Reason for posting: Selective serotonin reuptake inhibitors (SSRIs) have not previously been demonstrated, as a group, to be teratogenic.1 However, the results of an unpublished study2 by GlaxoSmithKline (GSK) has led the US Food and Drug Administration and Health Canada to warn that one SSRI, paroxetine, may increase the risk of major congenital malformations.3
The drug: Antidepressants, including paroxetine, are used to treat major depression, anxiety, obsessive–compulsive disorder and premenstrual dysphoria, all common disorders during the childbearing years.4 GSK, the manufacturer of paroxetine and another antidepressant, bupropion, recently completed an unpublished retrospective study of data from 2 US managed-care insurance databases. Pregnancy outcomes among 3581 expectant mothers aged 12– 49 years who were taking antidepressants were studied.
Initially, the study sought to investigate whether women prescribed buproprion had infants with higher rates of cardiovascular malformations than women either taking other antidepressants or taking the drug in the third trimester only. Any cardiac or serious congenital malformation was recorded for users of bupropion in the first or third trimester and users of any other antidepressants. The analysis excluded women with concurrent first-trimester use of known teratogens, including lithium, valproic acid and carbamazepine.
Children exposed to bupropion in the first or third trimester did not have increased rates of malformations relative to those taking any antidepressant. The FDA, however, requested a secondary analysis of specific rates of malformations among infants of users of all other antidepressants. There were 18 used in total, including SSRIs, tricyclics, serotonin–norepinephrine-reuptake inhibitors and other new antidepressants.
Only users of paroxetine had an increased risk of malformations higher than those of other antidepressants (Table 1). Various organ systems (gastrointestinal, genitourinary and central nervous system) were affected in roughly equal proportions. The most common cardiovascular malformations seen were ventricular septal defects.2
Table 1
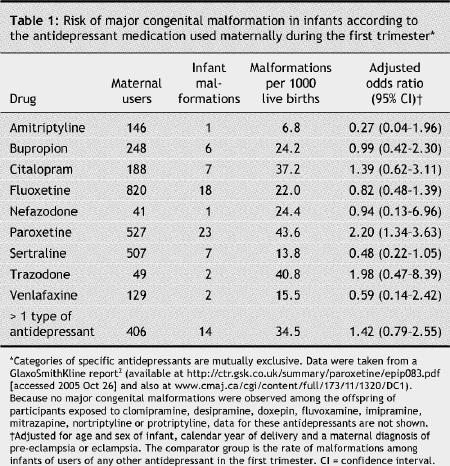
The absolute rate of major congenital seen in the first trimester for paroxetine users was 4%; of cardiovascular malformations, 2%.3 This study did not include controls of women not taking an antidepressant; however, the prevalence of major congenital and cardiovascular malformations for all births in the United States, regardless of drug exposure, are 3% and 1%, respectively.3
What to do: This study is limited by its retrospective design, its post hoc secondary analyses, the limited clinical details available in an insurance database, and its lack of controls. However, it is one of the first reasonably large epidemiologic studies to suggest possible teratogenicity of an SSRI. Why paroxetine may have this effect is not clear, and the results conflict with other epidemiologic studies performed to date.3 Although the relative risk increase of malformations is about twofold, the absolute risk increase over baseline malformation rates appears to be about 1% (i.e., about 100 pregnant users would be needed before additional harm would come to one infant). Any woman of childbearing age being treated with paroxetine should be counselled on these absolute and relative risks. If pregnancy is a real possibility, consideration should be given to switching medications.
Megan Williams Resident Department of Family Medicine University of Ottawa Ottawa, Ont. Eric Wooltorton CMAJ
Supplementary Material
Footnotes
Competing interests: None declared.
REFERENCES
- 1.Einarson TR, Einarson A. Newer antidepressants in pregnancy and rates of major malformations: a meta-analysis of prospective comparative studies. Pharmacoepidemiol Drug Saf 2005;Mar 1 [Epub ahead of print]. DOI:10.1002/pds.1084 [DOI] [PubMed]
- 2.GlaxoSmithKline study EPIP083. GSK medicine: bupropion and paroxetine. Epidemiology study: preliminary report on bupropion in pregnancy and the occurrence of cardiovascular and major congenital malformation. Available: http ://ctr.gsk.co.uk/summary/paroxetine/epip083.pdf (accessed 2005 Oct 26); also archived at www.cmaj.ca/cgi/content/full/173/11/1320/DC1 as an appendix.
- 3.Dillon AJ, for GlaxoSmithKline Inc., encorsed by Health Canada. New safety information regarding paroxetine. Findings suggest increased risk over other antidepressants, of congenital malformations, following first trimester exposure to paroxetine [Dear Health Care Professional letter]. Available: www.hc-sc.gc.ca/dhp-mps/medeff/advisories-avis/prof/paxil_3_hpc-cps_e.html (accessed 2005 Oct 26).
- 4.Hallberg P, Sjoblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects [review]. J Clin Psychopharmacol 2005;25:59-73. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


