Abstract
The abilities of the most common European genospecies of Borrelia burgdorferi sensu lato to survive blood meals taken by ticks feeding on birds were analyzed. A pattern of differential survival of the spirochetes in feeding ticks was observed. The result is consistent with the concept of selective transmission of Lyme borreliosis spirochetes.
The genospecies of Borrelia burgdorferi sensu lato and, at times, their variants are maintained in nature by different sets of hosts (1, 3, 6-10, 15, 18, 20, 21, 23; K. Hanincová, S. M. Schäfer, S. Etti, H.-S. Sewell, V. Taragelová, D. Ziak, M. Labuda, and K. Kurtenbach, submitted for publication). At present, the key determinant of this host association is considered to be the interaction of B. burgdorferi sensu lato with the alternative pathway of the host's complement system (14, 16, 19, 22, 25). A recently proposed model of transmission predicts the selection of spirochetes by complement in the gut of feeding ticks (17, 18). In the present study, an avian model was used to test this prediction.
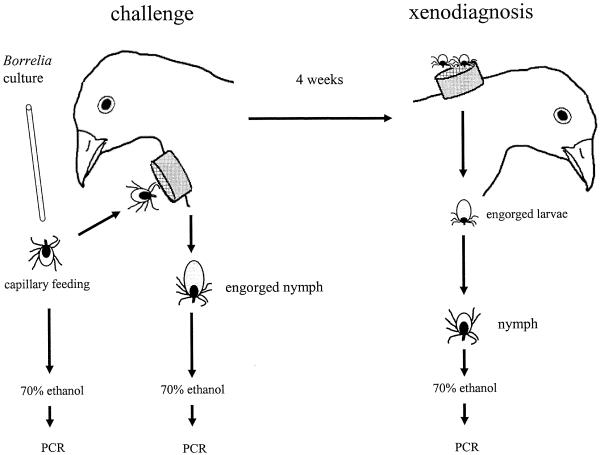
Two-week-old pheasants (Phasianus colchicus) were obtained from a breeder in Oxfordshire, United Kingdom. Ixodes ricinus larvae and nymphs were derived from a tick colony maintained at the NERC Centre for Ecology and Hydrology, Oxford, United Kingdom. Low-passage cultures of B. burgdorferi sensu stricto (ZS 7), Borrelia afzelii (ACA 1), Borrelia garinii (an isolate from Freiburg, Germany, with an ospA allele identical to that of the Rio2 strain [2]), and Borrelia valaisiana (strain UK) were expanded to a concentration of 107 cells per ml of culture medium. The cultures were used to infect questing nymphs with spirochetes by glass capillary feeding. Of those ticks which took up more than 100 spirochetes, as calculated by the volume of culture imbibed, 20 were introduced to each bird by being confined within a neoprene cell glued with latex to the shaved throat of each bird (Fig.1). Four groups composed of four birds each were challenged with ticks infected with one of the genospecies of B. burgdorferi sensu lato. Engorged nymphs were recovered, kept for 2 weeks, and then preserved in 70% ethanol.
For comparison, an additional four groups of 10 nymphs each (all of whom were preinfected with one of the four genospecies) were not allowed to feed on hosts but were starved for several weeks and then preserved in ethanol. Four weeks after the challenge with infected nymphs, the pheasants were tested by xenodiagnosis for Borrelia through the introduction of >50 noninfected I. ricinus larvae per animal. These larvae were also contained in neoprene cells and glued to the back of the neck of each pheasant. Engorged larvae were allowed to molt to nymphs. After xenodiagnosis, two skin biopsy samples were taken from each bird, one from the feeding site of ticks and one from the eyelid. The 5S-23S intergenic spacer and the ospA locus of B. burgdorferi sensu lato were amplified by nested PCR from DNA extracted from Borrelia cultures, ticks, and biopsy samples (5, 15), and the PCR products were sequenced. In addition, wild-type strains from three natural sources were analyzed: (i) ticks engorged on wild pheasants captured during 1996 in a woodland near Wimborne St. Giles, Dorset, United Kingdom (15), (ii) questing I. ricinus ticks collected in the summer of 2001 from the same site, and (iii) questing ticks collected in the spring of 2000 in a forest near Ville d'Eu, Normandy, France.
Of the 80 experimentally infected nymphs introduced to each group of birds, between 27 (34%) and 56 (70%) were recovered in a fully engorged state. Approximately half of the preinfected nymphs tested positive for B. burgdorferi sensu stricto, B. garinii, and B. valaisiana 2 weeks post-repletion (Fisher's exact test, P > 0.05) (Table 1). B. garinii and B. burgdorferi sensu stricto were also detected in xenodiagnostic ticks. Xenodiagnosis performed on birds challenged with B. valaisiana was unsuccessful because the larvae from this group of birds, all kept in one desiccator, did not survive. B. garinii was significantly more prevalent than B. burgdorferi sensu stricto in xenodiagnostic ticks (P < 0.05). By contrast, B. afzelii was not detected 2 weeks after repletion in either preinfected nymphs or xenodiagnostic ticks. However, B. afzelii as well as the other genospecies were detected in all starved nymphs that had taken up >100 spirochetes. One out of four and three out of eight biopsy samples taken from birds challenged by nymphs infected with B. garinii and B. valaisiana, respectively, tested positive, but all the birds exposed to nymphs preinfected with B. afzelii were negative. The ospA sequences of the B. garinii and B. valaisiana cultures were compared to ospA sequences detected in natural foci in the United Kingdom and France. In each pool from the field, the allele of the Rio2 strain of B. garinii (GenBank accession number AF227319; OspA serotype 3 [2]) and the allele of the UK strain of B. valaisiana (GenBank accession number AF09591) were found with considerable frequencies (Table 2).
TABLE 1.
Infection chains of B. burgdorferi sensu lato genospecies with the pheasant as the avian host model, infected I. ricinus nymphs as vectors, and larvae as xenodiagnostic ticksa
| Genospecies | No. positive/total no. examined (%)
|
||
|---|---|---|---|
| Vector ticks | Xenodiagnostic ticksb | Biopsy samples | |
| B. burgdorferi sensu stricto | 12/27 (44) | 6/44 (13) | NDc |
| B. afzelii | 0/56 (0) | 0/33 (0) | 0/8 |
| B. garinii | 14/31 (45) | 31/72 (43) | 1/4 |
| B. valaisiana | 23/38 (60) | NAd | 3/8 |
Four birds were examined for each genospecies tested.
Tested for infection after molting to nymphs.
ND, not determined.
N/A, not available. The larvae from this group of birds did not survive.
TABLE 2.
Frequency of ospA alleles of B. garinii and B. valaisiana in the field
| Sourcea | No. of B. garinii sequences | No. of B. garinii alleles | Frequency of Rio2 strain alleleb | No. of B. valaisiana sequences | No. of B. valaisiana alleles | Frequency of UK strain allele |
|---|---|---|---|---|---|---|
| A | 16 | 6 | 3/16 | 6 | 2 | 4/6 |
| B | 35 | 12 | 3/35 | 9 | 1 | 9/9 |
| C | 27 | 14 | 2/27 | 12 | 3 | 10/12 |
A, engorged nymphs of I. ricinus fed on male pheasants captured in Dorset, United Kingdom, in 1996 (15); B, questing nymphal and adult I. ricinus ticks collected in Dorset, United Kingdom; C, questing nymphal and adult I. ricinus collected in Ville d'Eu, Normandy, France.
Rio2 is the B. garinii strain which represents OspA serotype 3 (2).
The results show that B. garinii, B. valaisiana, and B. burgdorferi sensu lato survived the blood meal taken by ticks feeding on pheasants, whereas the B. afzelii strain was eliminated during the blood meal. The differential survival of spirochetes in the vector ticks was consistent with the xenodiagnostic data, confirming that pheasants are adequate reservoirs for B. garinii and B. burgdorferi sensu stricto (15). Although we did not assess the transmission of B. valaisiana from pheasants to ticks in this study, the survival of this genospecies in vector nymphs and its detection in three out of eight biopsy samples suggest that pheasants are also adequate reservoirs for B. valaisiana. This is strongly supported by the presence of this genospecies in ticks that fed on wild pheasants (15) (Table 2).
It is very unlikely that the elimination of the ACA 1 strain of B. afzelii in ticks feeding on birds was due to the loss of infectivity of this particular isolate, because starved ticks remained infected with this strain. Rather, the results indicate that the uptake of avian blood triggered the elimination of B. afzelii in the tick. Furthermore, B. afzelii has been detected only occasionally in bird-derived ticks anywhere in the world. The experimental and field-derived data taken together strongly suggest that all strains of B. afzelii are killed in ticks feeding on birds.
The pattern of differential survival found in this study is consistent with in vitro findings on resistance or sensitivity of spirochetes to avian complement of studies using the same cultures of B. burgdorferi sensu lato (16). The data validate our recent model of selective transmission of B. burgdorferi sensu lato, which suggests that B. afzelii is lysed by avian complement in the gut of the feeding tick (17, 18). It remains to be determined whether B. garinii and B. valaisiana are eliminated in ticks feeding on rodents, as would be predicted by the high sensitivity to complement of B. garinii strains (other than OspA serotype 4 strains or NT 29 ribotypes that are associated with rodents [8, 21]) and B. valaisiana (16).
Resistance of spirochetes to complement is now known to be mediated by the expression of complement regulator-acquiring surface proteins (11-13). In fact, a general property of the proteins encoded by genes of the erp gene family appears to be their ability to bind host-derived complement control proteins in a species-specific pattern (24). Furthermore, the erp genes are expressed in infected, feeding Ixodes scapularis ticks (4). Altogether, the combined information points to the tick gut as a crucial site of selection during the life cycle of B. burgdorferi sensu lato. Thus, we suggest that B. burgdorferi sensu lato comprises distinct ecotypes that are determined by their erp gene repertoires.
FIG. 1.
Flow chart illustrating the experimental protocol to assess reservoir competence of pheasants for B. burgdorferi sensu lato. The life stages of ticks and the points where samples were taken and preserved are indicated.
Acknowledgments
We thank M. M. Simon (Freiburg, Germany) for the supply of Borrelia cultures.
The study was supported by the Natural Environment Research Council and The Wellcome Trust, London, United Kingdom.
Editor: D. L. Burns
REFERENCES
- 1.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dobson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 2.Escudero, R., M. Barral, A. Pérez, M. M. Vitutia, A. L. García-Pérez, S. Jiménez, R. E. Sellek, and P. Anda. 2000. Molecular and pathogenic characterization of Borrelia burgdorferi sensu lato isolates from Spain. J. Clin. Microbiol. 38:4026-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gern, L., A. Estrada-Pena, F. Frandsen, J. S. Gray, T. G. T. Jaenson, F. Jongejan, O. Kahl, E. Korenberg, R. Mehl, and P. A. Nuttall. 1998. European reservoir hosts of Borrelia burgdorferi sensu lato. Zentbl. Bakteriol. 287:196-204. [DOI] [PubMed] [Google Scholar]
- 4.Gilmore, R. D., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 5.Guy, E. C., and G. Stanek. 1991. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain reaction. J. Clin. Pathol. 44:610-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gylfe, Å., B. Olsen, D. Straševièius, N. M. Ras, P. Weihe, L. Noppa, Y. Östberg, G. Baranton, and S. Bergström. 1999. Isolation of Lyme disease Borrelia from puffins (Fratercula arctica) and seabird ticks (Ixodes uriae) on the Faeroe Islands. J. Clin. Microbiol. 37:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gylfe, Å., S. Bergström, J. Lunstrom, and B. Olsen. 2000. Epidemiology—reactivation of Borrelia infection in birds. Nature 403:724-725. [DOI] [PubMed] [Google Scholar]
- 8.Hu, C. M., B. Wilske, V. Fingerle, Y. Lobet, and L. Gern. 2001. Transmission of Borrelia garinii OspA serotype 4 to BALB/c mice by Ixodes ricinus ticks collected in the field. J. Clin. Microbiol. 39:1169-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humair, P. F., D. Postic, R. Wallich, and L. Gern. 1998. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zentbl. Bakteriol. 287:521-538. [PubMed] [Google Scholar]
- 10.Humair, P. F., O. Rais, and L. Gern. 1999. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks, differential transmission pattern and overwintering maintenance. Parasitology 118:33-42. [DOI] [PubMed] [Google Scholar]
- 11.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 12.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2001. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int. Immunopharmacol. 1:393-401. [DOI] [PubMed] [Google Scholar]
- 13.Kraiczy, P. C. Skerka, V. Brade, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo, M. M., R. S. Lane, and P. C. Giclas. 2000. A comparative study of mammalian and reptilian alternative pathway of complement-mediated killing of the Lyme disease spirochete (Borrelia burgdorferi). J. Parasitol. 86:1223-1228. [DOI] [PubMed] [Google Scholar]
- 15.Kurtenbach, K., M. Peacey, S. G. T. Rijpkema, A. N. Hoodless, P. A. Nuttall, and S. E. Randolph. 1998. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl. Environ. Microbiol. 64:1169-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtenbach, K., H.-S. Sewell, N. H. Ogden, S. E. Randolph, and P. A. Nuttall. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66:1248-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtenbach, K., S. De Michelis, H.-S. Sewell, S. Etti, S. M. Schäfer, R. Hails, M. Collares-Pereira, M. Santos-Reis, K. Haninçová, M. Labuda, A. Bormane, and M. Donaghy. 2001. Distinct combinations of Borrelia burgdorferi sensu lato genospecies found in individual questing ticks from Europe. Appl. Environ. Microbiol. 67:4926-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtenbach, K., S. De Michelis, S. Etti, S. M. Schäfer, H.-S. Sewell, V. Brade, and P. Kraiczy. 2002. Host association of Borrelia burgdorferi sensu lato—-the key role of host complement. Trends Microbiol. 10:74-79. [DOI] [PubMed] [Google Scholar]
- 19.Lane, R. S., and G. B. Quistad. 1998. Borreliacidal factor in the blood of the western fence lizard (Sceloporus occidentalis). J. Parasitol. 84:29-34. [PubMed] [Google Scholar]
- 20.Le Fleche, A., D. Postic, K. Girardet, O. Peter, and G. Baranton. 1997. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 47:921-925. [DOI] [PubMed] [Google Scholar]
- 21.Marconi, R. T., S. Hohenberger, S. Jauris-Heipke, U. Schulte-Spechtel, C. P. Lavoie, D. Röβler, and B. Wilske. 1999. Genetic analysis of Borrelia garinii OspA serotype 4 strains associated with neuroborreliosis: evidence for extensive genetic homogeneity. J. Clin. Microbiol. 37:3965-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson, D. R., S. Rooney, N. J. Miller, and T. N. Mather. 2000. Complement-mediated killing of Borrelia burgdorferi by non-immune sera from sika deer. J. Parasitol. 86:1232-1238. [DOI] [PubMed] [Google Scholar]
- 23.Postic, D., M. Assous, P. A. D. Grimont, and G. Baranton. 1994. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int. J. Syst. Bacteriol. 44:743-752. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Dam, A. P., A. Oei, R. Jaspars, C. Fijen, B. Wilske, L. Spanjaard, and J. Dankert. 1997. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect. Immun. 65:1228-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]