Abstract
The QT interval on an electrocardiogram signifies the time required for the heart to repolarize after depolarization. It has long been appreciated that a long QT interval predisposes patients to life-threatening ventricular arrhythmia. Short QT syndrome is a newly described disease characterized by a shortened QT interval and by episodes of syncope, paroxysmal atrial fibrillation or life-threatening cardiac arrhythmias. The syndrome usually affects young and healthy people with no structural heart disease and may be present in sporadic cases as well as in families. Our understanding of a new disease has rarely benefitted so quickly from research in genetics, molecular biology and biophysics. It was first described in 2000 in a handful of patients, and since then 3 different genes associated with the disease and the biophysical basis have been described, and therapy has been made available. Here we review the current understanding of the pathophysiology, clinical presentation and treatment of short QT syndrome.
Since 2000, there has been increasing evidence that a short QT interval may at times be associated with an increased risk of life-threatening arrhythmic events. That year Gussak and associates1 described for the first time what has now been defined as a clinical syndrome. They identified a short QT interval in 3 people from the same family, one of whom had several episodes of paroxysmal atrial fibrillation. Similar ECG changes were also documented in another, unrelated patient with malignant arrhythmias and sudden death. The case report showed that a short QT interval could be familial and associated with atrial and ventricular arrhythmias. The definitive link between short QT syndrome and familial sudden death was described by Gaita and associates in 2003, with the clinical report of 2 families with short QT syndrome and a high incidence of sudden cardiac death.2 Just one year later, in 2004, the genetic and biophysical basis for the disease3 as well as a possible therapeutic approach were provided.2,4
The role of ion channels
Ion currents, ion channels, structural proteins and gap junctions are all involved in transmitting electrical impulses across cardiac myocytes to achieve a synchronized mechanical function. The discovery of the structure, function and pathophysiology of the ion channels has helped unravel the role played by the different ionic currents in electrical activity and electromechanical coupling.
Ion channels are essential units in cardiac excitability. They are glycoproteins embedded in the membrane of the cardiac myocytes that allow the flux of ions in and out of the cell to modulate the electrical gradient. The depolarizing currents are mediated mainly by channels that allow the entry of sodium and calcium ions into the cell, and the repolarizing currents are mediated by channels that allow the exit of potassium ions. The order of ion channel activation gives rise to the electrical current (Fig. 1). The electrical energy generated is responsible for myocyte excitability. Cardiac action potential requires a well-controlled ionic balance to prevent arrhythmogenesis. Therefore it is not surprising that these channels, when they do not work properly, have a tremendous potential to cause lethal arrhythmia.
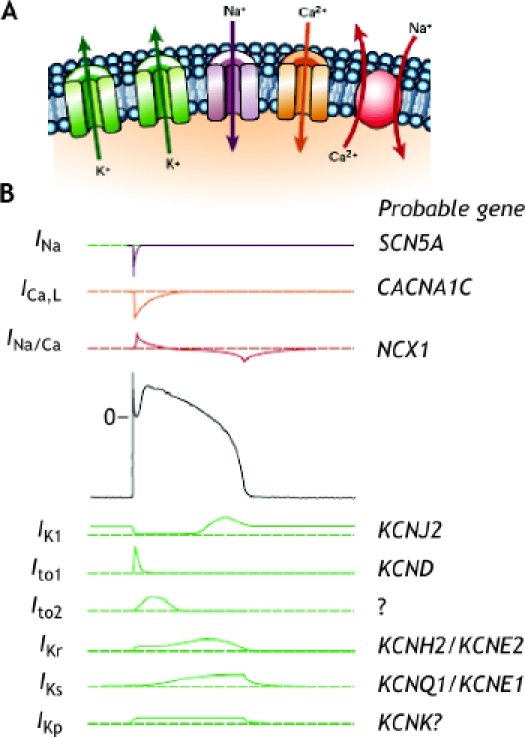
Fig. 1: A. Ion channels are embedded in the membrane and allow the flux of ions in and out of the cells following voltage gradients. The Na+/Ca2+ exchanger (red) is electrogenic, as it transports 3 sodium ions for each calcium ion across the surface membrane. B. Ionic currents and their corresponding genes; the generation of electrical activity by the different ionic currents will generate the cardiac action potential. Top: 3 depolarizing currents; centre: a ventricular action potential; bottom: repolarizing currents. Mutations in KCNJ2, which affects the IK1 current, result in the third form of short QT syndrome; mutations in KCNH2/KCNE2, affecting the IKr current, result in the syndrome's first form; mutations in KCNQ1/KCNE1, affecting the IKs current, result in the second form. Reprinted, with permission, from ref. 5.
Functional analysis of the ion channels involved in generating cardiac action potential explains the basic mechanisms of arrhythmia. However, our understanding of cardiac arrhythmias predisposing to sudden death, like long QT syndrome, Brugada syndrome and short QT syndrome, has also benefitted tremendously from the advances in genetics and molecular biology. These familial diseases allow the study of a pure form of a disease, in which a single abnormal protein is the critical factor in the risk of arrhythmogenecity. Genetic research has also provided new insights into how the abnormal and normal genes interact with their environment, drugs, and the damaged heart muscle and trigger the arrhythmia in the acquired forms.
Molecular genetics and electrophysiology
Short QT syndrome, as with most primary familial electrical diseases, is caused by mutations in genes that encode for cardiac ion channels. To date, 3 genes — KCNH2, KCNQ1 and KCNJ2 — encoding different potassium ion channels involved in repolarization have been linked to the syndrome,3,6,7 and the effect of their mutations on the action potential is shown in Fig. 2.
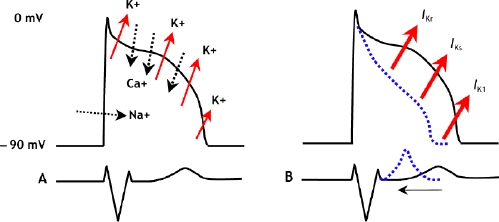
Fig. 2: A. Schematic representation of the normal action potential and the flux of ions. B. With gain-of-function mutations in any of 3 different potassium channels, the cardiac action potential shortens and the QT interval decreases.
KCNH2
The KCNH2 gene, often referred to as HERG, the human ether-a-go-go–related gene, expresses a protein that makes up the potassium channel responsible for the rapidly activating rectifier outward current (IKr). Our group identified 2 different missense mutations in the same residue in KCNH2 in 2 unrelated families.3 Both mutations resulted in the same substitution, of asparagine for lysine at codon 588, an area at the outer mouth of the channel pore.
To determine the mechanism by which the mutation, N588K, alters the outward potassium channel, we coexpressed N588K with KCNE2, which encodes a small, single transmembrane subunit to the KCNH2 channel. Analysis of the current generated after the mutated channels were transferred into human cells showed that the mutation abolished the inactivation of the KCNH2 channels, which thereby increased the IKr current. The mutation had similar effects on currents with and without coexpression of KCNE2.3 Analysis of the current–voltage relation showed that, with and without KCNE2, the current through the mutated channels failed to rectify in a physiologic range of voltages. During action potential clamp experiments, the kinetics of outward potassium flow through normal, or wild-type, channels demonstrated a typical “hump”-like waveform due to slow activation during phases 1 and 2 and a rapid increase in current during phase 3 as the inactivated channels recovered. In contrast, the currents found in the mutated channels were larger in all phases of the action potential. The biophysical analysis therefore showed that the mutation induced a “gain of function” in the IKr current, thus shortening the action potential (Fig. 3).3
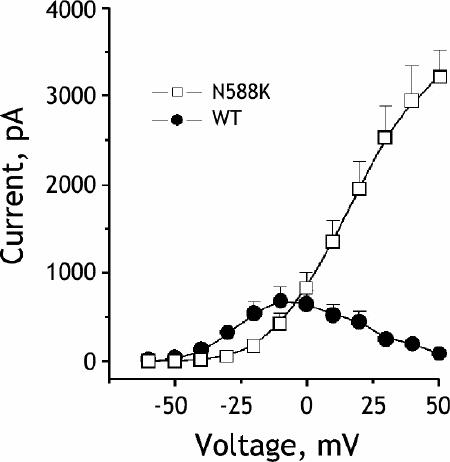
Fig. 3. Current–voltage relationship for steady state current measured at the end of the activating pulse. Steady state current amplitudes were measured at the end of the 800-ms test pulses. Mutation N588K removes rapid inactivation and significantly increases the amplitude of the rapid delayed rectifier current IKr in a physiologic range of membrane potentials. In wild type, or normal, currents, the current amplitude increased up to a test potential of –10 mV and then gradually decreased past –10 mV as the channels inactivated. In contrast, the N588K current increased linearly and did not rectify. N588K currents were much larger than wild type past 0 mV in the range of the ventricular action potential plateau. Reprinted, with permission, from ref. 8.
We also discovered that although the N588K mutation results in larger outward potassium currents in the ventricles, there is no effect on those in the Purkinje fibres8 (see the online figure, available at www.cmaj.ca/cgi/content/full/173/11/1349/DC1). Thus there is selective shortening of the ventricular action potentials and a substantial reduction in the refractory period of the ventricles but not of the Purkinje fibres. This heterogeneity in action potential durations and refractory periods is likely to create the substrate for reentrant arrhythmias.
The presence of paroxysmal atrial fibrillation in some affected patients suggests that the increased heterogeneity would also be present at the atrial level and may be responsible for the arrhythmia. More recently, our group demonstrated the definitive link between shortening of the action potential and atrial fibrillation by identifying the same genetic mutation in a third, unrelated family, in which atrial fibrillation was the only clinical manifestation of short QT syndrome.9
KCNQ1
The KCNQ1 gene encodes a subunit of the proteins responsible for the slowly activating delayed outward potassium current (IKs). The mutation was first identified by Bellocq and colleagues6 in a 70-year-old man with ventricular fibrillation and a QT interval of 290 ms after resuscitation. The patient had no inducible dysrhythmias at electrophysiologic study and no cardiac structural abnormalities.
Biophysical analysis showed that mutation in the KCNQ1 gene produced an outward potassium current with an amplitude similar to that of a normal channel. However, because of a pronounced shift of the half-activation potential, the mutated channel activated at more negative potentials and displayed accelerated activation kinetics, which led to the gain of function in the outward current that explains the short QT syndrome phenotype.6
Our group has recently identified a second mutation in KCNQ1 in a baby girl born at 38 weeks after delivery was induced because of bradycardia and irregular rhythm.10 The electrocardiogram revealed atrial fibrillation with slow ventricular response and a short QT interval. Genetic analysis identified a de novo missense mutation in the KCNQ1 gene. Voltage clamp experiments to characterize the physiologic consequences of this mutation revealed an instantaneous and voltage-independent potassium-selective current. This mutation would again lead to gain of function and shorten the action potential duration of ventricular myocytes.
KCNJ2
More recently, a third form of short QT syndrome has been linked to mutations in the KCNJ2 gene, which codes for the channel protein responsible for the inward rectifier current (IK1).7 The proband and her father, in whom the mutation was discovered, displayed short QT correction intervals of 315 and 320 ms respectively, and electrocardiogram recordings showed asymmetrical T waves with an abnormally rapid terminal phase. The proteins encoded by KCNJ2 are composed of 2 transmembrane segments linked by a long amino acid chain that forms the channel pore. When expressed in animal cells, the mutated channels generated electrical currents that did not rectify or decrease as much as those of the normal channels, whose functional positive range of potentials was –80 mV to –30 mV. This range of voltages corresponds to the very end of phase 3 repolarization and phase 4. Simulation of the effects of the mutated channels on the morphology of the ventricular action potential showed a selective speeding of late repolarization, which significantly shortened the action potential duration at 90% repolarization.
The findings of 3 forms of short QT syndrome, which link to 3 different potassium channels that alter currents at different voltages, demonstrate the heterogeneity of the disease.
Clinical manifestations
Most patients with short QT syndrome have short refractory periods, inducible ventricular fibrillation at electrophysiologic study, and a family history of sudden death or atrial fibrillation.1,2 The age at onset of clinical manifestations may be extremely young, with reports of malignant forms being responsible for even neonatal sudden cardiac deaths that may sometimes be attributed to sudden infant death syndrome.2
The characteristic sign of the disease is the presence of a very short QT interval on electrocardiogram. The T wave remains upright, and the interval between the peak and the end of the T wave is not prolonged (Fig. 4). The appearance of a well-separated U wave has also been reported in several cases. It is difficult to define the normal QT interval because the correcting equations have several limitations. Nevertheless, at a heart rate of 60 beats/min, the uncorrected, or normal, QT interval is usually higher than 360 ms.11 From the data shown in the familial forms of the syndrome, it is probably safe to say that the presence of a QT interval less than 330 ms should raise high suspicion about the disease.
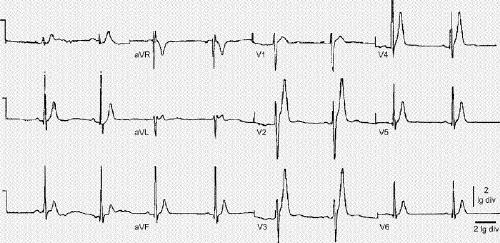
Fig. 4: Electrocardiogram of a patient with short QT syndrome. Observe the tall peaked T waves. Reprinted, with permission, from ref. 3.
The severity of the clinical manifestations is highly variable, ranging from no symptoms to atrial fibrillation and from recurrent syncope to sudden death.
Presence of atrial fibrillation
As indicated earlier, the first report by Gussak and associates made the link between short QT syndrome and atrial fibrillation.1 The proband in the family was a 17-year-old woman who, during surgery, developed rapid atrial fibrillation and pulmonary edema. She underwent cardioversion, and on follow-up her QT interval was 280 ms. Her mother was 51 years old and had had 3 episodes of sustained palpitations, 2 of which were later documented as atrial fibrillation. An electrocardiogram showed a QT interval of 230 ms. The proband's grandfather had died in 1999 at age 84. Later review of his medical records showed long-standing atrial fibrillation with a QT interval less than 300 ms in several electrocardiograms and left ventricular hypertrophy. The proband's brother, a 21-year-old with no history of palpitations, dizziness or syncope, was screened with an electrocardiogram and found to have a QT interval of 240 ms. During follow-up he has had some episodes of palpitations and brief episodes of atrial fibrillation with fast ventricular response.
Presence of sudden death
The association with sudden death was also provided by Gussak and associates with the description of a single case.1 A 37-year-old woman who had been found to have a short QT interval died suddenly while awaiting electrophysiologic study. However, the definitive familial link was provided by Gaita and associates2 with the description of 2 families with typical electrocardiographic patterns, a high incidence of sudden cardiac death at a young age, an absence of structural heart disease, and an abnormally high incidence of paroxysmal atrial fibrillation. The QT interval in the affected members ranged from 240 to 290 ms. The severity of the disease was patent in these families, with 2 members experiencing sudden cardiac death, which was aborted in one, in the first year of life.
Of the 6 people in the 2 families with short QT syndrome, 5 were inducible into ventricular fibrillation during electrophysiologic study, and all 6 received an implantable cardioverter defibrillator because of the strong family history of sudden cardiac death.
Disease management
Robust genotype–phenotype correlation data are not yet available for short QT syndrome, and thus clinical management must rely on clinical findings.
The disease is clinically highly heterogeneous, as indicated by the tremendous variation in symptoms and presentation in the 3 families with the same mutation3,9 and one isolated case.6 Preliminary data have shown that there may be effective pharmacologic therapy;4,12 the high incidence of sudden cardiac death, however, warrants the implantation of a cardioverter defibrillator, especially in people in whom sudden death has been aborted. The syndrome confers a risk of sudden death on young children, in whom implantation is not feasible. Recently, concern has been raised about inappropriate shocks, especially in relation to the presence of short- coupled prominent T waves. The adaptation of programming with decreased sensitivity levels and decay delays is warranted in these situations to prevent T-wave oversensing.13
Pharmacologic therapy
Numerous drugs block the repolarizing outward potassium current, including methanesulfonanilides, phosphodiesterase inhibitors,14 macrolide antibiotics,15 antifungal agents,16 and antihistamines;17 this is the basis for their prolonging effect on the QT interval and potential arrhythmogenicity. Because shortening of the QT interval is likely due to an increase in the outward current, blocking the current with class III antiarrhythmic drugs (which are known to increase the QT interval) may be a therapeutic approach for treating short QT syndrome. No large randomized trials have been conducted to date on drug therapies for the syndrome. The current evidence is derived from small studies.
In our study,3 sotalol, a class III antiarrhythmic with potent IKr blocking actions, was given to the proband as a preliminary test of this hypothesis. The QT interval at baseline was 291 ms and remained practically unchanged after sotalol was taken, which suggested that this dose of the IKr blocker was ineffective with this patient. Similar results were obtained in 2 other patients with the syndrome. In vitro analysis of sotalol in the heterologously expressed KCNH2/KCNE2 currents in normal and mutated channels showed that application of 100 μmol/L D-sotalol reduced the current in normal channels at +20 mV by 48%, as was expected given previously published data.18,19,20,21,22 Currents in the mutated channels, on the other hand, were reduced by only 9.0% (standard deviation [SD] 0.3%) and 27% (SD 0.3%) following application of 100 μmol/L and 500 μmol/L D-sotalol respectively. Thus, the mutation reduced the ability of D-sotalol to block the channel, a result consistent with the clinical findings. A similar decreased sensitivity to the drug was also observed when N588K was expressed without KCNE2.3
More recently, Gaita and colleagues showed in a clinical study that treatment with quinidine prolonged the QT interval and decreased inducibility, and the drug therefore has the potential to be an effective therapy.12 The use of flecainide, ibutilide or sotalol did not modify the QT interval. Consistent with these findings, electrophysiologic studies in heterologous expression systems showed that the N588K mutation reduced the affinity of the channels for quinidine by 5.8-fold, from 0.75 μmol/L in normal channels to 4.4 μmol/L in mutated channels, compared with a 20-fold reduction in affinity for D-sotalol,4 which provides evidence that quinidine may be superior to D-sotalol for treating the first form of the syndrome. Clinical follow-up in one family with paroxysmal atrial fibrillation indicates that the episodes respond well to treatment with class Ic agent propafenone.9
Future directions
In summary, 3 forms of short QT syndrome have been discovered to date. Each form is linked to mutations in different channels, which likely makes the therapeutic options unique to each form. Patients who present with atrial or ventricular arrhythmias and whose electrocardiogram shows a short QT interval that is not related to a correctible cause should be suspected as having short QT syndrome, especially if the family history suggests an inherited factor. These patients should be referred to a cardiologist with expertise in arrhythmia management for a correct diagnosis and treatment strategy. The severity of the disease, which may cause sudden cardiac death at a very young age, highlights the importance of aggressive risk stratification of patients and family members.
The first line of therapy, especially in people recovered from sudden cardiac death or with a history of syncopal episodes, is the implantation of cardioverter defibrillator. Although antiarrhythmics that prolong the QT interval, such as sotalol or quinidine, may be suitable for the first form of the syndrome, no known blocker can specifically target the other 2 forms. This establishes the syndrome's multigenic nature, which is similar to that of long QT syndrome, and highlights the need to identify the form, whether by genetic screening or clinical means, so that the right pharmacologic approach can be determined. Although the pharmacologic interactions for each form are not yet well identified or developed, knowing the genes and mechanisms involved hold the promise of a higher therapeutic specificity.
Supplementary Material
Acknowledgments
Ramon Brugada is supported in part by grants from the American Heart Association and the National Institutes of Health. Jonathan Cordeiro is supported in part by grants from the American Health Assistance Foundation.
Footnotes
This article has been peer reviewed.
Contributors: Ramon Brugada drafted the article. All of the authors made substantial contributions to its design, revised it critically, and gave final approval of the version to be published.
Competing interests: None declared.
Correspondence to: Dr. Ramon Brugada, Montreal Heart Institute, 5000 rue Belanger, Montréal QC H1T 1C8; ramon@brugada.org
REFERENCES
- 1.Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology 2000;94:99-102. [DOI] [PubMed]
- 2.Gaita F, Giustetto C, Bianchi F, et al. Short QT Syndrome: a familial cause of sudden death. Circulation 2003;108:965-70. [DOI] [PubMed]
- 3.Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation 2004;109:30-5. [DOI] [PubMed]
- 4.Wolpert C, Schimpf R, Giustetto C, et al. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol 2005;16:54-8. [DOI] [PMC free article] [PubMed]
- 5.Marban E. Cardiac channelopathies. Nature 2002;415:213-8. [DOI] [PubMed]
- 6.Bellocq C, van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation 2004;109:2394-7. [DOI] [PubMed]
- 7.Priori SG, Pandit SV, Rivolta I, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res 2005;96:800-7. [DOI] [PubMed]
- 8.Cordeiro JM, Brugada R, Wu YS, et al. Modulation of I(Kr) inactivation by mutation N588K in KCNH2: a link to arrhythmogenesis in short QT syndrome. Cardiovasc Res 2005;67:498-509. [DOI] [PubMed]
- 9.Hong K, Bjerregaard P, Gussak I, et al. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J Cardiovasc Electrophysiol 2005;16:394-6. [DOI] [PubMed]
- 10.Hong K, Piper DR, Diaz-Valdecantos A, et al. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc Res 2005 Aug 16; [Epub ahead of print]. [DOI] [PubMed]
- 11.Schulze-Bahr E, Breithardt G. Short QT interval and short QT syndromes. J Cardiovasc Electrophysiol 2005;16:397-8. [DOI] [PubMed]
- 12.Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol 2004;43:1494-9. [DOI] [PubMed]
- 13.Schimpf R, Wolpert C, Bianchi F, et al. Congenital short QT syndrome and implantable cardioverter defibrillator treatment: inherent risk for inappropriate shock delivery. J Cardiovasc Electrophysiol 2003;14:1273-7. [DOI] [PubMed]
- 14.Commerford PJ, Lloyd EA. Arrhythmias in patients with drug toxicity, electrolyte, and endocrine disturbances. Med Clin North Am 1984;68:1051-78. [DOI] [PubMed]
- 15.Volberg WA, Koci BJ, Su W, et al. Blockade of human cardiac potassium channel human ether-a-go-go-related gene (HERG) by macrolide antibiotics. J Pharmacol Exp Ther 2002;302:320-7. [DOI] [PubMed]
- 16.Dumaine R, Roy ML, Brown AM. Blockade of HERG and Kv1.5 by ketoconazole. J Pharmacol Exp Ther 1998;286:727-35. [PubMed]
- 17.Roy M, Dumaine R, Brown AM. HERG, a primary human ventricular target of the nonsedating antihistamine terfenadine. Circulation 1996;94:817-23. [DOI] [PubMed]
- 18.Lees-Miller JP, Duan Y, Teng GQ, et al. Molecular determinant of high-affinity dofetilide binding to HERG1 expressed in Xenopus oocytes: involvement of S6 sites. Mol Pharmacol 2000;57:367-74. [PubMed]
- 19.Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by Class III antiarrhythmic agents. J Gen Physiol 1990;96:195-215. [DOI] [PMC free article] [PubMed]
- 20.Ficker E, Jarolimek W, Kiehn J, et al. Molecular determinants of dofetilide block of HERG K+ channels. Circ Res 1998;82:386-95. [DOI] [PubMed]
- 21.Komeichi K, Tohse N, Nakaya H, et al. Effects of N-Acetylprocainamide and Sotalol on ion currents in isolated guinea-pig ventricular myocytes. Eur J Pharmacol 1990;187:313-22. [DOI] [PubMed]
- 22.Wettwer E, Grundke M, Ravens U. Differential effects of the new Class-III antiarrhythmic agents almokalant, e-4031 and d-Sotalol, and of quinidine, on delayed rectifier currents in guinea pig ventricular myocytes. Cardiovasc Res 1992;26 (11):1145-52. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


