Abstract
Human EBV-transformed B lymphocyte cell lines (LCLs) were used to measure the apoptotic response of individuals to γ radiation. The responses form a normal distribution around a median of 35.5% apoptosis with a range of 12-58%. This heterogeneous response has a genetic basis. LCLs from Caucasian donors and African American donors form distinct distributions of apoptotic response; all of the 11 LCLs comprising the lowest responding group (exhibiting between 12-20% apoptosis) are from Caucasian donors. The assay is capable of detecting significant effects of SNPs in two genes, MDM2 and AKT1, whose products are involved in controlling the p53 pathway and cellular response to DNA damage, suggesting that these data and this assay can be used to identify novel SNPs in other genes whose products impact the cellular response to radiation. Finally, the LCLs in the lowest apoptotic response group have the highest concentration of AKT1 protein and all harbor a haplotype in AKT1 that is present in Caucasians but absent in African Americans.
Keywords: AKT1, MDM2, p53
Research into inherited cancer susceptibility has primarily focused on the identification of mutations segregating with disease in large families. Individuals with Li-Fraumeni syndrome harbor a highly penetrant germ-line inactivating mutation in one allele of the tumor suppressor p53, develop tumors early in life, and frequently succumb to multiple tumors throughout their lifetimes (1, 2). Somatic inactivating mutations in p53 are found in approximately half of all tumors, lending support to the importance of the p53 pathway to cancer development (3). Because the p53 protein is a key transcriptional regulator of a signal transduction pathway that ultimately impacts the decision of a cell to divide, undergo growth arrest, or die (4), common SNPs in genes controlled by the p53 pathway have the capacity to impact the development of cancers. A candidate gene approach focusing on the p53 pathway should permit the discovery of low-penetrance alleles that increase cancer susceptibility or decrease age of onset of the disease.
Polymorphisms occur within the human genome an average of once every 1,000 nucleotides (5, 6). A candidate gene approach focusing on a known, relevant pathway considerably reduces the number of potential SNPs. However, one still needs a method to sort through potentially thousands of SNPs, the vast majority of which will have no detectable function, to find those polymorphisms that affect a phenotype. To accomplish this, an unbiased approach was chosen whereby polymorphisms in relevant genes were identified and the ability of those SNPs to affect a cancer-relevant phenotype, apoptosis, or programmed cell death in response to DNA damage was measured. The induction of apoptosis has been shown to be a critical function of the p53 pathway because failure to undergo p53-induced apoptosis in mice leads to tumor formation (7, 8) and a common defect in many types of human tumors is thought to be the failure of tumor cells to undergo apoptosis in response to stresses.
Methods
Response of EBV-Transformed B Cells to γ Radiation (γIR). A total of 120 EBV-transformed lymphoblast cell lines (LCLs) derived from anonymous, unrelated healthy volunteers were purchased from the Coriell Institute for Medical Research (Camden, NJ). Cells were cultured in RPMI medium 1640 supplemented with l-glutamine and 15% FBS (Sigma). Cells were seeded into T25 flasks at a density of 2 × 105 cells per ml and were grown at 37°C at 5% CO2 until the density reached 1 × 106 cells per ml (defined as one passage). Once the cultures reached a viability of 90% or greater, response to radiation was measured. For each cell line, two 10-ml cultures were seeded into T25 flasks at a density of 2 × 105 cells per ml and were grown for 48 h. One culture was irradiated with 5 Gy radiation (CIS BioInternational IBL 437C137Cs γ-radiation source), and the control culture was mock-irradiated. Twenty-four hours after irradiation, apoptosis was measured in both cultures by using a Guava Personal Cell Analysis flow cytometer (Hayward). Guava Viacount reagent, which differentially stains viable and nonviable cells based on their permeability to two dyes, was used to measure apoptosis. Control experiments on four LCLs demonstrated that assays of multicaspase activation, TUNEL staining, and nexin staining gave very similar results to Viacount (data not shown). The radiation-induced percent apoptosis for a culture was obtained by comparison of the irradiated to the mock-treated control culture. The average apoptotic response for each cell line was calculated from at least three measurements, each taken from a different passage. The total number of cell lines in our analysis was reduced to 113 after elimination of data from cell lines that never reached 90% viability, grew very slowly, underwent crisis during the experiment, or did not give reproducible results in the assay.
Genotyping. Genomic DNAs corresponding to the 113 LCLs were purchased from Coriell Institute. Genotyping of MDM2 SNP309 was performed as described (9). The five polymorphisms in AKT1 (10) (rs3803300, rs1130214, rs3730358, rs2498799, and rs2494732) were genotyped on an Applied Biosystems Prism 7000 using Applied Biosystems allelic discrimination assays. All genotype frequencies were in Hardy-Weinberg equilibrium.
Small Interfering RNA (siRNA) Gene Silencing. LCLs were grown to a density of 1 × 106 cells per ml and were transfected via electroporation using an Amaxa Nucleofector device according to the manufacturer's recommendations. Transfection efficiency was determined by fluorescent microscopy using Alexa Fluor 546 control nonsilencing duplex siRNAs (Qiagen). Typical transfection efficiency was 70-80%, and viability at 48 h after transfection was near 90%. AKT1 and p53 siRNA Smartpools (Dharmacon) were used to silence Akt1 and p53 expression. Lamin A/C and control nonsilencing duplex siRNAs (Qiagen) were used at the same concentration as AKT1 and p53 siRNAs (600 pmol). For radiation studies of siRNA-transfected cells, cells were irradiated (5 Gy) 24 h after transfection, and the percentage of apoptosis was measured 24 h after irradiation by comparing irradiated and mock-irradiated transfected cultures. Subsequently, the cells were lysed, and protein levels were analyzed as described below.
Western Analysis. Protein levels were analyzed from total cell extracts prepared by lysis in RIPA buffer (Sigma) in the presence of a protease and phosphatase inhibitor mixture (Sigma). Protein concentration was measured by using Bio-Rad's protein assay and spectrometry at 595 nm. Forty micrograms of total protein was separated on a 4-20% Tris/glycine gel and transferred to a poly(vinylidene difluoride) membrane. The following antibodies were used: AKT1 (2H10), Lamin A/C (no. 2032) and phospho-MDM2 Ser-166 (no. 3521) (Cell Signaling Technology); α-Tubulin (DM1A) (Sigma); and p53 (DO-1) and MDM2 (SMP14) (Santa Cruz Biotechnology). The proteins were visualized by using horseradish peroxidase-conjugated secondary antibodies (Pierce) and SuperSignal West Fento (Pierce).
Determination of AKT1 Protein Concentration by ELISA. For each cell line, cultures were seeded into T25 flasks at a density of 2 × 105 cells per ml and were grown for 72 h. Total cell protein extracts were prepared and quantitated as described above. AKT1 protein in each extract was determined by using the PathScan Total AKT1 Sandwich ELISA kit from Cell Signaling Technology according to the manufacturer's recommendations.
Statistical Analysis. Means and standard deviations of apoptotic responses of cell lines and protein levels were compared by unpaired t tests, one or two tailed as indicated in the text or by the Fisher's exact test when appropriate.
Determination of Haplotype Structure. Haplotypes were determined by expectation maximization using snphap software (http://www-gene.cimr.cam.ac.uk/clayton).
Results
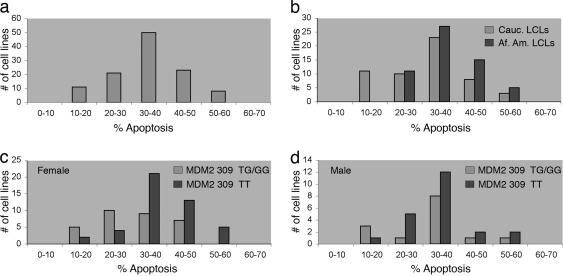
EBV-transformed B lymphocytes (LCLs) from normal, healthy human volunteers were used as surrogates for humans to measure the variation in response of individuals to radiation-induced cell death. Cell lines were exposed to 5 Gy of γIR to induce DNA damage, and apoptosis was measured 24 h later. To assess the reproducibility of the response, the experiment was repeated at least three times for each cell line. The data for each cell line was quite reproducible, with an average standard error of 2.2%. The identification, average apoptosis, and standard error for each cell line are presented in Table 1, which is published as supporting information on the PNAS web site. The average apoptosis for the population of 113 LCLs was 34.9% with a range from 12.3% to 58.9% apoptosis (Fig. 1a). The responses formed a normal distribution around a median of 35.5% apoptosis with a SD of 9.9%. In Fig. 1a, the cell lines are grouped based on their response to radiation and each grouping represents one SD (10%). The midpoint of the response of the 11 individuals in the lowest group (10-20% apoptosis) or the eight individuals in the highest group (50-60% apoptosis) is at least two SD away from the median. Thus, the response of the population to radiation is heterogeneous. Notably, the response of the 11 individuals at the low end of the spectrum is similar to that seen for Li-Fraumeni syndrome cancer patients who are carriers of germ-line p53 mutations (11). The frequency of apoptosis of an EBV-transformed LCL derived from a Li-Fraumeni patient was only 13.3% (data not shown), similar to that found by using peripheral blood lymphocytes from Li-Fraumeni patients (12).
Fig. 1.
The response to radiation is heterogeneous and is influenced by genetic factors. (a) A total of 113 LCLs from unrelated individuals were irradiated with 5Gy γIR to induce DNA damage, and apoptosis was measured 24 h later. Each cell line was measured at least three times. The identification, average apoptosis, and standard error for each cell line are presented in Table 1. Each cell line was placed in a bin based on its average apoptotic response. The bin size of 10% represents one standard deviation (9.9%) for the population. (b) LCLs are grouped by average response to radiation and by the self-identified race of the donor, either Caucasian or African American. (c) Female LCLs homozygous for the major allele at MDM2 SNP309 (TT) are grouped together; heterozygous (TG) LCLs are grouped with LCLs homozygous for the minor allele at MDM2 SNP309 (GG). (d) Male LCLs homozygous for the major allele at MDM2 SNP309 (TT) are grouped together; heterozygous (TG) LCLs are grouped with LCLs homozygous for the minor allele at MDM2 SNP309 (GG). Genotypes for all cell lines are included in Table 1.
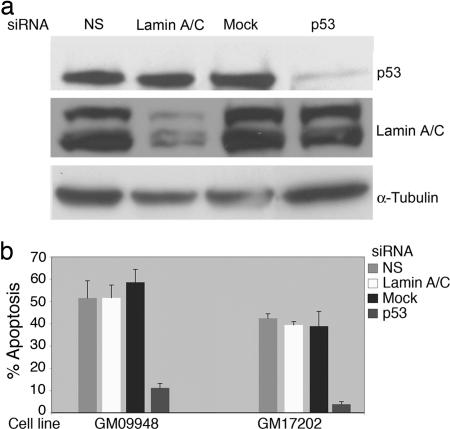
To further demonstrate the central role of the p53 pathway in this response, two LCLs with high apoptotic frequencies were transfected with p53 siRNA to reduce the expression of p53 and test whether this would impact the efficiency of apoptosis. Transfection of the GM09948 cell line with a nonspecific siRNA or with a siRNA directed against lamin resulted in no change in the response to γ IR (Fig. 2b). Transfection of the same cell line with a p53 siRNA that lowered the amount of p53 expression (Fig. 2a) resulted in a reduction of apoptotic response from >50% to 10% (Fig. 2b). A similar result was obtained with a second LCL, GM17202; p53 siRNA reduced apoptosis from >40% to <10% (Fig. 2b). Therefore, this assay measures p53-mediated apoptosis in response to γIR and the efficiency of that response is quite heterogeneous in the population. The p53-dependent transcriptional response of the 11 cell lines with the lowest apoptotic frequencies, shown in Fig. 1a, has been measured and appears to be similar to cell lines with higher apoptotic frequencies and distinct from a low transcriptional response of a p53 mutant LCL derived from a patient with Li-Fraumeni syndrome (W.H. and A.J.L., unpublished data). Together, these data suggest that individuals populating the low end of this distribution may show a decreased response to chemotherapeutic drugs designed to induce apoptosis or increased cancer susceptibility.
Fig. 2.
Reduction in expression of p53 by siRNA results in reduced apoptotic response in LCLs. (a) LCLs GM09948 and GM17202 were transfected with siRNAs specific for p53, lamin A/C, or a nonspecific siRNA (NS). Shown are Western blots of basal (no irradiation) whole cell extracts made from the GM09948 cell line 48 h after transfection. (b) GM09948 and GM17202 were treated with γIR 24 h after transfection with siRNAs, and apoptosis was measured 24 h after irradiation. Shown are the mean and standard deviation from two separate experiments.
Of the 113 LCLs shown in Fig. 1a, 76 are from female donors and 38 are from males. Male and female cell lines exhibit similar responses to radiation (P = 0.52; two-tailed t test). To maximize the genetic diversity and characterize the greatest number of polymorphisms, the cell lines were also chosen based on the race of the donor; each donor was self-identified as either Caucasian or African American. Fifty-five LCLs are derived from B cells from Caucasian donors, and 58 are derived from African American donors. Interestingly, the distributions of apoptotic response to radiation are different for Caucasian and African American cell lines (Fig. 1b), as all of the LCLs in the lowest response group (10-20% apoptosis) are Caucasian. This is a highly significant result (P = 0.0002; two-tailed Fisher's exact test) suggesting that one or more genetic factors that contribute to this low response vary in the two groups. This overrepresentation of Caucasians in the lowest response group is seen for both female and male LCLs (P = 0.0052 and 0.04, respectively; two-tailed Fisher's exact text).
As a first test of the hypothesis that SNPs in genes known to play a role in the p53 pathway and apoptosis might be detectable in this assay, the 113 cell lines were genotyped at MDM2 SNP309. Recently, it was demonstrated that the G allele of this SNP creates a binding site for the transcriptional activator SP1, resulting in increased expression of MDM2 (9). The MDM2 protein directly binds to and inhibits p53 by regulating its localization, stability, and activity as a transcriptional activator (13). Therefore, an increase in the expression of MDM2 results in reduced output from the p53 pathway. In Fig. 1c, the distribution of response of female LCLs homozygous for the major allele (T) at MDM2 SNP309 is compared to the distribution of response of female LCLs harboring one or two copies of the minor allele (G). The apoptotic response of LCLs harboring an MDM2 SNP309 G allele is shifted down to lower apoptotic frequencies. The shift in the distribution is significant (P = 0.001; one-tailed t test) and is supported by the known function of the SNP. Cancer cell lines homozygous for the minor allele (GG) of MDM2 exhibited very low levels of DNA damage-induced apoptosis due to decreased p53 function (9). Recently, it was shown that individuals with a germ-line mutation in one allele of the p53 gene (Li-Fraumeni syndrome) harboring one or two copies of the G allele at MDM2 SNP 309 are diagnosed with their first cancer at even younger ages (10 years earlier on average for breast cancers and 12 years earlier for soft tissue sarcomas) than Li-Fraumeni patients who have two copies of the T allele (9). Individuals within this high-risk group who were homozygous for the MDM2 SNP309 G allele had the greatest number of independent tumors (9). A significant, predicted effect of SNP309 can be detected in this in vitro B cell assay of DNA damage-induced apoptosis (Fig. 1c), thus providing a good proof-of-principle for the use of the assay to identify additional polymorphisms with similar phenotypes in cancer and providing support for the hypothesis that individuals within the lowest apoptotic response group may have an increased risk of developing cancers at an early age.
The frequency of MDM2 SNP309 in Caucasian LCLs is similar to previous reports (TT, 45%; TG, 44%; GG, 11%) (9). The frequency of the G allele in the African American population is much lower (TT, 74%; TG, 23%; GG, 3%). Examined independently, Caucasian LCLs and African American LCLs that harbor the G allele have a lower average response to radiation (P = 0.05 and 0.007; one-tailed t test for Caucasian and African American LCLs, respectively). Therefore, the polymorphism appears to be functional in both groups. However, the polymorphism is not functioning in this assay independent of gender. Female LCLs form two very different distributions based on MDM2 SNP 309 genotypes (P = 0.001; one-tailed t test) (Fig. 1c), but the difference between these distributions in male LCLs is reduced and not significant (P = 0.211, one-tailed t test) (Fig. 1d). Thus, whereas MDM2 SNP309 plays a role in the apoptotic response of female LCLs, the polymorphism may play only a weak or no role in male LCLs.
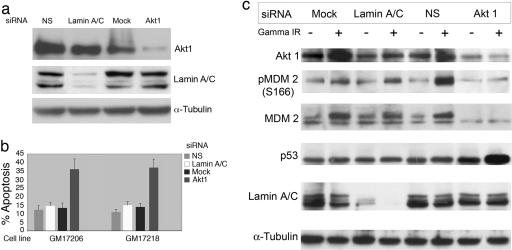
Recently, a haplotype within the AKT1 gene was reported to reduce the amount of AKT1 protein in lymphocytes and brain tissue of schizophrenic patients, and this haplotype was found in higher-than-expected frequencies in individuals with schizophrenia (10). AKT1 is an antiapoptotic protein kinase (14), and one of its substrates is the MDM2 protein (15-17). Phosphorylation of MDM2 by AKT1 at serine residues 166 and 188 inhibits MDM2 autoubiquitination, leading to the stabilization of MDM2 (15). MDM2 phosphorylation also promotes the movement of MDM2 into the nucleus where it can act to destabilize the p53 protein and reduce apoptosis (17). Thus, AKT1 can act in epistasis with MDM2 and functions to control the amount of MDM2 in a cell. To determine whether AKT1 activity impacts apoptotic frequency in B cells, two LCLs were transfected with siRNAs that were either nonspecific or directed against Lamin or the AKT1 transcript (Fig. 3). Transfection with AKT1 siRNA lowered the levels of AKT1 protein and lamin siRNA reduced the expression of the lamin protein; therefore, both of these siRNAs were specific for their targets (Fig. 3a). In both cell lines, reduction of AKT1 expression resulted in an increased apoptotic response (37% or 38%) compared to mock- or control-treated transfectants (11% or 12% apoptosis) (Fig. 3b), demonstrating that AKT1 expression impacts the efficiency of apoptosis in B cells.
Fig. 3.
Reduction in expression of AKT1 by siRNA results in increased apoptotic response in LCLs. (a) LCLs GM17206 and GM17218 were transfected with siRNAs specific for AKT1, lamin A/C, or a nonspecific siRNA (NS). Shown are Western blots of basal (no irradiation) whole cell extracts made from cell line GM17206 48 h after transfection. (b) GM17206 and GM17218 were treated with γIR (5 Gy) 24 h after transfection with siRNAs and apoptosis was measured 24 h after irradiation. Shown are the mean and standard deviation from three separate experiments. (c) Reduction in expression of AKT1 by siRNA results in decreased phosphorylation and stability of MDM2. Shown is a Western blot of whole cell protein extracts prepared 24 h after transfected GM17206 cells were exposed to γIR (5 Gy).
To determine whether the level of AKT1 affects apoptosis by altering MDM2 stability, AKT1 expression was reduced in an LCL by siRNA. GM17206 was transfected with siRNAs and cultures were split after transfection; half of each culture was irradiated (5 Gy), and half remained untreated. The cells were harvested 24 h after irradiation and prepared for Western blot analysis to detect total MDM2 protein, MDM2 phosphoserine 166, and total p53 (Fig. 3c). Upon DNA damage, the stability of p53 is increased and it becomes activated as a transcription factor (18); thus, the amount of p53 and the p53 target gene MDM2 are expected to rise after irradiation. In the mock, lamin siRNA, and nonspecific siRNA controls, quantitative densitometric scanning of the Western blot reveals that p53 protein level increased 50% after irradiation. This relatively low level of p53 induction may reflect the fact that transfection alone provides a stress that results in increased p53 levels compared to nontransformed, nonirradiated LCLs (data not shown). The level of MDM2 is increased after irradiation in the mock-treated, lamin siRNA-transfected, and nonspecific siRNA-transfected cells. In irradiated cells with reduced AKT1 expression, phosphorylation of MDM2 at Ser-166 was much reduced, and total MDM2 protein was reduced. At the same time, p53 levels were elevated in the AKT1 siRNA-transfected cells. This finding supports the hypothesis that AKT1 functions in B cells to phosphorylate and stabilize MDM2 leading to the decreased stability and activity of p53.
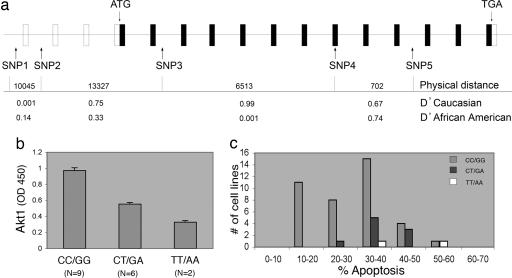
The LCLs were genotyped for SNPs across the AKT1 gene (SNPs 1-5, refs. 10 and 19) (Fig. 4a) to determine whether polymorphisms within AKT1 affect apoptosis in B cells. The physical distance between SNPs is given in nucleotides, and the pairwise linkage disequilibrium (LD) is presented as D′. A D′ value of 1.0 indicates complete LD, whereas a D′ value of 0 indicates that historical recombination events have occurred between the two SNPs such that they are only randomly present on the same chromosome. AKT1 SNP3 and -4 are in almost complete LD in Caucasians, but are not in LD in the African American population. Because of the strong LD between AKT1 SNP3 and -4 in Caucasians and the knowledge that AKT1 haplotypes associated with schizophrenia have been shown to alter AKT1 protein levels in lymphocytes (10), the effect of the SNP3-SNP4 haplotype on AKT1 protein levels was measured by using an ELISA assay specific for total AKT1 (Fig. 4b). Caucasian LCLs that are homozygous for the major SNP3-SNP4 haplotype (CC/GG) have higher amounts of AKT1 protein than do LCLs harboring the minor haplotype (TT/AA) (P = 8.0 × 10-8, two-tailed unpaired t test), and LCLs that are heterozygous at both positions have intermediate amounts of AKT1 protein (P = 6.6 × 10-5, two-tailed unpaired t test) compared to the minor allele homozygous cell lines.
Fig. 4.
The major AKT1 SNP3-4 haplotype is associated with higher basal AKT1 protein levels and decreased apoptotic response in Caucasian LCLs. (a) Genomic structure of the AKT1 locus and polymorphic sites used. Exons (boxes) and introns are not drawn to scale; open boxes represent noncoding sequences, filled boxes represent coding sequences. Pair-wise linkage disequilibrium between the SNPs calculated as D′ is shown, and the physical distance between SNPs is shown in nucleotides. Allele frequencies for individual SNPs are as follows: Caucasian SNP1 (A = 94.5, G = 5.5), SNP2 (G = 70.0, T = 30.0), SNP3 (C = 88.2, T = 11.8), SNP4 (G = 81.8, A = 18.2), SNP5 (A = 60.9, G = 39.1); African American SNP1 (A = 75.0, G = 25.0), SNP2 (G = 64.7, T = 35.3), SNP3 (C = 83.6, T = 16.4), SNP4 (G = 85.3, A = 14.7), SNP5 (A = 45.7, G = 54.3). (b) The AKT1 major SNP3-4 haplotype is associated with higher basal AKT1 protein levels. Mean ± SEM optical densities from an AKT1-specific ELISA are shown. The data include the results from two separate experiments. (c) The major AKT1 haplotype (CC/GG) is associated with lower apoptosis. Fifty Caucasian LCLs are grouped by average response to radiation and genotype at AKT1 SNPs 3 and 4. Five cell lines were eliminated from the analysis because they had AKT1 SNP3-4 genotype combinations other than those shown in c.
Because LCLs harboring the major SNP3-SNP4 haplotype have higher amounts of AKT1 protein, this haplotype would be predicted to associate with a decreased apoptotic response. In Fig. 4c, Caucasian LCLs are separated based on their genotypes at AKT1 SNP3 and SNP4 and their response to γIR. LCLs homozygous for the minor alleles at both SNP3 (TT) and SNP4 (AA) populate the high response end of the distribution, LCLs heterozygous for both SNP3 (CT) and SNP4 (GA) populate the middle of the distribution, whereas those LCLs homozygous for the major alleles at both SNP3 (CC) and SNP4 (GG) are found in a broad or biphasic distribution encompassing the lower half of the distribution that includes the 11 lowest apoptotic LCLs. The Caucasian LCLs homozygous for the major alleles (SNP3 CC, SNP4 GG) undergo, on average, less apoptosis in response to γIR than do the heterozygous (CT, GA) LCLs (P = 0.017, one-tailed t test). AKT1 SNP3 or SNP4 genotypes are not associated with apoptosis in African American LCLs. All 11 of the Caucasian LCLs in the 10-20% apoptotic response group share the major SNP3-SNP4 haplotype (CC, GG) (P = 0.014, one-tailed Fisher's exact test), suggesting that this haplotype is necessary but not sufficient to lower the apoptotic response of Caucasian LCLs to the 10-20% range because the same haplotype populates LCLs in the 20-30% and 30-40% region of the distribution in Fig. 4c. Notably, the broad distribution of apoptosis for Caucasian LCLs with the major AKT1 SNP3-SNP4 haplotype suggests that the low apoptosis phenotype may be modified by other genetic factors.
Discussion
EBV-transformed lymphoblasts have been used recently to demonstrate heritable variation in human gene expression (20), and specific germ-line determinants that regulate gene expression have been identified (21). Loci influencing response to chemotherapeutic drugs have also been identified by using human lymphoblastoid cell lines (22). Here, LCLs are used to demonstrate that considerable heterogeneity in the response to radiation-induced DNA damage exists within the population. Furthermore, the effects of individual polymorphisms that impact apoptosis can be detected in this assay. Using the list of cell lines and measured apoptotic responses provided in Table 1, polymorphisms in any gene that might impact the apoptotic response of B cells can be analyzed by simply genotyping the DNAs from the corresponding cell lines. Given that this assay has detected significant effects of SNPs in two genes (MDM2 and AKT1) that have clear clinical importance (9, 10), it is likely that the assay can be used to detect novel polymorphisms with similar phenotypes and that it provides a tool to perform ex vivo human genetic studies.
In addition, phenotypes that are observed in vitro provide hypotheses that can then be investigated in clinical samples. For example, the MDM2 SNP309 functions to reduce apoptosis in female LCLs to a much greater extent than in male LCLs. However, many more LCLs derived from females (n = 77) than from males (n = 36) were analyzed, so this conclusion will remain tentative and requires confirmation. Sexual dimorphism has been observed in the p53 pathway. In p53 mutant mice, the incidence of osteosarcoma was observed to be consistently higher in female mice than in male mice regardless of genetic background (23). In humans with Li-Fraumeni syndrome, tumors appear at higher frequencies in females than males, even when breast cancers are eliminated from the calculation (24).
A major finding of this work is that B cells harboring the major SNP3-SNP4 haplotype at AKT1 are relatively resistant to apoptosis compared to cells with the minor haplotype. It has not been shown that either SNP3 (located in intron 5) or SNP4 (a silent change at amino acid 242 in exon 11) represent a functional SNP with an ability to change either expression or activity of AKT1. Rather, these SNPs are markers for the region, and the linkage between a functional SNP and a marker SNP may be different in different populations. It is clear that the LD in this region may be complex. Whereas Emamian et al. (10) reported a core AKT1 haplotype involving the minor allele of SNP2 in transmission of schizophrenia in Americans of Northern European descent, AKT1 SNP 5 was shown to associate with schizophrenia in the Japanese population (19). Recently, the finding of Emamian et al. was replicated in an additional European sib-pair study (25). Single SNPs were tested for association with schizophrenia, and the minor allele of SNP2 was found to have a nominally significant overtransmission to affected sibs (P = 0.011). However, the minor allele of SNP3 was also significantly overtransmitted (P = 0.027). The data presented here suggest that AKT1 protein levels (and therefore apoptosis) are influenced by an AKT1 haplotype in Caucasians that includes SNP3 and SNP4. Whereas the allele frequencies of SNP3 and SNP4 are very similar in the Caucasian and African American populations, the apparent racial dimorphism in the effect of the AKT1 haplotype could be due to very different allele frequencies for the causative SNP in the two populations. Alternatively, the frequency of the functional SNP could be the same in both groups but the functional SNP may be linked to the marker SNPs (3 and 4) only in Caucasians. The major SNP3-SNP4 haplotype in AKT1 contributes to the low response of Caucasian LCLs to radiation. Its candidacy as a “race-specific” haplotype awaits further elucidation of the functional polymorphism(s) within the AKT1 gene.
Supplementary Material
Acknowledgments
This work was supported by grants from the Breast Cancer Research Foundation and National Institutes of Health Grant 5P01CA087497 (to A.J.L.).
Author contributions: S.L.H. and A.J.L. designed research; S.L.H., G.G., W.H., K.H., E.B., and G.B. performed research; S.L.H., G.G., H.R., and A.J.L. analyzed data; and S.L.H. and A.J.L. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: γIR, γ radiation; LCL, lymphoblast cell line; LD, linkage disequilibrium.
References
- 1.Malkin, D., Li, F. P., Strong, L. C., Fraumeni, J. F., Jr., Nelson, C. E., Kim, D. H., Kassel, J., Gryka, M. A., Bischoff, F. Z., Tainsky, M. A., et al. (1990) Science 250, 1233-1238. [DOI] [PubMed] [Google Scholar]
- 2.Garber, J. E., Goldstein, A. M., Kantor, A. F., Dreyfus, M. G., Fraumeni, J. F., Jr., & Li, F. P. (1991) Cancer Res. 51, 6094-6097. [PubMed] [Google Scholar]
- 3.Soussi, T. & Beroud, C. (2001) Nat. Rev. Cancer 1, 233-240. [DOI] [PubMed] [Google Scholar]
- 4.Harris, S. L. & Levine, A. J. (2005) Oncogene 24, 2899-2908. [DOI] [PubMed] [Google Scholar]
- 5.Chakravarti, A. (2001) Nature 409, 822-823. [DOI] [PubMed] [Google Scholar]
- 6.Sachidanandam, R., Weissman, D., Schmidt, S. C., Kakol, J. M., Stein, L. D., Marth, G., Sherry, S., Mullikin, J. C., Mortimore, B. J., Willey, D. L., et al. (2001) Nature 409, 928-933. [DOI] [PubMed] [Google Scholar]
- 7.Lowe, S. W., Schmitt, E. M., Smith, S. W., Osborne, B. A. & Jacks, T. (1993) Nature 362, 847-849. [DOI] [PubMed] [Google Scholar]
- 8.Symonds, H., Krall, L., Remington, L., Saenz-Robles, M., Lowe, S., Jacks, T. & Van Dyke, T. (1994) Cell 78, 703-711. [DOI] [PubMed] [Google Scholar]
- 9.Bond, G. L., Hu, W., Bond, E. E., Robins, H., Lutzker, S. G., Arva, N. C., Bargonetti, J., Bartel, F., Taubert, H., Wuerl, P., et al. (2004) Cell 119, 591-602. [DOI] [PubMed] [Google Scholar]
- 10.Emamian, E. S., Hall, D., Birnbaum, M. J., Karayiorgou, M. & Gogos, J. A. (2004) Nat. Genet. 36, 131-137. [DOI] [PubMed] [Google Scholar]
- 11.Camplejohn, R. S., Sodha, N., Gilchrist, R., Lomax, M. E., Duddy, P. M., Miner, C., Alarcon-Gonzalez, P., Barnes, D. M. & Eeles, R. A. (2000) Br. J. Cancer 82, 1145-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camplejohn, R. S. & Rutherford, J. (2001) Cell Prolif. 34, 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michael, D. & Oren, M. (2003) Semin. Cancer Biol. 13, 49-58. [DOI] [PubMed] [Google Scholar]
- 14.Bellacosa, A., Testa, J. R., Moore, R. & Larue, L. (2004) Cancer Biol. Ther. 3, 268-275. [DOI] [PubMed] [Google Scholar]
- 15.Feng, J., Tamaskovic, R., Yang, Z., Brazil, D. P., Merlo, A., Hess, D. & Hemmings, B. A. (2004) J. Biol. Chem. 279, 35510-35517. [DOI] [PubMed] [Google Scholar]
- 16.Milne, D., Kampanis, P., Nicol, S., Dias, S., Campbell, D. G., Fuller-Pace, F. & Meek, D. (2004) FEBS Lett. 577, 270-276. [DOI] [PubMed] [Google Scholar]
- 17.Mayo, L. D. & Donner, D. B. (2001) Proc. Natl. Acad. Sci. USA 98, 11598-11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bond, G. L., Hu, W. & Levine, A. J. (2005) Curr. Cancer Drug Targets 5, 3-8. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda, M., Iwata, N., Suzuki, T., Kitajima, T., Yamanouchi, Y., Kinoshiya, Y., Sekine, Y., Iyo, M., Harano, M., Komiyama, T., et al. (June 28, 2005) Int. J. Neuropsychopharmacol., 1-5. [DOI] [PubMed]
- 20.Cheung, V. G., Conlin, L. K., Weber, T. M., Arcaro, M., Jen, K. Y., Morley, M. & Spielman, R. S. (2003) Nat. Genet. 33, 422-425. [DOI] [PubMed] [Google Scholar]
- 21.Morley, M., Molony, C. M., Weber, T. M., Devlin, J. L., Ewens, K. G., Spielman, R. S. & Cheung, V. G. (2004) Nature 430, 743-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watters, J. W., Kraja, A., Meucci, M. A., Province, M. A. & McLeod, H. L. (2004) Proc. Natl. Acad. Sci. USA 101, 11809-11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donehower, L. A., Harvey, M., Vogel, H., McArthur, M. J., Montgomery, C. A., Jr., Park, S. H., Thompson, T., Ford, R. J. & Bradley, A. (1995) Mol. Carcinog. 14, 16-22. [DOI] [PubMed] [Google Scholar]
- 24.Hwang, S. J., Lozano, G., Amos, C. I. & Strong, L. C. (2003) Am. J. Hum. Genet. 72, 975-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwab, S. G., Hoefgen, B., Hanses, C., Hassenbach, M. B., Albus, M., Lerer, B., Trixler, M., Maier, W. & Wildenauer, D. B. (2005) Biol. Psychiatry 58, 446-450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.