to LPS but also to other bacterial components, such as macrophage-activating lipopeptide 2 or to a not-yet-identified component of the gram-positive L. monocytogenes (14, 30). We therefore investigated whether sensitization by P. acnes also exhibits positive and negative effects on the resistance to infection with L. monocytogenes. Groups of P. acnes-sensitized wild-type, LBP−/−, and IFN-γR−/− mice and unsensitized controls received graded doses of L. monocytogenes (102 to 106 CFU), and the outcome of infection was monitored over a period of 28 days. The cumulative lethality is shown in Table 2. In this study only protective effects of P. acnes treatment were observed. Thus, the unsensitized wild-type and LBP−/− mice were comparably susceptible to lethal infection, while the sensitized animals of both types were resistant to an infective dose of up to 106 CFU, as documented by the 100% survival of animals (Table 2). This indicates that LBP plays no role in the lethal outcome of L. monocytogenes infection, neither in sensitized nor in nonsensitized mice. Interestingly, the heat-killed L. monocytogenes, in contrast to heat-killed serovar Typhimurium, exhibited no toxicity in P. acnes sensitized mice (tested up to an equivalent of 109 CFU; not shown). Infection of IFN-γR−/− mice with L. monocytogenes resulted in an enhanced lethality; all mice succumbed to doses of 102 CFU of this microorganism (Table 2). This was true regardless of whether the mice had been treated with P. acnes or not, which is understandable since, in the absence of IFN-γ, sensitization does not occur and the protective effect of P. acnes pretreatment does not develop.
TABLE 2.
Susceptibility of LBP−/−, IFN-γR−/−, and wild-type mice to L. monocytogenes infection
| L. mono- cytogenes inoculum (CFU) | Lethality for mice (no. dead/total no. inoculated)
|
|||||
|---|---|---|---|---|---|---|
| LBP−/−
|
Wild type
|
IFN-γR−/−
|
||||
| Control | Sensitized | Control | Sensitized | Control | Sensitized | |
| 102 | 0/5 | 5/5 | 5/5 | |||
| 104 | 3/6 | 0/6 | 2/5 | 0/5 | 10/10 | 10/10 |
| 105 | 5/5 | 0/5 | 5/5 | 0/5 | ||
| 106 | 0/5 | 0/5 | ||||
DISCUSSION
The present study demonstrates that a preexisting LPS hypersensitivity may increase the resistance of the host to infection and lead to survival or, alternatively, accelerate infection lethality. Thus, in the serovar Typhimurium infection model, a sensitizing pretreatment with a standard dose of P. acnes completely protected 129Sv/Pas mice from a dose of bacteria that was otherwise 40% lethal (105 CFU). Similarly, priming with P. acnes protected BALB/c mice against a 100% lethal serovar Typhimurium dose (102 CFU; unpublished results). This is in agreement with an earlier study by Chedid and Parant, who observed that priming with Corynebacterium granulosum enhanced the resistance of mice to infection with Klebsiella pneumoniae (34). The protective effect of priming observed in the present study is explained by the enhanced LPS sensitivity of the host, which enables innate defense mechanisms to sense the minute amounts of LPS contained in the invading bacteria and to mobilize an efficient antibacterial defense. It is therefore not surprising that priming of LPS nonresponder BALB/c/l mice with P. acnes had only a minor effect (a somewhat-decreased kinetics of lethality) on the outcome of serovar Typhimurium infection (unpublished results).
The protective effect of LPS hypersensitivity was reversed when the number of infecting bacteria was increased. In this case, sensitized mice died faster of infection than unsensitized controls. The accelerated mortality was caused by the enhanced toxicity of the LPS already present in the relatively high numbers (106 and 107 CFU) of infecting bacteria. This is conclusively demonstrated by the fact that 106 and 107 killed microorganisms of serovar Typhimurium induced illness and 100% lethality, respectively, in the sensitized mice, while up to 109 CFU induced no lethality or apparent illness in unsensitized controls. The total amount of LPS present in 107 microorganisms of serovar Typhimurium was determined to be 0.27 μg. The same amount of purified LPS, as documented in the present study, was acutely lethal to hypersensitive mice.
In addition to the numbers of bacteria used for infection, also the degree of hypersensitivity determines whether the sensitization will be beneficial or deleterious for the infected host. This is shown in mice sensitized to different degrees by graded amounts of P. acnes and infected with an otherwise 100% lethal dose (107 CFU) of serovar Typhimurium. The highest sensitization accelerated infection lethality, while the medium and low sensitization were strongly and weakly protective, respectively. Thus, the degree of LPS hypersensitivity and size of the inoculum are the two factors determining the outcome of infection. They also determine the height of the response provoked in the host.
Given that LPS sensitivity influences the outcome of an infection, a similar role can be attributed to factors which modify LPS activity, such as LBP. In agreement with earlier findings (25), unsensitized LBP−/− mice were more susceptible to serovar Typhimurium infection than wild-type mice. In contrast, after P. acnes-priming, LBP−/− mice became much more resistant to infection than similarly primed wild-type mice. The protective effect of sensitization, which is reflected in the survival of an infection with up to 2 × 107 CFU, disappeared upon administration of LBP to the knockout mice at the time of infection. Importantly, in sensitized mice the absence of LBP reduced the LPS toxicity by a factor of approximately 50. Thus, in sensitized mice, the presence of LBP is important for the induction of LPS hyper-responses, which is in agreement with our earlier results (7) and which is detrimental for highly sensitized infected mice. It should be noted, however, that P. acnes-treated LBP−/− and wild-type mice exhibit the same enhanced susceptibility to the lethal effects of TNF-α (factor of approximately 300; not shown). This indicates that LBP plays no role in the mechanism of sensitization by P. acnes and is not involved in the induction of TNF-α effects. We conclude therefore that the remarkably high resistance of P. acnes-treated LBP−/− mice to serovar Typhimurium results from a moderate TNF-α response to infection but a strong TNF-α hypersensitivity. Thus, the role of LBP, at least during the early stages of infection, is pivotal to the outcome, beneficial or detrimental, depending on the extent to which it enhances the LPS response.
Recently, a role for LBP in human disease was indicated by a study in which a relationship between certain mutations in the LBP gene and a high mortality in septic patients was reported (24). This is an important finding, and it would be interesting to know how the mutations affect LPS binding and activity in such patients. LBP has also been reported to exert direct inhibitory effects on LPS activity when applied in excessive amounts (27, 46). Thus, administration of 100 μg of LBP to human monocytes or to mice in vivo inhibited cytokine production or protected mice from septic shock caused by LPS or gram-negative bacteria. Such high concentrations of LBP can occur in humans but have not been reported for mice. In humans therefore, strongly upregulated LBP may represent a protective mechanism.
In addition to LPS, other constituents of gram-negative bacteria, such as lipoprotein or DNA, could be expected to make a small contribution to the outcome of infection, separately or even in synergy with LPS. A synergism between LPS and bacterial DNA has been previously demonstrated (T. Sparwasser et al., Letter, Nature 386:336-337, 1997).
We showed earlier that priming with P. acnes also enhances the response of the sensitized mice to gram-positive bacteria such as L. monocytogenes. However, due to the absence of LPS, the enhanced activity (e.g., TNF-α response) of gram-positive bacteria was markedly lower than that of gram-negative microorganisms (30). In the present study the effect of P. acnes sensitization on L. monocytogenes infection was exclusively protective. The enhanced mortality observed in sensitized, serovar Typhimurium-infected mice was virtually absent from the L. monocytogenes-infected animals. We explain this by the absence of the direct toxicity of L. monocytogenes. In accordance, we show in this study that heat-killed L. monocytogenes (up to 109 CFU), in contrast to killed serovar Typhimurium, exhibits no toxicity in P. acnes-primed mice.
The mechanisms underlying the enhanced resistance or susceptibility of sensitized mice to infection were not specifically addressed in the present study. It can be safely assumed, however, that a higher inducibility of TNF-α and of other proinflammatory cytokines by invading bacteria and a higher susceptibility to endogenously produced TNF-α in hypersensitive mice are important factors. The importance of TNF-α in antibacterial defense is well documented (17, 37). Equally well documented is the high toxicity of this mediator for P. acnes-sensitized mice. Therefore, the difference in toxicity observed between serovar Typhimurium and L. monocytogenes (high and low, respectively) for P. acnes-sensitized mice might alone be explained by the difference in the TNF-α levels induced by the two pathogens. LPS hypersensitivity induced by P. acnes and other bacteria is mediated by IFN-γ. Although the precise mechanisms involved are not completely understood, IFN-γ is known to induce and/or enhance expression of genes involved in LPS recognition and mediator production and activity (16, 31, 38, 39, 42).
The direct relationship between P. acnes-induced hypersensitivity to bacterial components and P. acnes-induced effects on the susceptibility to infection is also demonstrated by the results obtained in IFN-γR−/− mice. Such mice, due to the absence of IFN-γ activity, do not develop hypersensitivity to LPS or other components of serovar Typhimurium, nor to components present in L. monocytogenes after P. acnes treatment (30). Consequently, pretreatment of IFN-γR−/− mice with P. acnes has no influence on an infection with either of the two pathogens.
The present study documents the complex relationship that exists between the factors determining host sensitivity to LPS and other bacterial components and susceptibility to infection. We conclude from the present results that the magnitude of the initial response of the host towards intruding microorganisms determines the further course and final outcome of infection and that a critical, narrow window decides whether the overall effect of sensitization will be beneficial or damaging to the infected animals.
Acknowledgments
The investigation was supported in part by BMBF-Gesundheit, project O1K19854/8, and by Deutsche Forschungsgemeinschaft-SPP 1110 (Angeborene Immunität), project Fr 448/4-1.
We are indebted to H. Stübig, N. Goos, and J. Kühnle for their excellent technical assistance and R. Silvestein and M. Hopman for their help in the preparation of the manuscript.
Editor: J. D. Clements
REFERENCES
- 1.Aggarwal, B. B., W. J. Kohr, P. E. Hass, B. Moffat, S. A. Spencer, W. J. Henzel, T. S. Bringman, G. E. Nedwin, D. V. Goeddel, and R. N. Harkins. 1985. Human tumor necrosis factor. Production, purification, and characterization. J. Biol. Chem. 260:2345-2354. [PubMed] [Google Scholar]
- 2.Akashi, S., H. Ogata, F. Kirikae, T. Kirikae, K. Kawasaki, M. Nishijima, R. Shimazu, Y. Nagai, K. Fukudome, M. Kimoto, and K. Miyake. 2000. Regulatory roles for CD 14 and phosphatidylinositol in the signaling via toll-like receptor 4-MD-2. Biochem. Biophys. Res. Commun. 268:172-177. [DOI] [PubMed] [Google Scholar]
- 3.Bihl, F., L. Lariviere, S. T. Qureshi, L. Flaherty, and D. Malo. 2001. LPS-hyporesponsiveness of mnd mice is associated with a mutation in Toll-like receptor 4. Genes Immun. 2:56-59. [DOI] [PubMed] [Google Scholar]
- 4.Brighbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 162:732-736. [DOI] [PubMed] [Google Scholar]
- 5.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 6.Doughty, L. A., K. B. Nguyen, J. E.Durbin, and C. A. Biron. 2001. A role for IFN-αβ in virus infection-induced sensitization to endotoxin. J. Immunol. 166:2658-2664. [DOI] [PubMed] [Google Scholar]
- 7.Freudenberg, M. A., M. Gumenscheimer, R. Jack, T. Merlin, C. Schütt, and C. Galanos. 1997. A strict requirement for LBP in the TNF-α hyperresponse of Propionibacterium acnes-sensitized mice to LPS. J. Endotoxin Res. 4:357-361. [Google Scholar]
- 8.Freudenberg, M. A., M. Kopf, and C. Galanos. 1996. Lipopolysaccharide-sensitivity of interferon-gamma-receptor deficient mice. J. Endotoxin Res. 3:291-298. [Google Scholar]
- 9.Freudenberg, M. A., Y. Kumazawa, S. Meding, J. Langhorne, and C. Galanos. 1991. Gamma interferon production in endotoxin-responder and -nonresponder mice during infection. Infect. Immun. 59:3484-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freudenberg, M. A., U. Meier-Dieter, T. Staehelin, and C. Galanos. 1991. Analysis of LPS released from Salmonella abortus equi in human serum. Microb. Pathog. 10:93-104. [DOI] [PubMed] [Google Scholar]
- 11.Freudenberg, M. A., T. Merlin, M. Gumenscheimer, C. Kalis, R. Landmann, and C. Galanos. 2001. Role of lipopolysaccharide susceptibility in the innate immune response to Salmonella typhimurium infection: LPS, a primary target for the recognition of Gram-negative bacteria. Microbes Infect. 3:1213-1222. [DOI] [PubMed] [Google Scholar]
- 12.Freudenberg, M. A., R. Salomao, A. Sing, I. Mitov, and C. Galanos. 1998. Reconciling the concepts of endotoxin sensitization and tolerance. Prog. Clin. Biol. Res. 397:261-268. [PubMed] [Google Scholar]
- 13.Galanos, C., and M. A. Freudenberg. 1993. Mechanisms of endotoxin shock and endotoxin hypersensitivity. Immunology 187:346-356. [DOI] [PubMed] [Google Scholar]
- 14.Galanos, C., M. Gumenscheimer, P. F. Mühlradt, E. Jirillo, and M. A. Freudenberg. 2000. MALP-2, a Mycoplasma lipopeptide with classical endotoxic properties: end of an era of LPS monopoly? J. Endotoxin Res. 6:471-476. [PubMed] [Google Scholar]
- 15.Galanos, C., O. Lüderitz, and O. Westphal. 1979. Preparation and properties of a standardized lipopolysaccharide from Salmonella abortus equi (Novo-Pyrexal). Zentbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 1 Orig. Reihe A 243:226-244. [PubMed] [Google Scholar]
- 16.Galve-de Rochemonteix, B., L. P. Nicod, and J. M. Dayer. 1996. Tumor necrosis factor soluble receptor 75: the principal receptor form released by human alveolar macrophages and monocytes in the presence of interferon gamma. Am. J. Respir. Cell. Mol. Biol. 14:279-287. [DOI] [PubMed] [Google Scholar]
- 17.Gross, A. S., J. C. Sadoff, N. Kelly, E. Bernton, and P. Gemski. 1989. Pretreatment with recombinant murine tumor necrosis factor α/cachectin and murine interleukin 1 a protects mice from lethal bacterial infection. J. Exp. Med. 169:2021-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunwald, U., X. Fan, R. S. Jack, G. Workalemahu, A. Kallies, F. Stelter, and C. Schutt. 1996. Monocytes can phagocytose Gram-negative bacteria by CD 14-dependent mechanism. J. Immunol. 157:4119-4126. [PubMed] [Google Scholar]
- 19.Hess, J., C. Ladel, D. Miko, and S. H. E. Kaufmann. 1996. Salmonella typhimurium aroA− infection in gene-targeted immunodeficient mice. J. Immunol. 156:3321-3326. [PubMed] [Google Scholar]
- 20.Heumann, D., P. Gallay, C. Barras, P. Zaech, R. J. Ulevitch, P. S. Tobias, M. P. Glauser, and J. D. Baumgartner. 1992. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J. Immunol. 148:3505-3512. [PubMed] [Google Scholar]
- 21.Hinshaw, L. B. (ed.). 1985. Handbook of endotoxin, vol. 2. Pathophysiology of endotoxin. Elsevier, Amsterdam, The Netherlands.
- 22.Hinshaw, L. B. 1990. Pathophysiology of endotoxin action: an overview, p. 419-426. In A. Nowotny, J. J. Spitzer, and E. J. Ziegler (ed.), Endotoxin research series, vol. 1. Cellular and molecular aspects of endotoxin reactions. Experta Medica, Amsterdam, The Netherlands.
- 23.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 24.Hubacek, J. A., F. Stuber, D. Frohlich, M. Book, S. Wetegrove, M. Ritter, G. Rothe, and G. Schmitz. 2001. Gene variants of the bacterial/permeability increasing protein and lipopolysaccharide binding protein in sepsis patients: gender-specific predisposition to sepsis. Crit. Care Med. 29:557-561. [DOI] [PubMed] [Google Scholar]
- 25.Jack, R. S., X. Fan, M. Bernheiden, G. Rune, M. Ehlers, A. Weber, G. Kirsch, R. Mentel, B. Furll, M. A. Freudenberg, and G. Schmitz. 1997. Lipopolisaccharide-binding protein is required in vivo to combat a Gram-negative bacterial infection. Nature 7:345-355. [DOI] [PubMed] [Google Scholar]
- 26.Katschinski, T., C. Galanos, A. Coumbos, and M. A. Freudenberg. 1992. Gamma interferon mediates Propionibacterium acnes-induced hypersensitivity to lipopolysaccharide in mice. Infect. Immun. 60:1994-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamping, N., R. Dettmer, N. W. J. Schröder, D. Pfeil, W. Hallatschek, R. Burger, and R. R. Schumann. 1998. LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J. Clin. Investig. 101:2065-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lien, E., and R. R. Ingalls. 2002. Toll-like receptors. Crit. Care Med. 30:1-11. [PubMed] [Google Scholar]
- 29.Matsuura, M., and C. Galanos. 1990. Induction of hypersensitivity to endotoxin and tumor necrosis factor by sublethal infection with Salmonella typhimurium. Infect. Immun. 58:935-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merlin, T., M. Gumenscheimer, C. Galanos, and M. A. Freudenberg. 2001. TNF-α hyper-response to Gram-negative and Gram-positive bacteria in Propionibacterium acnes primed or Salmonella typhimurium infected mice. J. Endotoxin Res. 7:157-163. [PubMed] [Google Scholar]
- 31.Merlin, T., R. Woelky-Bruggmann, C. Fearns, M. Freudenberg, and R. Landmann. 2002. Expression and role of CD14 in mice sensitized to lipopolysaccharide by Propionibacterium acnes. Eur. J. Immunol. 32:761-772. [DOI] [PubMed] [Google Scholar]
- 32.Michie, H. R., K. R. Manogue, D. R. Spriggs, A. Revhaug, S. Owyer, D. A. Dinarello, A. Cerami, S. M. Wolff, and D. W. Wilmore. 1988. Detection of circulating tumor necrosis factor after endotoxin administration. N. Engl. J. Med. 318:1481-1486. [DOI] [PubMed] [Google Scholar]
- 33.Muller, I., M. A. Freudenberg, P. Kropf, A. Kiderlen, and C. Galanos. 1997. Leishmania major infection in C57Bl/10 mice differing at the Lps locus: a new non-healing phenotype. Med. Microbiol. Immunol. (Berlin) 186:75-81. [DOI] [PubMed] [Google Scholar]
- 34.Parant, M., F. Parant, and L. Chedid. 1977. Inheritance of lipopolysaccharide-enhanced nonspecific resistance to infection and of susceptibility to endotoxic shock in lipopolysaccharide low-responder mice. Infect. Immun. 16:432-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poltorak, A., X. He, I. Smirnowa, M. Y. Lui, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57Bl/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 36.Poltorak, A., T. Merlin, P. J. Nilson, O. Sandra, I. Smirnova, I. Schupp, T. Boehm, C. Galanos, and M. A. Freudenberg. 2001. A point mutation in the IL12Rb2 gene underlies the IL-12 unresponsiveness of the LPS-defective C57Bl/10ScCr mice. J. Immunol. 167:2106-2111. [DOI] [PubMed] [Google Scholar]
- 37.Rothe, J., W. Lesslauer, H. Lotschet, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumor necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364:798-802. [DOI] [PubMed] [Google Scholar]
- 38.Ryu, S. Y., K. S. Jeong, B. N. Kang, S. J. Park, W. K. Yoon, S. H. Kim, and T. H. Kim. 2000. Modulation of transferring synthesis, transferring receptor expression, iNOS expression and NO production in mouse macrophages by cytokines, either alone or in combination. Anticancer Res. 20:3331-3338. [PubMed] [Google Scholar]
- 39.Salkowski, C. A., K. kopydlowski, J. Blanko, M. J. Cody, R. McNally, and S. N. Vogel. 1999. IL-12 is dysregulated in macrophages from IRF-1 and IRF-2 knockout mice. J. Immunol. 163:1529-1536. [PubMed] [Google Scholar]
- 40.Schramek, S., J. Kazar, Z. Sekeyova, M. A. Freudenberg, and C. Galanos. 1984. Induction of hypersensitivity to endotoxin in mice by Coxiella burnetii. Infect. Immun. 45:713-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schumann, R. R., S. R. Leong, G. W. Flaggs P. W. Gray, S. D. Wright, J. C. Mathison, P. S. Tobias, and R. J. Ulevitch. 1990. Structure and function of lipopolysaccharide binding protein. Science 249:1429-1431. [DOI] [PubMed] [Google Scholar]
- 42.Szabo, S. J., A. S. Dighe, U. Gubler, and K. M. Murphy. 1997. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 185:817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi, O., T. Kawai, P. F. Mühlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 44.Vogel, S. N. 1992. The LPS gene, p. 485-513. In B. Beutler (ed.), Tumor necrosis factors: the molecules and their emerging role in medicine. Raven Press, New York, N.Y.
- 45.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1995. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 46.Zweigner, J., H. J. Gramm, O. C. Singer, K. Wegscheider, and R. R. Schumann. 2001. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood 98:3800-3808. [DOI] [PubMed] [Google Scholar]