Abstract
The activation of innate immunity requires the amplification of signals induced by pattern-recognition receptors for bacterial products. We have investigated the role of the newly described cytokine IL-32 in the amplification of cytokine production induced by the two most clinically relevant families of microbial receptors, the cell-surface Toll-like receptors (TLRs) and the intracellular nuclear oligomerization domain (NOD) receptor family. IL-32 synergized with the NOD1- and NOD2-specific muropeptides of peptidoglycans for the release of IL-1β and IL-6 (a 3- to 10-fold increase). In contrast, IL-32 did not influence the cytokine production induced via TLRs. The synergistic effect of IL-32 and synthetic muramyl dipeptide (MDP) on cytokine production was absent in the cells of patients with Crohn's disease bearing the NOD2 frameshift mutation 3020insC, demonstrating that the IL-32/MDP synergism depends on NOD2. This in vitro synergism between IL-32 and NOD2 ligands was consistent with a marked constitutive expression of IL-32 in human colon epithelial tissue. In addition, the potentiating effect of IL-32 on the cytokine production induced by the synthetic muropeptide FK-156 was absent in NOD1-deficient macrophages, supporting the interaction between IL-32 and NOD1 pathways. When specific caspase inhibitors were used, the synergism between IL-32 and MDP/NOD2 depended on the activation of caspase 1. Only additive effects of IL-32 and muropeptides were observed for TNF-α production. The modulation of intracellular NOD2 pathways by IL-32, but not cell-surface TLRs, and the marked expression of IL-32 in colon mucosa suggest a role of IL-32 in the pathogenesis of Crohn's disease.
Keywords: Toll-like receptors, cytokines
IL-32 is a recently described cytokine produced mainly by T, natural killer, and epithelial cells that has the properties of a classical proinflammatory mediator (1). First, IL-32 is prominently induced by IFN-γ. Second, IL-32 also stimulates TNF-α and IL-8 production by the activation of NF-κB and p38 mitogen-activated protein (MAP) kinase (1). Because of the induction of IL-32 by IFN-γ and the subsequent proinflammatory effects of IL-32, we hypothesized that IL-32 may also provide an amplification mechanism in innate immune responses, particularly for the recognition signals induced by pattern-recognition receptors (PRRs) such as cell-surface Toll-like receptors (TLRs). In addition, we also considered the effect of IL-32 on the intracellular PRRs termed nucleotide oligomerization domain (NOD) receptors.
Initial sensing of microbial pathogens is mediated by the recognition of pathogen-associated molecular patterns (PAMPs), which are highly conserved structures expressed uniquely by the microbes of the same class (2). The most important of these recognition systems are the TLR and NOD families of proteins. Eleven TLRs have been identified in mammals, each with its own PAMP specificity (3). TLR-mediated signals both activate antimicrobial mechanisms of innate immunity and induce costimulatory signals for activation of specific immunity, thus representing a bridge between innate and acquired immune responses (4). Whereas TLRs are located mainly on the cell-surface membrane or the luminal side of the intracellular vesicles, proteins of the NOD family seem to function as intracytoplasmatic recognition systems (5). Thus, NOD1 and NOD2 are the PRRs for Gram-negative and Gram-positive peptidoglycans and their derived muropeptide components, with NALP1 (NACHT-LRR-PYD-containing protein 1) and NALP3 also proposed to recognize muramyl dipeptide (MDP).
Although recognition of pathogens by the various PRR families is required for activation of the innate host response, it became apparent that an efficient host defense requires proper amplification mechanisms of these signals. One of the most important amplification signals is provided by proinflammatory cytokines such as TNF-α, macrophage migration-inhibitory factor (MIF), and, particularly, IFN-γ. IFN-γ and TNF-α increase the expression of TLR2 and TLR4 in various cells types (6, 7), whereas MIF regulates innate immune responses through specific up-regulation of TLR4 (8). These effects result in synergistic effects with PAMPs such as LPS (9).
In the present study we investigated whether IL-32 also modulates the signals induced by specific TLR and NOD ligands. Surprisingly, whereas IL-32 had only additive effects on TLR-mediated induction of cytokines, IL-32 specifically synergized with NOD1 and NOD2 ligands for the synthesis of IL-1β and IL-6, but not TNF-α, through caspase 1-dependent mechanisms.
Materials and Methods
Expression and Purification of Recombinant IL-32 (rIL-32). Human rIL-32 isoform γ was prepared as previously reported (1). Briefly, rIL-32 was expressed in Escherichia coli and purified by a three-step purification: a Talon affinity column, size-exclusion chromatography, and ion-exchange chromatography. The rIL-32 activity was not due to LPS contamination, as demonstrated by experiments using polymyxin B: whereas polymyxin B completely blocked the stimulation of cytokines by 100 ng/ml E. coli serotype O55:B5 LPS (data not shown), it had only a negligible effect on cytokines induced by IL-32 (20 ng/ml). In contrast, boiling completely inactivated the rIL-32 activity, providing another argument that the heat-resistant LPS was not responsible for its activity. In addition, rIL-32 was fully able to activate cells of TLR2-/-, TLR4-/-, and NOD1-/- mice, demonstrating that its activity was not due to contamination with LPS, lipoprotein, or Gram-negative bacteria peptidoglycans (see below). To exclude the presence of LPS in the material, all experiments were performed in the presence of 1 unit/μl polymyxin B (Bedford Laboratories, Bedford, OH), with the exception of experiments in which IL-32/LPS synergism was investigated, in which no polymyxin B was used.
Isolation of Peripheral Blood Mononuclear Cells (PBMCs) and Stimulation of Cytokine Production. After informed consent was obtained, venous blood was drawn from the antecubital vein of seven healthy volunteers into heparin-containing tubes. The PBMC fraction was obtained by density centrifugation of blood diluted 1:1 in pyrogen-free saline over Histopaque (Sigma). Cells were washed twice in saline and suspended in culture medium (RPMI medium 1640) supplemented with penicillin (100 units/ml) and streptomycin (100 μg/ml).
PBMCs (5 × 105) in a 100-μl volume were added to flat-bottomed 96-well plates and incubated with 100 μl of either culture medium (negative control) or rIL-32γ (concentrations from 10 to 50 ng/ml) in combination with various TLR ligands: TLR2 with synthetic Pam3Cys lipopeptide (5 μg/ml), TLR3 with poly(I)·poly(C) (50 μg/ml), TLR4 with purified E. coli LPS (1 ng/ml), TLR5 with flagellin (10 ng/ml), TLR7 with R848 (resiquimod; 5 μg/ml), and TLR9 with CpG DNA (5 μg/ml). In separate experiments, the synergism among IL-32 and the bacterial muropeptides MDP (0.1–10 μg/ml) and diaminopimelic acid-containing muramyl tripeptide (Mur-Tri-DAP) (1 μg/ml) was investigated. All TLR and NOD ligands were checked for contamination with LPS during a Limulus amoebocyte lysate (LAL) assay and found to be negative. After a 24-h incubation at 37°C, supernatants were collected and stored at -70°C until cytokine assays were performed. For the measurement of cell-associated IL-1α, cells were lysed by 1% Triton X-100 and three cycles of freeze/thaw.
To test whether the monocyte or the lymphocyte population was responsible for the release of cytokines after IL-32 synergism with the TLR and NOD ligands, PBMCs were incubated for 2 h at 37°C. The nonadherent cells were mostly lymphocytes and were transferred to a different well, whereas the adherent cells were >90% monocytes. The synergism of IL-32 and the muropeptides was compared between monocytes and lymphocytes.
Cytokine concentrations were determined by specific electro-chemiluminescence assays, as previously described (10).
Genotyping of NOD2 Variants. Blood was collected from 74 patients with Crohn's disease and 10 healthy volunteers. PCR amplification of NOD2 gene fragments containing the polymorphic site 3020insC was performed in 50-μl reaction volumes containing 100–200 ng of genomic DNA, as previously described (11). The 3020insC polymorphism was analyzed by genescan analysis on an Applied Biosystems PRISM 3100 Genetic Analyzer according to the manufacturer's protocol. Four patients with Crohn's disease were found to be homozygous for the 3020insC mutation, and they were further investigated in the cytokine studies. As control groups, five patients with Crohn's disease who were homozygous for the WT allele and five healthy volunteers homozygous for the WT NOD2 allele were included.
Cytokine Production by Murine Peritoneal Macrophages. To exclude the possibility that the effects of IL-32 were due to microbial contaminants, we stimulated peritoneal macrophages of TLR2-/-, TLR4-/-, and NOD1-/- mice with purified rIL-32 and compared them with control macrophages. The cells were harvested 10 min after the i.p. injection of 4 ml of sterile PBS containing 0.38% sodium citrate. After centrifugation and washing, the cells were resuspended in RPMI medium 1640. Cells were cultured in 96-well microtiter plates at 1 × 105 cells per well, in a volume of 100 μl. After stimulation for 24 h with rIL-32 (10 ng/ml), cytokines were measured.
In additional experiments, resident peritoneal macrophages from either NOD1-/- or C57BL/6J (NOD1+/+ control) mice were harvested as described above. Cells were stimulated with IL-32 (10 ng/ml), the murine NOD1 ligand FK-156 (1 μM), or a combination of both. After a 24-h incubation at 37°C, supernatants were collected and stored at -70°C until cytokine assays were performed.
The Role of Caspases in the Synergism Between IL-32 and MDP. To investigate the role of caspases in the synergism between IL-32 and MDP, we added 20 μM zVAD-fmk, a fluoromethylketone pan-caspase inhibitor (Bachem) to the medium. In addition, either specific inhibition of caspase 1 with 20 μM Ac-Tyr-Val-Ala-Asp-2,6-dimethylbezoyloxymethylketone (YVAD, Alexis Biochemicals, San Diego, CA), or simultaneous inhibition of caspase 1 and caspase 5 with 20 μM Ac-Trp-Glu-His-Asp-aldehyde (Bachem) was performed. The role of endogenous IL-1β and IL-18 in the synergistic effects of IL-32 and MDP was investigated by blocking their activity with IL-1 receptor antagonist (IL-1Ra) (10 μg/ml) or IL-18 binding protein (IL-18BP) (10 μg/ml).
Statistical Analysis. The human experiments were performed in triplicate with blood obtained from seven volunteers. The mouse experiments were performed in 10 mice per group on two different occasions, and the data are presented as cumulative results. The differences among groups were analyzed by the Mann–Whitney U test and, where appropriate, by Kruskal–Wallis ANOVA. The level of significance among groups was set at P < 0.05. The data are given as means ± SEM.
Expression of IL-32 in Colon Mucosa by Immunohistochemistry (IHC). The tissue samples of normal colon from four individuals were fixed in 10% buffered formalin, routinely processed, and embedded in paraffin. Three sections from the paraffin blocks were used for IHC of IL-32 with the EnVision horseradish peroxidase detection system (DAKO). A previously described monoclonal mouse antibody against human IL-32α was used (1). IHC steps were carried out at room temperature. After deparaffinization, the sections were placed into a pressure cooker in 10 mM sodium citrate buffer (pH 6.0) at full power for 4 min, followed by treatment with 3% hydrogen peroxide for 10 min. The primary antibody (5 mg/ml) was diluted (1:16,000), incubated for 30 min with the tissue sections, and exposed to EnVision reagent (DAKO) for 30 min. The slides were then sequentially incubated with the chromogen reagent for 5 min, counterstained with Meyer's hematoxylin, and mounted. Negative control staining was performed by using mouse IgG1 isotype antibody. An Olympus (Melville, NY) microscope (BX50 model) equipped with a digital camera was used to prepare the microphotographs with magnifications of 200× and 400×.
Results
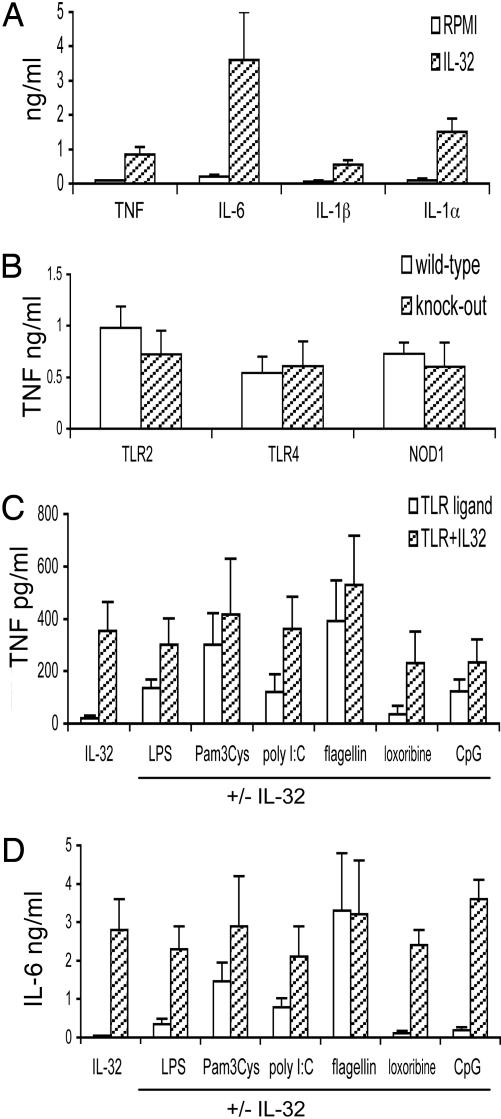
IL-32-Stimulated Production of Proinflammatory Cytokines. Purified rIL-32γ induced significant production of the proinflammatory cytokines TNF-α, IL-6, IL-1β, and IL-1α by human PBMCs (Fig. 1A). Polymyxin B completely blocked TNF-α induction by LPS (100 ng/ml), but it had no effect on IL-32γ-induced cytokines (data not shown), demonstrating that the stimulation of cytokines by IL-32 is not due to LPS contamination. To exclude the presence of microbial products as the source of stimulation by IL-32γ, TNF induction by IL-32 was tested in peritoneal macrophages isolated from TLR2, TLR4, and NOD1 knockout mice. As shown in Fig. 1B, IL-32γ-stimulated TNF-α production was nearly the same from cells isolated from knockout and WT mice. The production of IL-1β and IL-6 was also comparable in WT and knockout mice (data not shown). Thus, we conclude that TLR2 (bacterial lipoproteins), TLR4 (LPS), and NOD1 (Gram-negative bacteria peptidoglycan) ligands do not account for the ability of rIL-32γ to stimulate cytokine production.
Fig. 1.
IL-32 interaction with TLR pathways. (A) PBMCs were stimulated with IL-32γ (20 ng/ml) (hatched bars), and after 24 h the concentrations of TNF-α, IL-6, IL-1β, and intracellular IL-1α were measured. The presence of RPMI medium 1640 indicates unstimulated cells. (B) Peritoneal macrophages from TLR2-/-, TLR4-/-, and NOD1-/- mice (hatched bars) and the respective controls (open bars) were stimulated with 20 ng/ml IL-32γ.(C and D) Ligands of TLR4 (LPS), TLR2 (Pam3Cys), TLR3 [(poly(I)·poly(C)], TLR5 (flagellin), TLR7 (loxoribine), and TLR9 (CpG DNA) were added to PBMCs (open bars), with or without costimulation with IL-32 (hatched bars). Data are presented as means ± SEM (n = 7).
IL-32 Interaction with TLR Pathways. The amplification of signals induced by PRRs is crucial for the proper activation of immune responses to infection. To investigate whether IL-32 amplifies cytokine production induced by TLR recognition of microbial ligands, the role of IL-32 in combination with specific TLR ligands was investigated. Submaximal concentrations of the TLR ligands were used in the experiments, to allow the distinction of a putative synergistic effect with IL-32. The submaximal concentrations described in Materials and Methods were determined in pilot experiments (data not shown). IL-32 had only additive effects on the stimulation of cytokines by known ligands of TLR2 (Pam3Cys), TLR3 [poly(I)·poly(C)], TLR4 (LPS), TLR5 (flagellin), TLR7 (loxoribine), and TLR9 (CpG DNA) (Fig. 1 C and D), arguing against a major role of IL-32 for amplification of TLR signals.
Unique Amplification of Muropeptide-Induced Cytokine Production by IL-32. A second major family of pattern recognition receptors, recognizing peptidoglycans and their muropeptide derivatives, is the NLR (NACHT-LRR) family of intracellular proteins, to which NOD proteins belong. As shown in Fig. 2 A and B, IL-32 synergized with the muropeptide MDP, a synthetic derivative of Gram-positive peptidoglycan, as well as Mur-Tri-DAP, a synthetic derivative of Gram-negative peptidoglycan, for the release of IL-1β and IL-6. In contrast, only additive effects of IL-32 were observed when TNF-α or IL-1α production was measured (Fig. 2 C and D). Using a more detailed dose–response between IL-32γ and MDP, we observed a synergistic induction of IL-6 up to 10-fold over the additive value of IL-32γ plus MDP (Fig. 2E). In addition, we observed that the synergism of IL-32/muropeptide stimulation was entirely due to the monocyte population, because isolated lymphocytes from the same donors exhibited no cytokine release with either IL-32γ or MDP (Fig. 3). IL-32γ inactivation by boiling for 30 min eliminated its ability to stimulate cytokine production and its synergism with MDP, demonstrating that this effect was due to IL-32 and not to contamination with heat-resistant microbial ligands (data not shown).
Fig. 2.
IL-32 amplification of cytokine production stimulated by muropeptides. (A–D) In human PBMCs, IL-32γ (10 ng/ml) (hatched bars) was coincubated with MDP or Mur-Tri-DAP (both at 1μg/ml), and production of IL-1β (A), IL-6 (B), TNF-α (C), and IL-1α (D) is shown. MDP or Mur-Tri-DAP stimulation alone is represented by open bars. (E). The effects on IL-6 production when using different concentrations of IL-32 and MDP are shown. Open bars represent PBMCs stimulated with 5ng/ml IL-32γ plus the corresponding MDP concentration inμg/ml; hatched bars represent PBMCs stimulated with 10 ng/ml IL-32γ plus the corresponding MDP concentration; solid bars represent 20 ng/ml IL-32γ plus MDP; stippled bars represent 50 ng/ml IL-32γ plus MDP concentration. The data are presented as fold increase in which the nanograms of IL-32-induced IL-6 and the nanograms of MDP-induced IL-6 from individual PBMC cultures were added and assigned a value of 1.0. The measured value of IL-6 induced by the combination of IL-32γ plus MDP was then calculated as fold increase over 1.0. Data are presented as means ± SEM (n = 7). *, P < 0.01.
Fig. 3.
Monocyte-dependent IL-32/MDP synergism. PBMCs, adherent monocytes, or nonadherent lymphocytes were stimulated with MDP/IL-32 as described for Fig. 2. After 24 h, IL-1β (A) and IL-6 (B) were measured. Data are presented as means ± SD (n = 7). *, P < 0.01.
The Synergism Between IL-32 and Bacterial Muropeptides Is Mediated by NOD1 and NOD2. MDP and Mur-Tri-DAP are ligands of both NOD2 and NOD1 (12, 13), as well as of the NALP molecules NALP1 and NALP3 (14). To investigate whether the synergism between IL-32 and muropeptides is mediated by NOD1/NOD2 or NALP1/NALP3 molecules, PBMCs were isolated from patients with Crohn's disease who were homozygous for the 3020insC NOD2 mutation. The clinical picture of Crohn's disease differs profoundly from that of Muckle–Wells syndrome, which is caused by gain-of-function NALP1 mutations. In addition, peritoneal macrophages were isolated from NOD1-deficient mice.
Combinations of IL-32 and muropeptides were incubated with either human or murine cells. As shown in Fig. 4A, IL-32 synergistically enhanced MDP-induced cytokine production in healthy volunteers and in patients with Crohn's disease who bear the WT NOD2 gene. However, this synergism was absent in patients with Crohn's disease possessing the 3020insC frameshift mutation. Similarly, IL-32 enhanced cytokine production of the murine NOD1 ligand FK-156 in WT mice in a synergistic manner, but this synergism was absent from NOD1-deficient animals (Fig. 4B). Therefore, these results in humans and mice are consistent with the conclusion that synergism between IL-32 and muropeptides is mediated by NOD1 and NOD2 and not NALPs.
Fig. 4.
The synergism among IL-32 and muropeptides is mediated by NOD1 and NOD2. (A) PBMCs isolated from five healthy controls, five Crohn's patients bearing the WT NOD2 allele (Crohn-WT), and four patients homozygous for the frameshift mutation 3020insC NOD2 (Crohn-FS) were stimulated with combinations of IL-32γ (10 ng/ml) and MDP (10 μg/ml) for 24 h. Data are presented as means ± SEM. (B) IL-32γ (10 ng/ml) and the murine NOD1 ligand FK-156 (1 μM) were added together to peritoneal macrophages harvested from control NOD1+/+ mice and NOD1-/- animals. After 24 h, IL-6 concentration was measured. Data are presented as means ± SEM (n = 10). *, P < 0.05.
The Synergism Between IL-32 and MDP Is Caspase 1-Dependent. The proinflammatory caspases 1 and 5 are associated with the NLR proteins (15); these caspases process the precursors of IL-1β and IL-18 into their biologically active forms. As shown in Fig. 5A, the presence of the pan-caspase inhibitor reduced (from 4- to 1.5-fold) the synergistic effect of IL-32γ plus MDP. To distinguish more precisely whether caspase 1 or caspase 5 is responsible, the effect of two specific inhibitors, either of caspase 1 alone or of both caspases 1 and 5, was investigated. The specific caspase 1 inhibitor was as effective for the inhibition of IL-32/MDP synergism as the pan-caspase inhibitor, whereas the dual inhibitor of caspases 1 and 5 did not result in an additional reduction over that of caspase 1 (Fig. 5 A and B). Unfortunately, no specific caspase 5 inhibitor is currently available, which precluded us from adding a separate condition providing caspase 5 inhibition alone.
Fig. 5.
The role of proinflammatory caspases for the IL-32 effect on NOD2 stimulation. A pan-caspase inhibitor, a caspase 1 inhibitor, or a caspase 1 and 5 inhibitor (each at 20 μM) was added to the stimulation of PBMCs with IL-32γ (open bars), MDP (hatched bars), or a combination of both (filled bars). (A–C) Concentrations of IL-1β (A), IL-6 (B), and TNF-α (C) were measured after a 24-h stimulation. (D) IL-1Ra (10 μg/ml) or IL-18BP (10 μg/ml) was added to the IL-32/MDP stimulation. Data are presented as means ± SEM (n = 7). *, P < 0.05.
Because IL-6 production is often IL-1-dependent (16), the effect of inhibitors of caspases 1 and/or 5 was also assessed. As shown in Fig. 5B, a similar reduction was observed to be consistent with IL-1β/caspase-dependent production of IL-6. Furthermore, the combination of IL-32γ plus MDP for TNF-α production was unaffected by adding inhibitors of caspases 1 and/or 5 (Fig. 5C). These observations suggest that caspase 1, rather than caspase 5, is required for the synergistic stimulation of IL-1β and IL-6 by IL-32/MDP.
Because caspase 1 processes both pro-IL-1β and pro-IL-18 in their active forms, we investigated whether the effect of IL-32/MDP synergism on IL-6 production is secondarily induced by release of endogenous IL-1β or IL-18. Therefore, the activity of IL-1β was blocked by the presence of IL-1Ra, and that of IL-18 with IL-18BP. Whereas IL-18BP had no effects on IL-6 release, IL-1Ra inhibited the synergistic effects of IL-32/MDP on IL-6 production (62% inhibition, P < 0.02; Fig. 5D). Therefore, the synergistic stimulation of IL-6 by IL-32/MDP is due to intermediary release of endogenous IL-1β.
IL-32 Expression in the Epithelial Cells of Colon Mucosa. Because IL-32 specifically synergizes with NOD ligands, and NOD2 is a prominent susceptibility gene in Crohn's disease, we have investigated IL-32 expression in the colon mucosa. As shown in Fig. 6, IL-32 is strongly expressed in the mucosal epithelial cells of the colon. The pattern of expression shows it is expressed mainly in the differentiated epithelial cells of colon and located in both nuclei and the cytoplasm.
Fig. 6.
IL-32 expression in the epithelial cells of colon mucosa. Biopsies of the colon mucosa of human large intestine were stained with a murine anti-human IL-32 monoclonal antibody. The staining of colon tissue of one representative individual of four healthy volunteers tested is shown. IL-32 is largely expressed in the differentiated colon epithelial cells, whereas the control antibody showed no staining. (Magnification: A, ×200; B, ×400. Scale bar: 100 μm.)
Discussion
In the present study we demonstrate that IL-32γ specifically synergizes with the muropeptide components of peptidoglycan, which is common to all clinically relevant bacteria, including mycobacteria. The synergism was observed for the production of IL-1β and IL-6, leading to an increase in cytokine production up to 10-fold greater than the additive value of IL-32γ or muropeptide stimulation alone. Furthermore, this synergism between IL-32γ and muropeptides depended on NOD1 and NOD2, two intracellular recognition receptors for muropeptides. Additionally, we show that the IL-32/NOD synergism is caspase 1-dependent. In contrast, synergism was not observed between IL-32 and the specific ligands of TLR2, TLR3, TLR4, TLR5, TLR7, and TLR9. Thus, these findings support the concept that IL-32 specifically modulates NOD1/NOD2 pathways, while having no effect on TLR-mediated activation. Because NOD2 is likely the most important susceptibility gene for Crohn's disease, and because IL-32 is expressed by the epithelial cells of intestinal mucosa, IL-32 may prove to be a vital contributor in the pathogenesis of Crohn's disease.
The implications of these findings are fundamental to understanding postsignal transduction by IL-32. On the one hand, IL-32γ is without effect on cytokine production induced by the ligation of cell-surface TLRs by a broad variety of TLR ligands (Fig. 1). On the other hand, IL-32γ apparently has a positive influence on the function of the intracellular receptors NOD1 and NOD2 (Fig. 2). Thus, MDP initiates gene transcription and synthesis of IL-1β after binding to the intracellular receptor NOD2, but, in the presence of IL-32, the synthesis of IL-1β is enhanced in a synergistic manner. Apparently, this property of IL-32 is unique to the signal transduction of NOD2, but not that of TLRs. The synergistic effect of IL-32/MDP on IL-6 production is partially due to intermediary production of endogenous IL-1β, rather than a direct action of IL-32 on IL-6 production. It is presently unknown whether the IL-32 signal is directed at the transcription or translation of IL-1β. IL-32 alone induced only a marginal stimulation of NOD1 and NOD2 expression (data not shown), which is unlikely to explain the synergistic effects.
It seems, therefore, that IL-32 potentiates one or more of the intracellular pathways induced by NOD ligands. MDP engagement of NOD2 results in the activation of two signaling pathways. First, NOD2 activates the serine/threonine kinase receptor-interacting protein 2/RICK/CARDIAK (Rip2), and this leads to NF-κB activation and transcription of proinflammatory cytokine genes such as those encoding TNF-α, IL-1β, and IL-6 (17). Second, the CARD domain of NLR proteins interacts with caspases 1 and 5, and this interaction results in the processing of cytokine precursors such as pro-IL-1β and pro-IL-18 (15, 18). The synergistic activity of IL-32/MDP stimulation on IL-1β and IL-6 production, but not on TNF-α release, strongly suggests that caspase-mediated mechanisms, rather than Rip2/NF-κB stimulation of transcription (which should lead to synergistic effects on both IL-1β and TNF-α), are responsible for the effect of IL-32. Indeed, both a pan-caspase inhibitor and a specific caspase 1 inhibitor significantly reduced (by 50–75%) the synergism between IL-32 and MDP, demonstrating an essential role for caspase 1. Additional inhibition of caspase 5 by a dual inhibitor of caspases 1 and 5 did not have an additional effect on the IL-32/MDP stimulation, suggesting that caspase 1 alone, and not caspase 5, mediates the synergistic effect. These observations are consistent with a report showing that NOD1 interacts with caspase 1, enhancing the pro-IL-1β processing (18). However, a low level of synergy between IL-32 and MDP remained even after the inhibition of caspase 1, suggesting that caspase 1-independent mechanisms may also be secondarily involved in this phenomenon.
Proinflammatory cytokines, especially IFN-γ, prime cells for stimulation with TLR ligands (9). This effect is due to the increase in surface expression of TLR2 and TLR4 by IFN-γ, with the result of synergistic effects on cytokine induction by bacterial components such as LPS (7, 19). We have hypothesized that IL-32, which is strongly stimulated by IFN-γ, could be equally potent in the amplification of signals induced by TLRs. However, this hypothesis is not supported by the present data; IL-32 displayed only additive effects on cytokine production when cells were stimulated by ligands of TLR2, TLR3, TLR4, TLR5, TLR7, or TLR9. In separate experiments, no stimulatory effects of IL-32 on TLR2 and TLR4 expression were observed (data not shown), accounting for the lack of synergism between IL-32 and TLR ligands. On the one hand, these findings demonstrate that IL-32 differs from other proinflammatory cytokines such as IFN-γ, TNF-α, and macrophage migration-inhibitory factor (MIF) in the ability to amplify TLR signals. On the other hand, because only additive effects were observed with combinations of IL-32 plus TLR ligands, we conclude that IL-32 signal transduction lacks the ability to modify TLR-induced cytokine production. IFN-γ is an effective inducer of IL-32 release (1). However, the lack of effect by IL-32 on TLR signaling argues that it is highly unlikely that IL-32 mediates the IFN-γ potentiation of TLR-induced cytokine production.
In addition to TLRs, the second major recognition system used by the cells to recognize pathogens is represented by the NLR family of proteins (20). NOD1 and NOD2 are members of the NLR family, which mediates recognition of muropeptide components from peptidoglycans of Gram-negative and Gram-positive bacteria and mycobacteria. Stimulation of NOD2 by MDP, or of NOD1 by Mur-Tri-DAP, induces NF-κB activation and cytokine release (12, 13, 21). Moreover, mice deficient in NOD2 fail to respond with cytokine production after challenge with MDP (22). In addition, mutations in NOD2 have been implicated in the pathogenesis of Crohn's disease (23, 24), and patients homozygous for the 3020insC mutated allele have defective cytokine responses to MDP and peptidoglycan (11, 25, 26). It has been shown that NALP1 and NALP3, two additional members of the NLR family, can also interact with MDP and induce cytokine upon stimulation (14). In the present study, the stimulation of freshly isolated PBMCs with a combination of IL-32 plus either MDP or Mur-Tri-DAP resulted in synergistic effects on the production of IL-1β and IL-6, whereas only additive effects were observed for the release of TNF-α and IL-1α. Monocytes in PBMC cultures are responsible for the release of cytokines when stimulated with IL-32 and muropeptides, whereas lymphocytes do not respond to either stimulant or their combination.
To differentiate whether IL-32 amplifies signals induced by either NOD1/NOD2 or NALP1/NALP3, receptors known to interact with muropeptides, we have investigated the IL-32/MDP synergism in cells isolated from patients homozygous for the 3020insC NOD2 loss-of-function mutation and in peritoneal macrophages of NOD1-deficient mice. Synergism for IL-1β and IL-6 was observed with the combination of IL-32γ plus MDP in healthy volunteers and patients with Crohn's disease bearing the WT NOD2 allele, whereas synergism was absent from the PBMCs from patients with Crohn's disease homozygous for the 3020insC NOD2 mutation. Similarly, the synergism between IL-32 and FK-156, an agonist molecule of murine NOD1, was absent from macrophages isolated from NOD1-deficient mice. Thus, the synergism of IL-32 with muropeptides depends on activation of NOD1 and NOD2. Whether NALP1 or NALP3 is also involved in this process remains to be investigated.
IL-32 induces degradation of IκB and NF-κB activation, as well as phosphorylation of p38 mitogen-activated protein kinase (MAPK), two pathways characteristic of the signaling route of proinflammatory cytokines (1). In the present study, caspase 1 inhibition reduced by 70% the IL-32-induced IL-1β. This finding suggests that IL-32 activates a third pathway after stimulation of monocytes, namely stimulation of caspase 1 leading to processing of pro-IL-1β. Caspase 1 is responsible for processing both pro-IL-1β and pro-IL-18 into their respective biologically active forms. Using specific inhibitors of IL-1β and IL-18 activity, we demonstrate that, whereas caspase 1 activation is responsible for the synergistic effects of IL-32 and MDP on IL-1β production, it is IL-1β, not IL-18, that accounts for the secondary effect on IL-6 synthesis.
The in vitro findings of the present study have clinical implications. Signals induced through pathogen recognition receptors such as TLRs are crucial for the integrity of intestinal mucosa (27). Similarly, NOD2 signaling plays a protective role in Crohn's disease by inducing production of defensins and cytokines, thus playing a role in antimicrobial defense in the gut, as well as maintaining the barrier function of the epithelium (28, 29). Mutations of NOD2 lead to greatly increased risk of developing Crohn's disease (23, 24, 30), and NOD2-deficient mice have an increased susceptibility to lethality when infected with intracellular pathogens (29). Moreover, increased release of IL-1β has been reported in mice bearing a mutation equivalent to the human 3020insC NOD2 mutation, which confers increased susceptibility to colitis (31). Because of the specific amplification of NOD2-induced IL-1β by IL-32, we speculate that IL-32 may play an important role in intestinal homeostasis and/or inflammation. This hypothesis is also suggested by the high expression of IL-32 in the epithelial cells lining the colon lumen (Fig. 6). However, whether IL-32 is involved in the pathogenesis of Crohn's disease and the possible mechanisms of its effects in vivo remain to be investigated.
Acknowledgments
This work was supported by National Institutes of Health Grants AI-15614, HL-68743, and CA-046934 (to C.A.D.). M.G.N. was supported by a Vidi grant from the Netherlands Organization for Scientific Research (NWO-ZonMW). D.-Y.Y. was supported by a grant from the International Cooperation Program of the Ministry of Science and Technology (Korea).
Author contributions: M.G.N., C.A.D., and S.-H.K. designed research; M.G.N., T.A., G.F., M.W., and J.-M.K. performed research; T.A., S.E.G., J.-S.P., E.A., D.-Y.Y., and S.-H.K. contributed new reagents/analytic tools; M.G.N., G.F., and S.-H.K. analyzed data; and M.G.N., C.A.D., and S.-H.K. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: PRR, pattern-recognition receptor; TLR, Toll-like receptor; NOD, nuclear oligomerization domain; MDP, muramyl dipeptide; r, recombinant; PBMC, peripheral blood mononuclear cell; Mur-Tri-DAP, diaminopimelic acid-containing muramyl tripeptide; IL-1Ra, IL-1 receptor antagonist; IL-18BP, IL-18 binding protein.
References
- 1.Kim, S. H., Han, S. Y., Azam, T., Yoon, D. Y. & Dinarello, C. A. (2005) Immunity 22, 131-142. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov, R. (2001) Nat. Rev. Immunol. 1, 135-145. [DOI] [PubMed] [Google Scholar]
- 3.Beutler, B. (2004) Nature 430, 257-263. [DOI] [PubMed] [Google Scholar]
- 4.Kapsenberg, M. (2003) Nat. Rev. Immunol. 3, 984-993. [DOI] [PubMed] [Google Scholar]
- 5.Inohara, N., Ogura, Y. & Nunez, G. (2002) Curr. Opin. Microbiol. 5, 76-80. [DOI] [PubMed] [Google Scholar]
- 6.Abreu, M. T., Arnold, E. T., Thomas, L. S., Gonsky, R., Zhou, Y., Hu, B. & Arditi, M. (2002) J. Biol. Chem. 277, 20431-20437. [DOI] [PubMed] [Google Scholar]
- 7.Faure, E., Thomas, L., Xu, H., Medvedev, A. E., Equils, O. & Arditi, M. (2001) J. Immunol. 166, 2018-2024. [DOI] [PubMed] [Google Scholar]
- 8.Roger, T., David, J., Glauser, M. P. & Calandra, T. (2001) Nature 414, 920-924. [DOI] [PubMed] [Google Scholar]
- 9.Bosisio, D., Polentarutti, N., Sironi, M., Bernasconi, S., Miyake, K., Webb, G. R., Martin, M. U., Mantovani, A. & Muzio, M. (2002) Blood 99, 3427-3431. [DOI] [PubMed] [Google Scholar]
- 10.Puren, A. J., Fantuzzi, G., Gu, Y., Su, M. S.-S. & Dinarello, C. A. (1998) J. Clin. Invest. 101, 711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netea, M. G., Kullberg, B. J., de Jong, D., Francke, B., Sprong, T., Naber, T., Drenth, J. P. H. & Van der Meer, J. W. M. (2004) Eur. J. Immunol. 34, 2052-2059. [DOI] [PubMed] [Google Scholar]
- 12.Girardin, S. E., Boneca, I. G., Viala, J., Chamaillard, M., Labigne, A., Thomas, G., Philpott, D. J. & Sansonetti, P. J. (2003) J. Biol. Chem. 278, 8869-8872. [DOI] [PubMed] [Google Scholar]
- 13.Girardin, S. E., Boneca, I. G., Carneiro, L. A. M., Antignac, A., Jehanno, M., Viala, J., Tedin, K., Taha, M.-K., Labigne, A., Zahringer, U., et al. (2003) Science 300, 1584-1587. [DOI] [PubMed] [Google Scholar]
- 14.Martinon, F., Agostini, L., Meylan, E. & Tschopp, J. (2004) Curr. Biol. 14, 1929-1934. [DOI] [PubMed] [Google Scholar]
- 15.Martinon, F. & Tschopp, J. (2004) Cell 117, 561-574. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello, C. A. (2005) J. Exp. Med. 201, 1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, K., Inohara, N., Hernandez, L. D., Galan, J. E., Nunez, G., Janeway, C. A., Medzhitov, R. & Flavell, R. A. (2002) Nature 416, 194-199. [DOI] [PubMed] [Google Scholar]
- 18.Yoo, N. J., Park, W. S., Kim, S. Y., Reed, J. C., Son, S. G., Lee, J. Y. & Lee, S. H. (2002) Biochem. Biophys. Res. Commun. 299, 652-658. [DOI] [PubMed] [Google Scholar]
- 19.Wolfs, T. G., Buurman, W. A., van Schadewijk, A., de Vries, B., Daemen, M. A., Hiemstra, P. S. & van't Veer, C. (2002) J. Immunol. 168, 1286-1293. [DOI] [PubMed] [Google Scholar]
- 20.Kufer, T. A., Fritz, J. H. & Philpott, D. J. (2005) Trends Microbiol. 13, 381-388. [DOI] [PubMed] [Google Scholar]
- 21.Chamaillard, M., Hashimoto, M., Horie, Y., Masumoto, J., Qiu, S., Saab, L., Ogura, Y., Kawasaki, A., Fukase, K., Kusumoto, S., et al. (2003) Nat. Immunol. 4, 702-707. [DOI] [PubMed] [Google Scholar]
- 22.Pauleau, A. L. & Murray, P. J. (2003) Mol. Cell. Biol. 23, 7531-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hugot, J.-P., Chamaillard, M., Zouali, H., Lesage, S., Cezard, J.-P., Belaiche, J., Almer, S., Tysk, C., O'Morain, C. A., Gassull, M., et al. (2001) Nature 411, 599-603. [DOI] [PubMed] [Google Scholar]
- 24.Ogura, Y., Bonen, D. K., Inohara, N., Nicolae, D. L., Chen, F. F., Ramos, R., Britton, H., Moran, T., Karalluskas, R., Duerr, R. H., et al. (2001) Nature 411, 603-606. [DOI] [PubMed] [Google Scholar]
- 25.Netea, M. G., Ferwerda, G., De Jong, D. J., Jansen, T., Jacobs, L., Kramer, M., Naber, T. H. J., Drenth, J. P. H., Girardin, S. E., Kullberg, B. J., et al. (2005) J. Immunol. 174, 6518-6523. [DOI] [PubMed] [Google Scholar]
- 26.van Heel, D. A., Ghosh, S., Hunt, K., Lundberg, A. M., Ahmad, T., McGovern, D. P., Onnie, C., Negoro, K., Goldthorpe, S., Foxwell, B. M., et al. (2005) Lancet 365, 1794-1796. [DOI] [PubMed] [Google Scholar]
- 27.Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. (2004) Cell 118, 229-241. [DOI] [PubMed] [Google Scholar]
- 28.Hisamatsu, T., Suzuki, M., Reinecker, H.-C., Nadeau, W. J., McCormick, B. A. & Podolsky, D. K. (2003) Gastroenterology 124, 993-1000. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi, K. S., Chamaillard, M., Ogura, Y., Henegariu, O., Inohara, N., Nunez, G. & Flavell, R. A. (2005) Science 307, 731-734. [DOI] [PubMed] [Google Scholar]
- 30.Hampe, J., Grebe, J., Nikolaus, S., Solberg, C., Croucher, P. J. P., Mascheretti, S., Jahnsen, J., Moum, B., Klump, B., Krawczak, M., et al. (2002) Lancet 359, 1661-1665. [DOI] [PubMed] [Google Scholar]
- 31.Maeda, S., Hsu, L.-C., Liu, H., Bankston, L.-A., Iimura, M., Kagnoff, M. F., Eckmann, L. & Karin, M. (2005) Science 307, 734-738. [DOI] [PubMed] [Google Scholar]