Abstract
Inflammation plays a major pathological role in spinal cord injury (SCI). Although antiinflammatory treatment using the glucocorticoid methyprednisolone sodium succinate (MPSS) improved outcomes in several multicenter clinical trials, additional clinical experience suggests that MPSS is only modestly beneficial in SCI and poses a risk for serious complications. Recent work has shown that erythropoietin (EPO) moderates CNS tissue injury, in part by reducing inflammation, limiting neuronal apoptosis, and restoring vascular autoregulation. We determined whether EPO and MPSS act synergistically in SCI. Using a rat model of contusive SCI, we compared the effects of EPO [500-5,000 units/kg of body weight (kg-bw)] with MPSS (30 mg/kg-bw) for proinflammatory cytokine production, histological damage, and motor function at 1 month after a compression injury. Although high-dose EPO and MPSS suppressed proinflammatory cytokines within the injured spinal cord, only EPO was associated with reduced microglial infiltration, attenuated scar formation, and sustained neurological improvement. Unexpectedly, coadministration of MPSS antagonized the protective effects of EPO, even though the EPO receptor was up-regulated normally after injury. These data illustrate that the suppression of proinflammatory cytokines alone does not necessarily prevent secondary injury and suggest that glucocorticoids should not be coadministered in clinical trials evaluating the use of EPO for treatment of SCI.
Keywords: cytokines, glucocorticoids, inflammation, neuroprotection, trauma
The emotional and economic costs of spinal cord injury (SCI) are large, especially for the long-term nursing care necessitated by profound disability. Despite multiple therapeutic approaches that target different aspects of the pathophysiologic cascade contributing to SCI, there have been no major advances in clinical care that reliably attenuate injury or restore function. Despite initial clinical data suggesting that treatment using MPSS within 8 h of injury provides benefit (1), the current consensus is that MPSS offers, at best, limited improvement and may actually cause harm, e.g., acute corticosteroid myopathy (2) or infection (3, 4), particularly in high-risk patients. Although great promise exists for the potential use of stem cell transplantation, much additional preclinical work is required before moving into clinical trials (reviewed by Kulbatski et al. in ref. 5). Clearly, other therapeutic alternatives merit current exploration.
From a pathophysiological perspective, SCI has historically been divided into two distinct phases. Primary (mechanical) injury directly disrupts tissues but, in the acute phase, frequently causes only limited cell death surrounding the lesion epicenter. However, vigorous inflammation initiated as a response to primary injury subsequently causes extensive demyelination, tissue edema, and irreversible cell loss (reviewed by Kwon in ref. 6). As a general response to serious injury, the hypothalamic-adrenal axis is activated, and endogenous cortisol is secreted to potentially control excessively destructive inflammation. As a cortisol substitute, the synthetic glucocorticoid methyprednisolone sodium succinate (MPSS) attenuates spinal cord damage in preclinical models by reducing lipid peroxidation produced by free-radical and reactive oxygen species generated by cellular metabolism, especially within the milieu of ischemia followed by reperfusion (7). MPSS has also been reported to reduce proinflammatory cytokine production and tissue edema (8), inducible nitric oxide synthase activity, neuronal apoptosis (9), free-radical formation, and release of excitatory amino acids and vasoactive molecules and also provides beneficial modulation of calcium and sodium transcellular fluxes.
However, several unresolved issues exist in consideration of MPSS for the treatment of SCI. For example, in cats, the observed dose-response is an inverted U-shaped curve, with higher doses (e.g., 60 mg/kg) being ineffective (reviewed by Hall and Springer in ref. 10). Other species, e.g., rodents, have received less attention, but the results suggest a different outcome, with only a small window of opportunity for reducing tissue injury and with no improvement in motor outcome (11). Additional detrimental effects of glucocorticoids have been clearly documented, including potentiation of ischemic (12) and inflammatory (13) neuronal injury and potent suppression of neurotrophic factors, e.g., glial-derived neurotrophic factor (14).
The cytokine erythropoietin (EPO) (previously known for its hormonal effects on the bone marrow), which maintains an adequate circulating red-cell mass, also functions as a markedly potent, locally produced molecule ameliorating metabolic stress in many tissues (reviewed by Brines and Cerami in ref. 15). Studies have demonstrated robust EPO-mediated protection of the spinal cord from ischemia in the rabbit (16) or mechanical trauma in the rabbit, rat, and mouse (17-19). EPO mediates tissue protection via multiple, interacting pathways (reviewed by Ghezzi and Brines in ref. 20), including a reduction of both apoptotic cell death and the reactive increases in proinflammatory cytokines (21), mobilization of endothelial progenitor cells, promotion of angiogenesis (22) and healing (23), restoration of vascular autoregulation (24-26), and reduction of lipid peroxidation (19).
Although a marked protection in SCI by EPO has been observed, more needs to be learned about the therapeutic window, optimal site of administration, and dose-ranging characteristics. In anticipation of the study of EPO in human clinical trials, we assessed these parameters using a well defined mechanical-injury model in the rat. Because of the current clinical use of MPSS in SCI, we also evaluated the effects of this compound in the absence or presence of EPO, determining the extent of anatomical injury, hindlimb motor function 1 month after injury, and concentrations of key proinflammatory cytokines that are important mediators of secondary injury within the spinal cord.
A wide therapeutic temporal and dosage range for EPO was observed, and, although both agents reduced the production of proinflammatory cytokines equivalently, MPSS was completely ineffective in preventing secondary injury. Unexpectedly, coadministration of MPSS with EPO resulted in a complete loss of EPO-mediated tissue protection, despite fully suppressed proinflammatory cytokine levels.
Materials and Methods
Animals. Adult Sprague-Dawley rats (Charles River Laboratories) weighing 240-260 g were used. Animals were kept under standard housing conditions (22 ± 2°C, 65% humidity, and lights from 6:00 a.m. to 8:00 p.m.) and were fed a standard dry diet; water was available ad libitum. All experimental protocols were approved by the Animal Review Committee of the University of Milan and the Kenneth S. Warren Institute and met the Italian guidelines for laboratory animals [which conform to the European Communities Directive of November, 1986 (86/609/EEC) and the Guide for the Care and Use of Laboratory Animals, National Research Council].
Serum EPO Levels. For determining pharmacokinetics, animals were injected with recombinant human (rh)EPO (Dragon Pharmaceuticals, Vancouver, BC, Canada) at the dose and route indicated in the text. Serum samples were serially withdrawn via the tail vein and human EPO concentration determined by using an ELISA that does not cross-react with rat EPO (Quantiquine, R & D Systems).
SCI with UTS-Impactor and Drug Treatment. Traumatic SCI was performed by means of the UTS-impactor, which is fully described in the supporting information for ref. 17. The core of the UTS-impactor is a 2.3-mm end-diameter stainless steel rod that is precisely driven into the spinal cord with a specified force and displacement. The movement and impact is monitored by means of a miniaturized piezoelectric dynamometer present within a section of the impacting rod and linked to a computer that drives the device and records and manages the data. The impounding piston was positioned 1 mm above the exposed cord at T9 and set for an excursion of 3 mm. A force of 1 Newton for 1 second was applied, followed by an automatic return of the impaction rod. Animals were maintained under halothane anesthesia and positioned over a mat kept at the temperature of 38°C and, before awakening, were treated with buprenorphine [0.03 mg/kg of body weight (kg-bw)] for pain and penicillin G (10,000 units/kg-bw) as an antimicrobial agent. Each experimental group contained at least 18 animals. After SCI, the rats were housed two per cage and underwent manual bladder evacuation three times daily. rhEPO (Epoietin Alpha, Ortho Biotech, Milan) was administered as a single treatment within 30 min after injury. Methylprednisolone sodium succinate (Sigma) was administered at a dose of 30 mg/kg-bw by i.p. injection. Notably, a dose of 60 mg/kg proved to be lethal, killing all of the animals treated (n = 8) within the first week after induction of SCI.
Functional Assessment. All outcome measures were obtained in blinded fashion by four investigators and averaged. Neurological function was evaluated at 24 h after injury and then twice a week thereafter, by open-field testing using the methodology of Basso, Beattie, and Bresnahan (27).
Histology and Immunocytochemistry. At the end of the experimental period, animals were anesthetized by inhalation of halothane and perfused with 4% paraformaldehyde in isotonic PBS at pH 7.4 by transcardial perfusion. The spinal cord encompassing the full injury site was postfixed (24 h) with the same paraformaldehyde-containing solution, and segments of the spinal cord were embedded in paraffin and 8-μm sections cut transversely. Every 20th section was stained with hematoxylin and eosin. One cross section containing the lesion epicenter and the total T9-segment cavitation was analyzed by using computer-assisted image analysis (nih image, National Institutes of Health, Bethesda) through a Leica DG 100 camera mounted on a Leica microscope). The extent of cavitation was calculated as the area of cavitated tissue divided by the area of the total cross-section at the level of the injury (five per experimental group). Vibratome sections (40 μm) were also collected onto glass slides and processed for immunocytochemistry. Primary antibodies for glial fibrillary acidic protein (GFAP) and ED-1 (1:500; Chemicon) were applied overnight at 4°C and incubated for 3 h at room temperature with FITC-conjugated rabbit anti-mouse antibody (Chemicon). Vibratome sections were incubated with a polyclonal antibody to EPO (H-162; 1:300; Santa Cruz Biotechnology) and processed according to the peroxidase-antiperoxidase method.
Cytokine ELISA. At the indicated times, spinal cord tissue was homogenized and sonicated (Branson Sonifier 250) in a 7.4 pH buffer solution containing 10 mM Tris, 0.032 mM sucrose, 0.5 mM EDTA, 2 mM EGTA, 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. The processing was performed at 4°C. Proteins were determined by the Lowry-Ciocalteau technique (28). The tissue concentration of inflammatory cytokines [macrophage inflammatory protein (MIP)-2 (homologue of human IL-8), TNF-α, IL-6, and IL-1β] were assayed at the site of injury by means of specific ELISA kits (BioSource Europe, Nivelles, Belgium) following the manufacturer's instructions. At least six animals per group were included for analysis.
Western Blotting. Proteins were prepared from spinal cord tissue obtained from rostral, lesion epicenter, and caudal regions, and protein-concentration determination was performed according to the Lowry-Ciocalteau technique (28). Proteins (75 μg) were separated on a 10% polyacrylamide gel and transferred onto nitrocellulose membranes. Membranes were subsequently incubated with a polyclonal antibody directed against EPO receptor (EPOR) (H-194; 1:200, Santa Cruz Biotechnology) and bands visualized by using the Kodak Image Station 440.
Statistical Analysis. Data are expressed as the mean ± SD. Multiple group comparisons of the differences in quantitative measurements were made by ANOVA, followed by Dunnett's t test (two-tailed). Statistical significance was accepted for a P < 0.05 level.
Results
Serum EPO Levels. A dose of 1,000 units/kg-bw rhEPO, administered via the tail vein as a bolus injection, reached a peak level of ≈2,070 ng/ml at the first sample point (15 min) and decayed slowly thereafter (Fig. 1). In contrast, an i.p. dose of 1,000 units/kg-bw reached a peak of ≈80 ng/ml at about 2 h and then slowly decreased. A dose of 500 units/kg-bw i.v. peaked at ≈100 ng/ml, and a dose of 5,000 units/kg-bw via either route reached equivalent serum levels only after ≈15 h (data not shown).
Fig. 1.
Pharmakokinetics of rhEPO (5,000 units/kg-bw) administered via the i.v. or i.p. routes (mean of three animals per group).
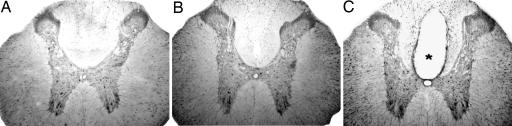
Inflammatory Response at the Lesion Epicenter. At baseline, scattered white matter cells and large pyramidal motoneurons expressed EPO (Fig. 2A). Four weeks after SCI, the EPO immunoreactivity was greatly increased in white matter astrocytes, whereas surviving neurons appeared unchanged (Fig. 2B). The staining intensity of neurons and astrocytes did not appear to change after an i.p. dose of 5,000 units/kg-bw EPO (Fig. 2B). In Fig. 2C, a typical section of the posttraumatic cavity is also visible, and its size is markedly reduced by EPO treatment (Fig. 2B). Four weeks after SCI, quantitative evaluation of the percentage of spared tissue at the lesion epicenter showed a significant reduction by EPO treatment but not if MPSS was applied alone or in addition to EPO (Table 1). Posttraumatic gliosis and scar formation (data not shown) was also reduced by EPO treatment. In the normal spinal cord, GFAP-positive cells are mainly located around the central canal and are only sparsely distributed in the white matter (Fig. 3 A-C). After injury, however, many GFAP-positive cells are present throughout the white and gray matter and around the cavitation (Fig. 3 D-F). Treatment with rhEPO (5,000 units/kg-bw i.p.) greatly reduced gliosis (Fig. 3 G-I) and inflammatory-cell recruitment into the site of injury: The number of cells expressing ED-1 (i.e., microglia) within the lesion epicenter after 7 days was markedly reduced by EPO treatment (Fig. 4).
Fig. 2.
Localization of EPO-expressing neurons and astrocytes at 4 weeks after SCI in vibratome sections of spinal cords at 3 mm rostral from the center of the lesion site by immunoperoxidase technique from control (A) and lesioned rats with (B) or without (C) rhEPO (5,000 units/kg-bw i.p.) treatment. In unlesioned rats subjected to only a laminectomy, positive labeling is observed in neurons throughout the gray matter and astrocytes in the white matter (A). A posttraumatic cavity (*) is present in lesioned rats treated with saline. (C) Astrocytes are more intensively stained, especially in the dorsal columns, whereas neurons are not. (B) In rats treated with rhEPO, the cavity is markedly reduced, and astrocytes hyperexpress EPO.
Table 1. Percentage of spared tissue at 4 weeks after SCI.
| Treatment | Lesion epicenter, % spared tissue | Throughout, % spared tissue |
|---|---|---|
| Saline | 42.1 + 2.3 | 49.9 + 3.6 |
| EPO | 56.2 + 4.0* | 66.4 + 3.0* |
| MPSS | 43.4 + 5.2 | 48.2 + 6.7 |
| EPO plus MPSS | 42.7 + 3.2 | 51.4 + 4.8 |
EPO at the dose of 5,000 units/kg-bw i.p. and MPSS 30 mg/kg-bw i.p. *, P < 0.01 compared with saline, MPSS, and EPO plus MPSS. Data are given as means ± SD.
Fig. 3.
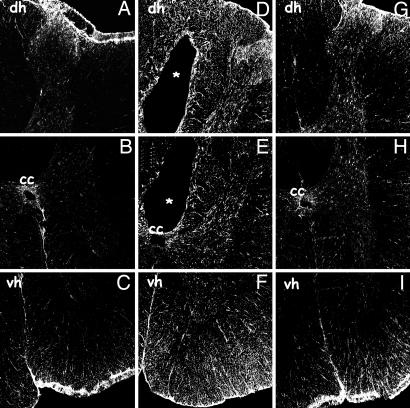
Immunolocalization of GFAP-positive cells at 3 mm rostral from the center of the SCI lesion site by immunofluorescence in vibratome sections of spinal cords obtained 4 weeks after SCI from control (A-C) and lesioned rats with (G-I) or without (D-F) rhEPO treatment. (A-C) In control spinal cords, some positive cells are present close to the meninges of the dorsal horn (dh) (A), around the central canal (cc) (B), and in the ventral white matter (vh) and meninges (C). (D-F) In lesioned saline-treated rats, GFAP-positive cells are present throughout the cord and around the posttraumatic cavity (D and E)(*) and in the ventral white matter (F). (G-I) Treatment with rhEPO greatly attenuates the GFAP expression in the injured cord.
Fig. 4.
Immunolocalization of ED-1-positive cells at 2 mm rostral from the center of the SCI lesion site by immunofluorescence in cryostat sections of spinal cords from lesioned rats 7 days after SCI with (EPO) or without (SALINE) rhEPO treatment. The number of ED-1-positive cells is greatly reduced throughout the lesion site in rhEPO-treated rats.
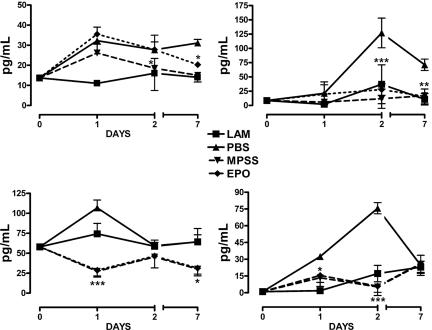
Tissue Cytokine Concentrations. Spinal cord concentrations of inflammatory cytokines determined by ELISA within the lesion epicenter at 1, 2, or 7 days after SCI (Fig. 5) exhibited time-dependent increases after injury, with MIP-2 (IL-8) increasing markedly within the first day and maintained up to 7 days. Treatment with MPSS or EPO was associated with a gradual decrease in MIP-2 levels beginning 2 days after SCI. MIP-2 is a recruitment signal for leukocytes, and histological evaluation at day 7 was remarkable for an almost complete absence of these inflammatory cells (Fig. 4), suggesting that secondary injury would also be reduced.
Fig. 5.
Cytokine profiles within the spinal cord after injury show that both EPO and MPSS suppress proinflammatory cytokine production to an equivalent degree. (Upper Left) MIP-2. (Upper Right) TNF-α.(Lower Left) IL-1β.(Lower Right) IL-6. LAM, laminectomy alone. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared with PBS).
TNF-α peaked by 2 days and subsequently decreased slightly but remained elevated at much higher levels, compared with the controls, for at least 7 days after injury. Both EPO and MPSS administration prevented these increases (Fig. 5). IL-6 also peaked within 2 days but then decreased rapidly. Both EPO and MPPS reduced the maximum IL-6 levels to that of the control. The posttraumatic increase of IL-1β occurred in the first day and was counteracted by both agents.
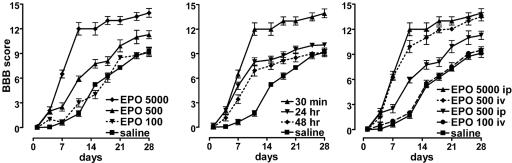
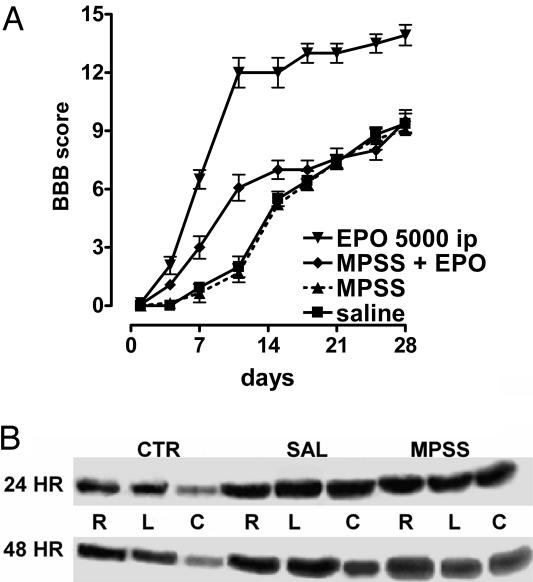
Recovery of Hindlimb Function. Open-field locomotor testing confirmed previous studies indicating that single doses of EPO improved recovery of hindlimb function for at least 4 weeks postinjury. The beneficial effects of EPO (5,000 units/kg-bw i.p.), administered within 30 min of lesioning, were obvious between 4 and 8 days after SCI and reached a plateau between 12 and 16 days (Fig. 6 Left). When treatment was delayed by 24 or 48 h, only a transient improvement was observed during the early posttraumatic stages of recovery, and, in both cases, motor function was not significantly different from saline-treated controls after 16 days (Fig. 6 Center). A lower dose of EPO, administered by means of an i.p. injection within 30 min of SCI, was less effective. A dose of 500 units/kg-bw i.p. yielded a smaller but significant improvement (Fig. 6 Right). When EPO was administered via the i.v. route, the minimum effective dose decreased to 500 units/kg-bw, with lower doses being ineffective (Fig. 6 Right). MPSS administration in combination with EPO, however, affected greatly the efficacy of EPO, because it failed to enhance the rate of hindlimb recovery (Fig. 7A), showing only a transient improvement (similar to the delayed treatment). MPPS alone also did not affect the rate of recovery of function and was identical to saline. Western blotting performed for EPOR using proteins obtained adjacent to the lesion epicenter showed marked increases at all levels examined 1 day after injury, maintained for at least an additional 24 h (Fig. 7B). Treatment with MPSS did not affect EPOR protein abundance.
Fig. 6.
Open-field motor scores after injury show dose-, time-, and route-dependency of EPO. (Left) For single doses delivered i.p. both 5,000 units/kg-bw and 500 units/kg-bw were significantly better than saline, whereas 100 units/kg-bw was not. (Center) Although EPO (5,000 units/kg-bw) administered up to 48 h after injury was associated with a significantly better motor score for the first 3 weeks after injury, by 28 days, 24- and 48-h delays in treatment were not different from saline. (Right) EPO administered i.v. at a lower dose of 500 units/kg-bw was significantly better than the same dose administered i.p.
Fig. 7.
MPSS neutralizes EPO-mediated spinal cord protection. (A) MPSS antagonizes the protective effects of EPO in recovery of open-field motor scores. Although early recovery was significantly better than saline, by 3 weeks, animals receiving both MPSS and EPO were not different from those receiving saline. (B) Immunoblotting for EPOR within the spinal cord at 24 or 48 h after a sham injury (laminectomy and impactor positioning only) (CTR) revealed the most pronounced expression of EPOR protein in the rostral (R), lesion site (L), and caudal (C) portions of the cord. Subsequent to impaction injury, EPOR immunoreactivity increased markedly at the three levels examined. No differences were noted between MPSS- versus saline (SAL)-treated animals.
Discussion
EPO and its nonerythropoietic derivatives have been shown to be remarkably effective in tempering injury in diverse tissues and organs (reviewed by Brines and Cerami in ref. 15). For EPO in experimental stroke, the presence of the blood-brain barrier has required high doses, in the range of ≈500-5,000 units/kg-bw administered i.v. or i.p. for effective triggering of neuroprotection. Similarly, the data obtained here and our previous observations (17) show that rhEPO, administered i.p., is effective in SCI at a minimum effective dose of ≈500 units/kg-bw. The fact that high doses of EPO i.v. or i.p. are therapeutic when administered within 30 min, despite a much slower rise of serum levels after i.p. injection, is consistent with a broad therapeutic temporal window in which delayed dosing is still effective in SCI. A dose of 500 units/kg-bw of EPO, administered i.v., that peaked at ≈100 ng/ml was as efficacious as a dose of 5,000 units/kg-bw i.p. that produced serum concentrations of ≈50-80 ng/ml. In contrast, lower doses of EPO administered either i.v. or i.p. were ineffective, suggesting that peak serum concentrations of ≈50 ng/ml are required for significant protection. Unlike EPO's action in erythropoiesis, in which the area-under-the-curve predicts biological efficacy (29) attainment of a minimum peak concentration appears to be critical for tissue protection.
We have shown that a wide therapeutic time window exists for the nonerythropoietic analogue of EPO (carbamylated EPO), administered in a multiple-dosage regimen after compressive SCI (30) and for a single dose of EPO, administered i.v. at 5,000 units/kg-bw (31). In this study, the temporal window for a single dose of EPO administered via the i.p. route appears narrower. Although an initial improvement was noted for animals treated with EPO, it was transient, and, after 14 days, the slope of the recovery curve lessened, and the motor score at 1 month was not different from the saline group. This observation suggests that traumatic impact to the spinal cord triggers processes that cannot be modulated by a single dose of exogenous EPO administered with a delay of >12 h. The qualitatively different type of injury (with an especially prominent vascular component) produced by the aneurysm-clip model appears to lack this critical-injury component, because a single dose can be delayed for 24 h without a significant loss in efficacy (31).
A complicated interplay of pro- and antiinflammatory molecules orchestrates the pathophysiology of the spinal cord after injury. Within the first hours, neutrophils appear (32), with lymphocytes and microglia only later mobilized into the injury site (at 24-48 h). Waves of oligodendrocyte apoptosis begins later (peaking at about day 8) and are completed by 12-14 days (33, 34). Our data show that even a single dose of EPO can markedly reduce the influx of microglia (ED-1-positive cells) into the lesion site within 1 week. The presence of microglia is associated with cavitation and marked scarring, as we have observed for the saline-treated animals, and was not seen in the EPO-treatment groups.
Much attention has focused on the potential key roles of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 for amplification of damage after a CNS injury (35). In our experiments, the TNF-α and IL-6 in untreated animals peaked by day 2 and thereafter declined, even without treatment. The observed peaks are delayed compared with the reports in other studies of a range of 1-6 h of neurons and microglia, respectively (36, 37). Furthermore, Pan et al. (38) have shown rapid induction (minutes) of proinflammatory cytokine mRNAs in SCI arising from the spinal cord itself. An explanation for these differences is currently lacking but could depend on the severity of injury and, therefore, the pathophysiological mechanism of cytokine production. Despite obvious differences in peak cytokine production, there is a consensus in the literature that MPSS suppresses inflammation (reviewed in ref. 39).
However, it is interesting that, although MPSS markedly reduced cytokine concentrations, both locally within the epicenter and more distally, no improvement in outcome was observed in our studies. Thus, permanent injury does not invariably depend on the presence of inflammatory processes. It is especially surprising that MPSS-mediated suppression of IL-6 was not associated with an improved motor score, because changes of this cytokine have been associated with a parallel modulation of injury in experimental SCI (40, 41). It should be noted that, in contrast to MPSS, other experimental treatments attenuating the acute inflammatory response, e.g., the hydroxymethylglutaryl CoA reductase inhibitor atorvastatin (42) or antithrombin (43), among others, show significant benefits in preclinical models.
When MPSS was coadministered with a dose of EPO otherwise sufficient to promote recovery, an early improvement in motor score was observed over the first 2 days, similar to what is seen with delayed administration of EPO alone, suggesting that MPSS neutralized early EPO-mediated protective responses, likely noninflammatory actions of EPO. A number of potential explanations exist. For example, MPSS might blunt the up-regulation of EPOR, a critical mediator of responsiveness to injury, for both endogenous and exogenous EPO. On the basis of the EPOR Western blot data obtained from the injured spinal cord, EPOR is present in this model not only at baseline but increases after the impaction in a similar manner, with or without MPSS. Of note, however, the antibody used detects the EPOR monomer, whereas our work suggests that tissue protection is mediated by a heteromeric receptor consisting of the EPOR and the β common subunit receptor (18) of IL-3. Protein analysis based on EPOR alone, therefore, would not detect changes of the β common subunit abundance. Alternatively, MPSS could interfere with assembly of the hetercomplex transducing tissue protection. Another receptor-based explanation could be that, although MPSS decreases cytokine expression, it might not do the same for cytokine receptors. Therefore, trace levels of cytokines could still signal biologically. Currently, there are no data available to evaluate this possibility.
SCI is associated with an early loss of vascular autoregulation, which, if antagonized, leads to a rapid motor recovery (44, 45) that is very similar temporally to what has been observed after EPO administration. In other preclinical injury models, e.g., the spasm of the basilar artery induced by subarachnoid hemorrhage (46, 47) or the splanchnic-artery constriction in the setting of septic shock (24), are reversed by single doses of EPO. It is notable that high-dose glucocortoid administration has been associated with vascular dysfunction (reviewed by Yand and Zhang in ref. 48). A major mechanism appears to be disordered nitric oxide production and function (49, 50).
Furthermore, MPSS might increase the clearance of EPO from the vasculature, for example, by augmenting renal excretion. This explanation appears very unlikely, because an EPO analogue with an exceedingly short half-life (several minutes) is very effective when administered as a single bolus immediately after compressive injury of the spinal cord (51). Although high-dose MPSS could inhibit the sympathetic discharge after an injury (52) (which, secondarily, could suppress IL-10-mediated antiinflammatory effects) (53, 54), there is no evidence supporting this possibility. Other potential endocrine-mediated systemic effects of MPSS are possible and will require evaluation.
High-dose glucocorticoid administration has been shown to inhibit not only proinflammatory cytokines but also neuroprotective growth factors, e.g., glial-derived neurotrophic factor (14), brain-derived neurotrophic factor (BDNF), and NT-3 (55). A recent study showing that the neuroprotection activities of EPO for hippocampal neurons in vitro depends on the production of BDNF (56) is directly relevant. In this view, MPSS could suppress EPO-mediated neurotrophin production, accounting for the deleterious effects of glucocorticoids in saline-treated animals (i.e., interfering with endogenously produced EPO and with exogenous EPO). These explanations for the antagonizing effects of MPSS on the beneficial effects of EPO require further evaluation.
In sum, these studies show a wide dose- and time window for the use of EPO in SCI. MPSS appears to negate critical early-phase actions of EPO. Although further study is required to understand the effects of multiple dosing of MPSS, prudence dictates that MPSS or other glucocorticoids be avoided in clinical trials to determine the efficacy of EPO in human spinal cord disease. Whether similar neutralizing effects of MPSS occur with other therapeutics is unknown but should be considered a possibility.
Acknowledgments
Confocal microscopy was carried out at the Centro Interdipartimentale di Microscopia Avanzata, University of Milan. This work was supported by the Italian Ministry of Education and Research (COFIN 2003) and 2002 Istituto di Recovera e Cura a Carattere Scientifico (Italian Ministry of Health) (A.G.), with additional funding provided by the Kenneth S. Warren Institute.
Author contributions: A.G., A.C., and M.B. designed research; A.G., L.M., B.D.S., S.C., A.M.D.G., S.D.B., T.C., and M.B. performed research; A.G., L.M., B.D.S., S.C., A.M.D.G., S.D.B., T.C., and M.B. analyzed data; and A.G., T.C., A.C., and M.B. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: EPO, erythropoietin; EPOR, EPO receptor; GFAP, glial fibrillary acidic protein; kg-bw, kg of body weight; MPSS, methylprednisolone sodium succinate; rhEPO, recombinant human EPO; MIP, macrophage inflammatory protein; SCI, spinal cord injury.
References
- 1.Bracken, M. B., Shepard, M. J., Collins, W. F., Jr., Holford, T. R., Baskin, D. S., Eisenberg, H. M., Flamm, E., Leo-Summers, L., Maroon, J. C., Marshall, L. F., et al. (1992) J. Neurosurg. 76, 23-31. [DOI] [PubMed] [Google Scholar]
- 2.Qian, T., Guo, X., Levi, A. D., Vanni, S., Shebert, R. T. & Sipski, M. L. (2005) Spinal Cord 43, 199-203. [DOI] [PubMed] [Google Scholar]
- 3.Short, D. J., El Masry, W. S. & Jones, P. W. (2000) Spinal Cord 38, 273-286. [DOI] [PubMed] [Google Scholar]
- 4.American Association of Neurological Surgeons (2002) Neurosurg. Suppl. 50, S63-S72. [Google Scholar]
- 5.Kulbatski, I., Mothe, A. J., Nomura, H. & Tator, C. H. (2005) Curr. Drug Targets 6, 111-126. [DOI] [PubMed] [Google Scholar]
- 6.Kwon, B. K., Borisoff, J. F. & Tetzlaff, W. (2002) Mol. Intervent. 2, 244-258. [DOI] [PubMed] [Google Scholar]
- 7.Szabo, C. (1996) Shock 6, 79-88. [DOI] [PubMed] [Google Scholar]
- 8.Merola, A., O'Brien, M. F., Castro, B. A., Smith, D. A., Eule, J. M., Lowe, T. G., Dwyer, A. P., Haher, T. R. & Espat, N. J. (2002) J. Orthop. Trauma 16, 155-161. [DOI] [PubMed] [Google Scholar]
- 9.Yu, Y., Matsuyama, Y., Nakashima, S., Yanase, M., Kiuchi, K. & Ishiguro, N. (2004) NeuroReport 15, 2103-2107. [DOI] [PubMed] [Google Scholar]
- 10.Hall, E. D. & Springer, J. E. (2004) NeuroRx 1, 80-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabchevsky, A. G., Fugaccia, I., Sullivan, P. G., Blades, D. A. & Scheff, S. W. (2002) J. Neurosci. Res. 68, 7-18. [DOI] [PubMed] [Google Scholar]
- 12.Sapolsky, R. M. & Pulsinelli, W. A. (1985) Science 229, 1397-1400. [DOI] [PubMed] [Google Scholar]
- 13.Diem, R., Hobom, M., Maier, K., Weissert, R., Storch, M. K., Meyer, R. & Bahr, M. (2003) J. Neurosci. 23, 6993-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima, S., Matsuyama, Y., Yu, Y., Kiuchi, K. & Ishiguro, N. (2004) NeuroReport 15, 2337-2340. [DOI] [PubMed] [Google Scholar]
- 15.Brines, M. & Cerami, A. (2005) Nat. Rev. Neurosci. 6, 484-494. [DOI] [PubMed] [Google Scholar]
- 16.Celik, M., Gokmen, N., Erbayraktar, S., Akhisaroglu, M., Konakc, S., Ulukus, C., Genc, S., Genc, K., Sagiroglu, E., Cerami, A. & Brines, M. (2002) Proc. Natl. Acad. Sci. USA 99, 2258-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorio, A., Gokmen, N., Erbayraktar, S., Yilmaz, O., Madaschi, L., Cichetti, C., Di Giulio, A. M., Vardar, E., Cerami, A. & Brines, M. (2002) Proc. Natl. Acad. Sci. USA 99, 9450-9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brines, M., Grasso, G., Fiordaliso, F., Sfacteria, A., Ghezzi, P., Fratelli, M., Latini, R., Xie, Q. W., Smart, J., Su-Rick, C. J., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 14907-14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaptanoglu, E., Solaroglu, I., Okutan, O., Surucu, H. S., Akbiyik, F. & Beskonakli, E. (2004) Neurosurg. Rev. 27, 113-120. [DOI] [PubMed] [Google Scholar]
- 20.Ghezzi, P. & Brines, M. (2004) Cell Death Differ. 11, S37-S44. [DOI] [PubMed] [Google Scholar]
- 21.Villa, P., Bigini, P., Mennini, T., Agnello, D., Laragione, T., Cagnotto, A., Viviani, B., Marinovich, M., Cerami, A., Coleman, T. R., et al. (2003) J. Exp. Med. 198, 971-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahlmann, F. H., De Groot, K., Spandau, J. M., Landry, A. L., Hertel, B., Duckert, T., Boehm, S. M., Menne, J., Haller, H. & Fliser, D. (2004) Blood 103, 921-926. [DOI] [PubMed] [Google Scholar]
- 23.Buemi, M., Vaccaro, M., Sturiale, A., Galeano, M. R., Sansotta, C., Cavallari, V., Floccari, F., D'Amico, D., Torre, V., Calapai, G., et al. (2002) Acta Derm. Venereol. 82, 411-417. [DOI] [PubMed] [Google Scholar]
- 24.Squadrito, F., Altavilla, D., Squadrito, G., Campo, G. M., Arlotta, M., Quartarone, C., Saitta, A. & Caputi, A. P. (1999) Br. J. Pharmacol. 127, 482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grasso, G., Sfacteria, A., Passalacqua, M., Morabito, A., Buemi, M., Macri, B., Brines, M. L. & Tomasello, F. (2005) Neurosurgery 56, 821-827. [DOI] [PubMed] [Google Scholar]
- 26.Cayli, S. R., Kocak, A., Yilmaz, U., Tekiner, A., Erbil, M., Ozturk, C., Batcioglu, K. & Yologlu, S. (2004) Eur. Spine J. 13, 724-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basso, D. M., Beattie, M. S. & Bresnahan, J. C. (1995) J. Neurotrauma 12, 1-21. [DOI] [PubMed] [Google Scholar]
- 28.Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. (1951) J. Biol. Chem. 193, 265-275. [PubMed] [Google Scholar]
- 29.Besarab, A. (2000) Semin. Nephrol. 20, 364-374. [PubMed] [Google Scholar]
- 30.Leist, M., Ghezzi, P., Grasso, G., Bianchi, R., Villa, P., Fratelli, M., Savino, C., Bianchi, M., Nielsen, J., Gerwien, J., et al. (2004) Science 305, 239-242. [DOI] [PubMed] [Google Scholar]
- 31.Grasso, G., Sfacteria, A., Cerami, A. & Brines, M. (2004) Neuroscientist 10, 93-98. [DOI] [PubMed] [Google Scholar]
- 32.Schnell, L., Fearn, S., Klassen, H., Schwab, M. E. & Perry, V. H. (1999) Eur. J. Neurosci. 11, 3648-3658. [DOI] [PubMed] [Google Scholar]
- 33.Liu, X. Z., Xu, X. M., Hu, R., Du, C., Zhang, S. X., McDonald, J. W., Dong, H. X., Wu, Y. J., Fan, G. S., Jacquin, M. F., et al. (1997) J. Neurosci. 17, 5395-5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shuman, S. L., Bresnahan, J. C. & Beattie, M. S. (1997) J. Neurosci. Res. 50, 798-808. [DOI] [PubMed] [Google Scholar]
- 35.Merrill, J. E. & Benveniste, E. N. (1996) Trends Neurosci. 19, 331-338. [DOI] [PubMed] [Google Scholar]
- 36.Yang, L., Jones, N. R., Blumbergs, P. C., Van Den Heuvel, C., Moore, E. J., Manavis, J., Sarvestani, G. T. & Ghabriel, M. N. (2005) J. Clin. Neurosci. 12, 276-284. [DOI] [PubMed] [Google Scholar]
- 37.Yang, L., Blumbergs, P. C., Jones, N. R., Manavis, J., Sarvestani, G. T. & Ghabriel, M. N. (2004) Spine 29, 966-971. [DOI] [PubMed] [Google Scholar]
- 38.Pan, J. Z., Ni, L., Sodhi, A., Aguanno, A., Young, W. & Hart, R. P. (2002) J. Neurosci. Res. 68, 315-322. [DOI] [PubMed] [Google Scholar]
- 39.Fu, E. S. & Saporta, S. (2005) J. Neurosurg. Anesthesiol. 17, 82-85. [DOI] [PubMed] [Google Scholar]
- 40.Lacroix, S., Chang, L., Rose-John, S. & Tuszynski, M. H. (2002) J. Comp. Neurol. 454, 213-228. [DOI] [PubMed] [Google Scholar]
- 41.Okada, S., Nakamura, M., Mikami, Y., Shimazaki, T., Mihara, M., Ohsugi, Y., Iwamoto, Y., Yoshizaki, K., Kishimoto, T., Toyama, Y. & Okano, H. (2004) J. Neurosci. Res. 76, 265-276. [DOI] [PubMed] [Google Scholar]
- 42.Pannu, R., Barbosa, E., Singh, A. K. & Singh, I. (2005) J. Neurosci. Res. 79, 340-350. [DOI] [PubMed] [Google Scholar]
- 43.Hirose, K., Okajima, K., Uchiba, M., Nakano, K. Y., Utoh, J. & Kitamura, N. (2004) Thromb. Haemost. 91, 162-170. [DOI] [PubMed] [Google Scholar]
- 44.Mautes, A. E., Weinzierl, M. R., Donovan, F. & Noble, L. J. (2000) Phys. Ther. 80, 673-687. [PubMed] [Google Scholar]
- 45.Noble, L. J., Donovan, F., Igarashi, T., Goussev, S. & Werb, Z. (2002) J. Neurosci. 22, 7526-7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Springborg, J. B., Ma, X., Rochat, P., Knudsen, G. M., Amtorp, O., Paulson, O. B., Juhler, M. & Olsen, N. V. (2002) Br. J. Pharmacol. 135, 823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grasso, G., Buemi, M., Alafaci, C., Sfacteria, A., Passalacqua, M., Sturiale, A., Calapai, G., De Vico, G., Piedimonte, G., Salpietro, F. M. & Tomasello, F. (2002) Proc. Natl. Acad. Sci. USA 99, 5627-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang, S. & Zhang, L. (2004) Curr. Vasc. Pharmacol. 2, 1-12. [DOI] [PubMed] [Google Scholar]
- 49.Iuchi, T., Akaike, M., Mitsui, T., Ohshima, Y., Shintani, Y., Azuma, H. & Matsumoto, T. (2003) Circ. Res. 92, 81-87. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell, B. M., Dorrance, A. M., Mack, E. A. & Webb, R. C. (2004) J. Cardiovasc. Pharmacol. 43, 8-13. [DOI] [PubMed] [Google Scholar]
- 51.Erbayraktar, S., Grasso, G., Sfacteria, A., Xie, Q. W., Coleman, T., Kreilgaard, M., Torup, L., Sager, T., Erbayraktar, Z., Gokmen, N., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 6741-6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown, M. R. & Fisher, L. A. (1986) Life Sci. 39, 1003-1012. [DOI] [PubMed] [Google Scholar]
- 53.Elenkov, I. J. & Chrousos, G. P. (2002) Ann. N.Y. Acad. Sci. 966, 290-303. [DOI] [PubMed] [Google Scholar]
- 54.Woiciechowsky, C., Asadullah, K., Nestler, D., Eberhardt, B., Platzer, C., Schoning, B., Glockner, F., Lanksch, W. R., Volk, H. D. & Docke, W. D. (1998) Nat. Med. 4, 808-813. [DOI] [PubMed] [Google Scholar]
- 55.Hayashi, M., Ueyama, T., Nemoto, K., Tamaki, T. & Senba, E. (2000) J. Neurotrauma 17, 203-218. [DOI] [PubMed] [Google Scholar]
- 56.Viviani, B., Bartesaghi, S., Corsini, E., Villa, P., Ghezzi, P., Garau, A., Galli, C. L. & Marinovich, M. (2005) J. Neurochem. 93, 412-421. [DOI] [PubMed] [Google Scholar]