Abstract
The neuropeptide galanin mediates its effects through the receptor subtypes Gal1, Gal2, and Gal3 and has been implicated in anxiety- and depression-related behaviors. Nevertheless, the receptor subtypes relevant to these behaviors are not known because of the lack of available galanin-selective ligands. In this article, we use behavioral, neurochemical, and electrophysiological approaches to investigate the anxiolytic- and antidepressant-like effects of two potent small-molecule, Gal3-selective antagonists, SNAP 37889 and the more soluble analog SNAP 398299. Acute administration of SNAP 37889 or SNAP 398299 enhanced rat social interaction. Furthermore, acute SNAP 37889 was also shown to reduce guinea pig vocalizations after maternal separation, to attenuate stress-induced hyperthermia in mice, to increase punished drinking in rats, and to decrease immobility and increase swimming time during forced swim tests with rats. Moreover, SNAP 37889 increased the social interaction time after 14 days of treatment and maintained its antidepressant effects during forced swim tests with rats after 21 days of treatment. In microdialysis studies, SNAP 37889 partially antagonized the galanin-evoked reduction in hippocampal serotonin (5-hydroxytryptamine, 5-HT), as did the 5-HT1A receptor antagonist WAY100635. Their combination produced a complete reversal of the effect of galanin. SNAP 398299 partially reversed the galanin-evoked inhibition of dorsal raphe cell firing and galanin-evoked hyperpolarizing currents. These results indicate that Gal3-selective antagonists produce anxiolytic- and antidepressant-like effects, possibly by attenuating the inhibitory influence of galanin on 5-HT transmission at the level of the dorsal raphe nucleus.
Keywords: anxiety, depression, galanin, neuropeptide, dorsal raphe
Galanin is a 29- to 30-aa-containing neuropeptide (1) involved in a variety of peripheral and central physiological and pathophysiological processes, including gastrointestinal motility, cardiovascular contraction, neuroendocrine function, feeding behavior, pain perception, learning, memory, anxiety, and depression (2, 3). The physiological actions of galanin are mediated by Gal1, Gal2, and Gal3 G-protein-coupled receptor subtypes (4), which are uniquely distributed throughout the CNS and periphery. Gal1 and Gal2 mRNAs are widely expressed throughout the rat brain but differ significantly in their specific distribution patterns (5). In contrast, central Gal3 mRNA distribution is more discrete, with a prominent representation in the hypothalamus and lower levels in some limbic regions including the locus ceruleus, the dorsal raphe, and the midbrain central gray (6, 7). It is worth noting that Gal3 mRNA has also been found in such diverse tissues as the pancreas, adrenal gland, kidney, and lung (7), suggesting complex and varied physiological actions in the periphery. Nevertheless, the distinct Gal3 localization in the brain suggests that it may mediate some of the mood-regulating effects of galanin. In support of this view, galanin is synthesized in the serotonergic dorsal raphe nucleus (DRN) and the noradrenergic locus ceruleus (8, 9), and central galaninergic transmission has been implicated in the regulation of affective states, probably through its influence on these neurotransmitter systems (10-15). Interestingly, galanin has been shown to interact with central serotonergic transmission at the molecular (16), neurochemical (17), and behavioral (18, 19) levels after ventricular administration but not to affect presumed norepinephrine-related behaviors that are mediated by the locus ceruleus (20). These findings suggest that galanin may influence anxiety- and depression-related behaviors by negatively modulating central serotonergic transmission at the level of the DRN and that the galanin system is a potential target for the treatment of affective disorders. Nevertheless, the current lack of subtype-selective ligands (21) has hampered the elucidation of specific roles for the galanin receptor subtypes in the physiological effects of galanin.
In this article, we describe the in vitro and in vivo characterization of two small-molecule Gal3 receptor antagonists, SNAP 37889 and SNAP 398299, using a number of acute and chronic rodent models of anxiety and depression, as well as by using neurochemical and electrophysiological techniques. Importantly, the behavioral results were compared with those of a classic benzodiazepine anxiolytic, chlordiazepoxide (CDP), and with a selective serotonin reuptake inhibitor (SSRI) antidepressant, fluoxetine. The results of these studies are discussed in the context of current therapies for the treatment of affective disorders and highlight the potential therapeutic advantages that Gal3 receptor antagonists may offer over existing treatments.
Materials and Methods
Animals. Behavioral studies were performed in adult male Sprague-Dawley rats, adult C57BL/6J mice, and infant (2 weeks old) Hartley guinea pigs (for details see Supporting Materials and Methods, which is published as supporting information on the PNAS web site). All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee of the participating laboratories.
Receptor Binding and in Vitro Functional Antagonism. Binding affinities for SNAP 37889 and SNAP 398299 at the human Gal1, Gal2, and Gal3 receptors were determined by using the 125I-galanin displacement assay described in ref. 22. Additionally, SNAP 37889 was tested for binding in a broad cross-reactivity panel (NovaScreen, Hanover, MD) that included G-protein-coupled receptors, ion channels, enzymes, and transporters. The ability of SNAP 37889 to antagonize functional responses to galanin was examined in modified HEK-293 cells (PEAKrapid cells, Edge Biosystems, Gaithersburg, MD) transiently cotransfected with the Gal3 receptor and Gαz by measuring the inhibition of adenylyl cyclase activity (for details see Supporting Materials and Methods).
Forced Swim Test (FST). Rats received a single oral (p.o.) dose (1 ml/kg) of vehicle (1 ml/kg of 100% N,N-dimethylacetamide), SNAP 37889 (1, 3, and 10 mg/kg), or fluoxetine (10 mg/kg) 2 h before the start of the 5-min test session. A separate group of animals was tested after 21 days of dosing (i.p. injections) with vehicle (1 ml/kg) or SNAP 37889 (30 mg/kg) (for details see Supporting Materials and Methods).
Social Interaction Test. Rats received p.o. treatments (1 ml/kg) of vehicle (5% DMSO in 1% hydroxypropylmethyl-β-cellulose), CDP (5 mg/kg), or SNAP 37889 (3, 10, or 30 mg/kg) and were tested under bright light and in unfamiliar conditions 1 h after dosing. A separate group of rats was tested after 14 days of dosing (i.p. injections) with vehicle (1 mg/kg), CDP (5 mg/kg), or SNAP 37889 (30 mg/kg) (for details see Supporting Materials and Methods).
Vogel Conflict Test. Rats had restricted access to water for 3 days (1 h of access per day) and on day 4 were tested in the Vogel conflict assay, 1 h after receiving a single i.p. injection (1 ml/kg) of vehicle (1 ml/kg 5% DMSO + 1% hydroxypropylmethyl-β-cellulose, vol/vol), CDP (5 mg/kg), or SNAP 37889 (3 or 10 mg/kg). The number of licks and shocks received during the test session were recorded (for details see Supporting Materials and Methods).
Mouse Stress-Induced Hyperthermia and Guinea Pig Vocalization Test. SNAP 37889 was also tested in these behavioral models. Methods and figures are presented in full in Supporting Materials and Methods.
In Vivo Microdialysis. Rats were anesthetized with a ketamine/xylazine mixture (i.p.), and guide cannulae were implanted into the ventral hippocampus (vHip) and lateral ventricle.
The night before the experiment, microdialysis probes were inserted into the guide cannulae, and animals were placed in the testing environment. On the following day, artificial cerebrospinal fluid (aCSF) was delivered at a rate of 1.5 μl/min for 1-2 h before collecting the samples. Samples were collected over 30 min. After a 120-min baseline period, rats were divided into four groups and treated with vehicle (5% DMSO + 0.25% hydroxypropylmethyl-β-cellulose), SNAP 37889 (30 mg/kg, p.o.), WAY100635 (0.6 mg/kg in saline, s.c.), or SNAP 37889 + WAY100635. After a 120-min collection period, galanin was infused [(1.5 nmol/1 μl, intracerebroventricularly (i.c.v.)], and samples were collected for an additional 210 min. HPLC with electrochemical detection was used for sample analysis (full details are provided in Supporting Materials and Methods).
In Vivo Electrophysiology. Extracellular single-unit recordings from DRN cells were made as described in ref. 23. DRN cells were studied 5.5-6.5 mm below the dura. Waveform and firing rate were recorded with micro 1041 and spike 2 software (Cambridge Electronic Design, Cambridge, U.K.). After attaining a stable firing rate (baseline), animals received 1.5 μl of galanin (i.c.v.). Vehicle (5% N,N-dimethylacetamide and 10% polyethylene glycol in water) or SNAP 398299 was delivered (i.v.) after the effect of galanin reached its plateau. See Supporting Materials and Methods for details.
In Vitro Whole-Cell Recordings. The recordings from the neurons of DRN were made under conditions similar to those described in ref. 24, except that the recording was done in the whole-cell configuration (for details see Supporting Materials and Methods).
Statistical Analyses. Statistical analyses were carried out by using the prism package (GraphPad, San Diego). Behavioral and microdialysis data were analyzed by using a one-way ANOVA with treatment as the main factor. When a statistically significant effect was found, post hoc analysis was carried out by using the Student-Newman-Keuls post hoc test. Electrophysiological and chronic FST data were analyzed by using a two-tailed Student t test.
Drugs. SNAP 37889 (1-phenyl-3-[[3-(trif luoromethyl)pheny-l]imino]-1H-indol-2-one) and SNAP 398299 (1-[3-(2-pyrro-lidinylethoxy)phenyl]-3-{[3-(trif luoromethyl)phenyl]aza-methylene}benzo[d]azolin-2-one) were synthesized as described in refs. 25 and 26. Porcine galanin was purchased from Bachem; WAY100635 was purchased from Sigma-Aldrich; and 125I-labeled porcine galanin was purchased from Amersham Pharmacia Biosciences.
Results
SNAP 37889 and SNAP 398299 Are Selective, High-Affinity, Competitive Antagonists of Gal3. SNAP 37889 binds with high affinity to membranes from transiently transfected LMTK- cells expressing the human Gal3 receptor (Ki = 17.44 ± 0.01 nM; n > 100) and is highly selective for Gal3 over the Gal1 and Gal2 subtypes (Ki > 10,000 nM for each subtype; n = 46 of each subtype). Cross-reactivity was determined for a broad collection of G-protein-coupled receptors, channels, enzymes, and transporters including monoamine oxidase A, monoamine oxidase B, serotonin (5-hydroxytryptamine, 5-HT) transporter, norepinephrine transporter, and dopamine transporter. Selectivity in each instance was >100-fold (see Table 1, which is published as supporting information on the PNAS web site). SNAP 398299, a highly water-soluble analog of SNAP 37889, also shows selective, high-affinity binding to the human Gal3 receptor (Gal3 Ki = 5.33 ± 0.28 nM; Gal1 and Gal2 Ki > 1,000 nM; n = 2).
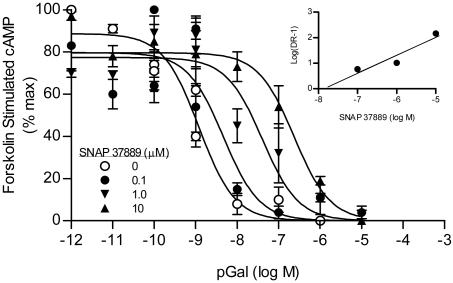
When tested for the antagonism of galanin-evoked inhibition of adenylyl cyclase, SNAP 37889 (0.1-10 μM) produced concentration-dependent rightward shifts of the concentration-effect curve to galanin (Fig. 1). Schild regression afforded a line with a slope not different from unity (0.92 ± 0.04; n = 4). Constraining the slope to 1 afforded a pKb estimate of 7.54 (Kb = 29 nM; see Fig. 1).
Fig. 1.
SNAP 37889 is a competitive antagonist at the Gal3 receptor, producing dose-related shifts in the concentration-effect curve to galanin in the adenylyl cyclase assay. (Inset) Schild regression of the mean EC50 values affords a slope of 0.92 ± 0.04 and, when constrained to a unit slope, a pKb value of 7.54 (Kb = 29 nM; n = 4).
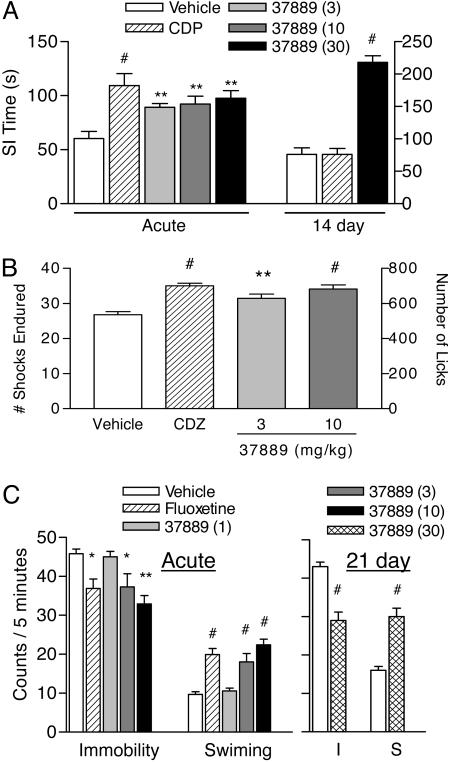
Gal3 Antagonists Show Acute and Chronic Efficacy in Rodent Models of Anxiety and Depression. In the social interaction test, acute (1 h) administration of SNAP 37889 (3, 10, and 30 mg/kg) produced dose-related increases in active social interaction time, with significant effects at all doses examined (Fig. 2A) [F(4, 39) = 5.728; P < 0.0010]. The effect at 30 mg/kg was comparable to that of the benzodiazepine CDP (5 mg/kg). Rearing behavior was not affected at any dose of SNAP 37889, suggesting that increases in social interaction were not secondary to a nonspecific increase in locomotor activity (see Fig. 6, which is published as supporting information on the PNAS web site). When tested in a separate group of animals after chronic (14 day) administration, SNAP 37889 (30 mg/kg) again produced an increase in social interaction time (Fig. 2 A) [F(2, 23) = 42.35; P < 0.0001]. The effect was significantly greater than that for animals receiving CDP (5 mg/kg), which were not different from vehicle-treated controls. Rearing behavior was not significantly affected with chronic administration of SNA P 37889 (Fig. 6). SNAP 398299 is a more water-soluble analog of SNAP 37889 that is better suited for i.v. or in vitro dosing. SNAP 398299 also increased active social interaction time at doses of 1 and 10 mg/kg (see Fig. 7, which is published as supporting information on the PNAS web site) [F(4, 38) = 5.297; P < 0.0017]; the effect was again comparable to that of CDP.
Fig. 2.
The Gal3-selective antagonist SNAP 37889 displays acute anxiolytic- and antidepressant-like activity in animal models. (A) Acute (1 h) treatment with SNAP 37889 (3, 10, and 30 mg/kg, p.o.) produced a dose-dependent increase in social interaction time. The maximum effect was comparable to that of the positive control, CDP (5 mg/kg, p.o.). After 14 days of chronic administration, SNAP 37889 (30 mg/kg, i.p.) significantly increased social interaction time, whereas social interaction time in rats receiving CDP (5 mg/kg per day, i.p.) was not different from the control (n = 9 per group). (B) Pretreatment with SNAP 37889 increased punished responding during Vogel conflict test in rats. The Gal3 antagonist produced an increase in drinking in water-deprived animals at each dose tested (3 and 10 mg/kg, i.p.). The increase in drinking was accompanied by an increase in the number of shocks tolerated. In each case, the effect at the highest dose tested was equivalent to that of the positive control, CDP (5 mg/kg, i.p.) (n = 10 per group). (C) Acute (1 h) pretreatment with SNAP 37889 produced a dose-dependent decrease in immobility and parallel increase in swimming time during the 5-min test. The maximum effect was similar to the control antidepressant agent, fluoxetine (10 mg/kg, p.o.). Animals treated chronically (21 days) with SNAP 37889 (30 mg/kg, i.p.) also exhibited a significant decrease in immobility (I) and increase in swimming (S) time relative to vehicle-treated controls (n = 10 per group). Data are represented as mean ± SEM for each dose. *, P < 0.05; **, P < 0.01; #, P < 0.001 relative to the vehicle control group.
Anxiolytic-like activity was seen in the Vogel test, during which acute dosing with SNAP 37889 (3 and 10 mg/kg) resulted in a significant increase in punished drinking, as assessed by the number of licks (Fig. 2B) [F(3, 32) = 13.67; P < 0.0001] and number of shocks tolerated (Fig. 2B) [F(3, 32) = 12.98; P < 0.0001]. The highest dose tested produced an effect equivalent to that of CDP.
SNAP 37889 was also effective in reducing stress-induced hyperthermia in mice. Animals given a 1-h pretreatment (0.3, 3, and 30 mg/kg, p.o.) showed a significant attenuation of the hypothermic effects of handling stress (see Fig. 8, which is published as supporting information on the PNAS web site) [F(4, 36) = 5.055; P < 0.0024], similar to mice receiving CDP (10 mg/kg). Post hoc analysis indicates a significant effect of each dose tested when compared with the vehicle control.
Finally, SNAP 37889 reduced anxiety-related vocalizations of guinea pig pups in response to maternal separation (Fig. 9, which is published as supporting information on the PNAS web site) [F(5, 54) = 13.06; P < 0.0001]. Vocalizations in vehicle-treated pups were significantly reduced relative to untreated pups. Nevertheless, SNAP 37889 produced a further reduction in the number of emitted vocalizations at 3, 10, and 30 mg/kg relative to vehicle-treated animals, similar to the positive control, buspirone.
In FST, rats dosed acutely (1 h) with SNAP 37889 exhibited a dose-dependent decrease in immobility [F(4, 45) = 6.098; P < 0.0005] and increase in swimming [F(4, 45) = 15.81; P < 0.0001] relative to vehicle-treated animals (Fig. 2C). The effects were significant at 3 and 10 mg/kg and comparable to those of the reference antidepressant, fluoxetine (10 mg/kg). In a separate set of animals assessed in FST after chronic (21 days) administration, SNAP 37889 (30 mg/kg) produced a significant decrease in immobility (Fig. 2C) (t14 = 4.941; P < 0.0002) and increase in swimming (Fig. 2C) (t14 = 5.516; P < 0.0001) compared with vehicle-treated control rats.
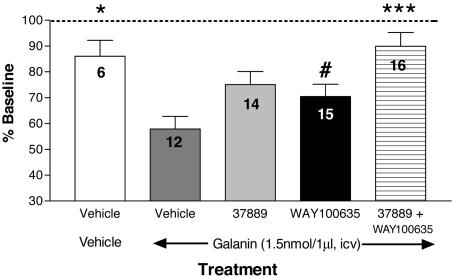
Gal3 Antagonists Reverse the Galanin-Evoked Inhibition of Ventral Hippocampal 5-HT Output and Dorsal Raphe Firing in Vivo. Infusion of galanin (1.5 nmol/1 μl) into the lateral ventricle of freely moving rats resulted in a significant reduction in ventral hippocampal 5-HT output, to 58 ± 3.5% of baseline control values, and this effect was attenuated by a 2-h pretreatment with either the Gal3 receptor antagonist SNAP 37889 or the 5-HT1A receptor antagonist WAY100635 (Fig. 3) [F(4, 58) = 5.657; P < 0.0006). Post hoc comparisons revealed a nonsignificant attenuation of the effects of galanin after pretreatment with SNAP 37889 or WAY100635, probably because of the small number of animals in the vehicle - vehicle-treated group. Nevertheless, combined pretreatment with SNAP 37889 and WAY100635 resulted in significant and additive effects, reversing the galanin-induced reduction in extracellular 5-HT by 77%. Extracellular levels of 5-HT (percent baseline ± SEM) were not significantly altered from baseline values over the 2 h after the systemic administration of vehicle [2-h area under the curve (AUC), 107 ± 13.54], SNAP 37889 (2-h AUC, 114.7 ± 9.7), WAY100635 (2-h AUC, 106.9 ± 10.37), or the combination of SNAP 37889 + WAY100635 (108.7 ± 8.68). The pretreatment injection resulted in a small, transient increase in extracellular 5-HT, which returned to baseline values within 2 h after administration, before an i.c.v. galanin challenge.
Fig. 3.
Gal3- and 5-HT1A-receptor interactions modulate the reduction of extracellular 5-HT output in vHip produced by central injection of galanin. Galanin (1.5 nmol/1 μl, i.c.v.) caused a significant reduction in basal vHip 5-HT in vehicle-pretreated animals. Pretreatment with either SNAP 37889 or WAY100635 resulted in an attenuation of the galanin-mediated effect. Combined administration of SNAP 37889 and WAY100635 produced an additive and significant reversal of the galanin-induced decrease in vHip 5-HT. The number of animals used in each pretreatment group is indicated within each bar. Data are represented as the mean ± SEM for each treatment over the 3.5-h sampling period, beginning 1 h after galanin administration. *, P < 0.05; ***, P < 0.001 when compared with the vehicle - galanin control group. #, P < 0.05 when compared with the SNAP 37889 + WAY100635 pretreatment group.
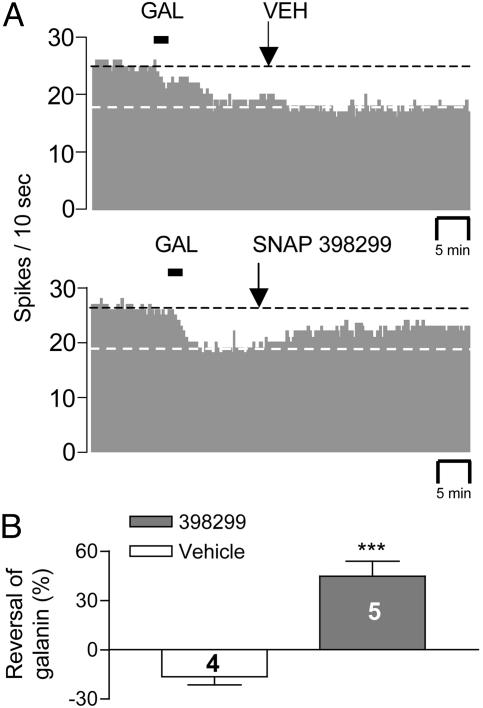
Single-unit in vivo extracellular recordings of spontaneous firing from DRN soma were conducted in anesthetized rats in an attempt to correlate changes in ventral hippocampal 5-HT output with DRN neuronal firing. The firing rate of DRN was decreased in response to galanin (1.5 nmol/1.5 μl, i.c.v.) in 14 of 19 cells tested. Among the 14 galanin-responders, complete firing cessation was observed in one cell, and this cell was excluded from statistic analysis. Galanin produced significant, albeit partial, inhibition of firing in the remaining 13 cells (Fig. 4A) (66 ± 4.6% of baseline control values; P < 0.001). SNAP 398299 alone (2 mg/kg, i.v.) had no effect on basal firing but significantly reversed galanin's inhibitory effect (P < 0.001), whereas infusion of saline (i.v.) resulted in a modest insignificant decrease in cell firing at equivalent cumulative volume (Fig. 4B).
Fig. 4.
The soluble Gal3-selective antagonist, SNAP 398299, reverses the in vivo electrophysiological effects of galanin in the DRN. (A) (Upper) Representative histogram from a single DRN neuron demonstrating that vehicle (i.v.) administration has no effect on the galanin-induced (i.c.v.) reduction of spike activity. (Lower) Representative histogram from a single DRN neuron indicating that infusion of SNAP 398299 (i.v.) attenuates the galanin-induced reduction in spike output. (B) Mean effect of vehicle and SNAP 398299 in four to five DRN neurons. Data are depicted as spikes per 10 s (A) or the mean ± SEM (B). ***, P < 0.001 when compared with the vehicle control group.
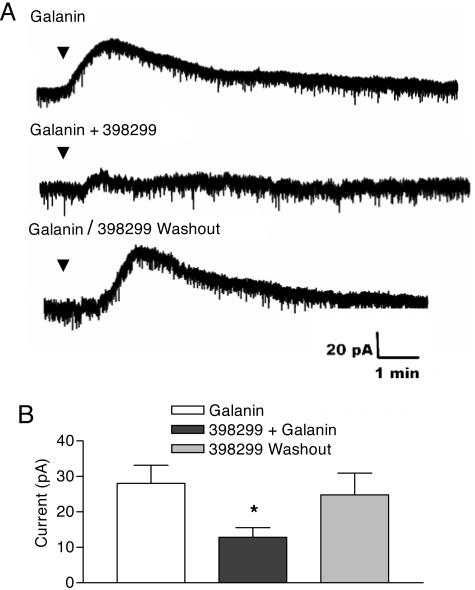
SNAP 398299 Reverses Galanin-Evoked Outward Currents in Vitro. During whole-cell recording, micropipette application of galanin (1 μM) produced a reversible outward current (mean = 33.25 ± 2.18 pA) in 52 of 129 (40%) of the cells tested. These results agree well with the results discussed in ref. 24. The antagonistic effect of SNAP 398299 was examined in nine galanin-responsive cells. The response to galanin (28 ± 5.1 pA) was inhibited significantly by 53.8 ± 5.2% in five cells after preincubation with SNAP 398299 (1 μM) for 3-5 min (Fig. 5) (12.8 ± 2.8 pA). The response to galanin returned completely on washout of the antagonist.
Fig. 5.
Representative traces demonstrating that SNAP 398299 attenuates the galanin-mediated increase in outward currents in the DRN in vitro. (A) Galanin evoked an outward current in the DRN slice preparation (top trace), and this effect was partially attenuated after application of SNAP 398299 (1 μM) (middle trace). The effects of SNAP 398299 are reversible, as indicated by the return of the galanin-induced outward current after washout of the antagonist (bottom trace). (B) SNAP 398299 attenuates the galanin-evoked outward current. Data are represented as mean ± SEM for data from five cells. *, P < 0.05 when compared with the vehicle control group.
Discussion
Several studies have implicated galanin neurotransmission in the modulation of anxiety, mood, and emotion (10-15); however, previous studies have not specifically addressed the role of Gal3 in these effects. This article demonstrates that selective Gal3 antagonists display both acute and chronic anxiolytic- and antidepressant-like behavioral profiles in a number of predictive animal models. Moreover, additional studies suggest that the behavioral effects of Gal3-selective antagonists may be due, in part, to the blockade of Gal3 receptors located in the DRN.
The acute and chronic efficacy of SNAP 37889 in models of anxiety and depression were generally noted over doses ranging from 3 to 30 mg/kg for most behavioral assays; however, the compound was shown to be efficacious at 0.3 mg/kg in the mouse stress-induced hyperthermia test. In each case, the maximum effect of SNAP 37889 was indistinguishable from the positive control used for each test. Moreover, the fact that SNAP 37889 did not affect rearing behavior at doses up to 30 mg/kg in the rat social interaction test suggests that its efficacy in this model cannot be attributed to a nonspecific influence on motor activity or exploratory behavior.
A role for galanin transmission in the regulation of anxiety states has been shown to depend on the route and site of peptide administration. For instance, i.c.v. administration of galanin elicits anxiolytic effects in the punished drinking test (10), whereas local administration of galanin into the central nucleus of the amygdala produces anxiogenic effects (13). The latter result points to this brain region as an important player in galanin's ability to modulate affect; however, the responsible galanin receptor subtype remains unclear. The significant increase in punished drinking after systemic administration of SNAP 37889, demonstrated in this article, could reflect blockade of Gal3-mediated galaninergic transmission within the central nucleus of the amygdala. Nevertheless, Khoshbouei et al. (14) have reported that the immobilization stress-induced increase in norepinephrine release in the central nucleus of the amygdala and the parallel recruitment of galanin release produce anxiolytic-like effects in the elevated plus maze test. This apparent discrepancy may be related to the type of stressor involved. Thus, in conditions of uncontrollable neurolimbic stress, galanin released from noradrenergic locus ceruleus afferents in the ventral tegmental area decreases the firing of dopamine neurons, leading to behavioral depression (11). In fact, uncontrollable stress results in activation of the DRN, an effect compensated for by prefrontal cortical input under conditions of controllable stress (27), suggesting that the behavioral outcome of galanin-5-HT interactions may depend on the type of stressor involved.
The present study demonstrates that blocking galaninergic transmission both acutely and chronically with a selective Gal3 antagonist decreases immobility time during FST in the rat, a test commonly used to predict antidepressant activity. Nevertheless, the nonselective Gal1/Gal2 agonist galnon has also been shown to produce antidepressant-like effects in FST (15). Furthermore, chronic (14 day) treatment of rats with fluoxetine was shown to elevate galanin mRNA in the DRN and locus ceruleus and to increase Gal2, but not Gal1, binding in the DRN (15). Taken together, these results suggest that chronic antidepressant treatment may, in part, shift the influence of galanin transmission from Gal3- to Gal1/Gal2-mediated signaling.
Galanin is thought to modulate hippocampal 5-HT output by its actions in the DRN. Thus, galanin injected i.c.v., as well as directly into the DRN, results in a significant reduction in extracellular 5-HT output in the vHip, whereas injection into the vHip is without effect (17, 28). In this article, we demonstrate the ability of SNAP 37889 to partially reverse the galanin-evoked decrease in 5-HT outflow within the vHip. We also confirmed previous reports demonstrating that pretreatment with the 5-HT1A antagonist WAY100635 attenuates the galanin-evoked decrease in extracellular vHip 5-HT (28), an effect probably mediated by somatodendritic 5-HT1A autoreceptors located within the DRN (29). These results indicate that at least some of the effects of galanin are indirectly related to 5-HT1A-receptor stimulation. The synergistic interaction following coadministration of SNAP 37889 and WAY100635 to reverse the galanin-evoked reduction of vHip 5-HT output suggests that galanin acts on at least two receptor subtypes to inhibit neuronal firing: Gal3, which has direct inhibitory effects, and another Gal subtype, which promotes 5-HT release within the DRN to inhibit firing by the 5-HT1A receptor. This interpretation is supported by electrophysiological evidence for a direct inhibitory effect of galanin on DRN neurons, as well as an indirect influence on 5-HT autoinhibition (24). It was suggested that galanin evoked outward potassium currents by a postsynaptic influence at a Gi-coupled galanin receptor subtype other than Gal1 (24). In the present in vitro and in vivo electrophysiology studies, SNAP 398299 produced significant but partial antagonism of galanin-evoked inhibitory actions on DRN neurons, indicating a role for Gal3-mediated effects in this context. The lack of neurochemical or electrophysiological effects of SNAP 37889 and SNAP 398299 in the absence of exogenously applied galanin points to the lack of significant tone at the Gal3 receptor under resting conditions. Thus, the anxiolytic- and antidepressant-like profile of Gal3 antagonists noted in this article directly identifies Gal3 as a participant in the inhibitory actions of galanin in anxious or depressed states and raises the possibility that Gal3-mediated signaling plays a prominent role in the disease state.
A growing body of evidence suggests that hypergalaninergic transmission and/or repeated physiological insult may be involved in the underlying pathophysiology of anxiety and depression. For instance, Flinder's Sensitive Line rats, which are considered to be a genetic model of depression, exhibit increased galanin binding in the DRN and increased immobility time during FST (16). Moreover, galanin-overexpressing mice have been reported to show an increase in immobility time during FST (30). Under conditions of chronic social stress, preprogalanin mRNA levels were shown to increase in the locus ceruleus (20), and galanin-overexpressing mice exposed to repeated FST display enhanced norepinephrine and 5-HT release in the vHip (31). Collectively, these results suggest that excessive galanin transmission may be an underlying condition in disease states involving repeated physiological insult. Furthermore, it is possible that this hypergalaninergic state may be a prerequisite for the anxiolytic and antidepressant efficacy of Gal3 antagonists. It will be important, therefore, to establish whether such a state is characteristic in major depression in humans.
The demonstration of both acute and chronic efficacy in models of anxiety and depression has important therapeutic implications. Current strategies for the treatment of these disorders include benzodiazepine and SSRI regimens. Although well documented as having acute effects in humans, the protracted use of benzodiazepines has serious side effects and can cause a tolerance to develop, as well as having the potential for abuse liability (32, 33). Although not associated with tolerance or abuse liabilities, the therapeutic effects of SSRIs are generally not apparent until 2-3 weeks of continuous treatment (34). Thus, an important finding of the present study is that Gal3 antagonists exhibit an efficacy profile that is distinct from that of benzodiazepines and SSRIs, showing acute anxiolytic- and antidepressant-like activity that persists after chronic administration in animal models. These findings suggest that Gal3-selective antagonists may represent an additional class of therapeutic agents for the treatment of anxiety and depression.
Supplementary Material
Acknowledgments
K.Z., Z-Q.D.X., and T.H. are supported by the Swedish Research Council (04X-2887), the Marianne and Marcus Wallenberg Foundation, and European Union Grant LHSM-CT-2003-503474.
Conflict of interest statement: No conflicts declared.
Abbreviations: DRN, dorsal raphe nucleus; SSRI, selective serotonin reuptake inhibitor; CDP, chlordiazepoxide; 5-HT, serotonin (5-hydroxytryptamine); FST, forced swim test; vHip, ventral hippocampus; p.o., oral(ly) (per os); i.c.v., intracerebroventricular(ly).
References
- 1.Tatemoto, K., Rökaeus, Å., Jörnvall, H., McDonald, T. J. & Mutt, V. (1983) FEBS Lett. 164, 124-128. [DOI] [PubMed] [Google Scholar]
- 2.Bartfai, T., Hökfelt, T. & Langel, U. (1993) Crit. Rev. Neurobiol. 7, 229-274. [PubMed] [Google Scholar]
- 3.Crawley, J. N. (1995) Regul. Pept. 59, 1-16. [DOI] [PubMed] [Google Scholar]
- 4.Branchek, T. A., Smith, K. E., Gerald, C. & Walker, M. W. (2000) Trends Pharmacol. Sci. 21, 109-117. [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell, D., Ahmad, S., Wahlestedt, C. & Walker, P. (1999) J. Comp. Neurol. 409, 469-481. [PubMed] [Google Scholar]
- 6.Mennicken, F., Hoffert, C., Pelletier, M., Ahmad, S. & O'Donnell, D. (2002) J. Chem. Neuroanat. 24, 257-268. [DOI] [PubMed] [Google Scholar]
- 7.Bard, J. A., Borowsky, B., Smith, K. E., Branchek, T. A., Gerald, C. P. G. & Jones, K. A. (2001) U.S. Patent 6,329,197.
- 8.Melander, T., Hökfelt, T., Rökaeus, Å., Cuello, A. C., Oertel, W. H., Verhofstad, A. & Goldstein, M. (1986) J. Neurosci. 6, 3640-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holets, V., R., Hökfelt, T., Rökaeus, Å., Terenius, L. & Goldstein, M. (1988) Neuroscience 24, 893-906. [DOI] [PubMed] [Google Scholar]
- 10.Bing, O., Möller, C., Engel, J. A., Soderpalm, B. & Heilig, M. (1993) Neurosci. Lett. 164, 17-20. [DOI] [PubMed] [Google Scholar]
- 11.Weiss, J. M., Bonsall, R. W., Demetrikopoulos, M. K., Emery, M. S. & West, C. H. K. (1998) Ann. N.Y. Acad. Sci. 863, 364-382. [DOI] [PubMed] [Google Scholar]
- 12.Fuxe, K., Jansson, A., Diaz-Cabiale, Z., Andersson, A., Tinner, B., Finnman, U.-B., Misane, I., Razani, H., Wang, F.-H., Agnati, L. F. & Ögren, S. O. (1998) Ann. N.Y. Acad. Sci. 863, 274-290. [DOI] [PubMed] [Google Scholar]
- 13.Möller, C., Sommer, W., Thorsell, A. & Heilig, M. (1999) Neuropsychopharmacology 21, 507-512. [DOI] [PubMed] [Google Scholar]
- 14.Khoshbouei, H., Cecchi, M., Dove, S., Javors, M. & Morilak, D. A. (2002) Pharmacol. Biochem. Behav. 71, 407-417. [DOI] [PubMed] [Google Scholar]
- 15.Lu, X., Barr, A. M., Kinney, J. W., Sanna, P., Conti, B., Behrens, M. M. & Bartfai, T. (2005) Proc. Natl. Acad. Sci. USA 102, 874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellido, I., Diaz-Cabiale, Z., Jimenez-Vasquez, P. A., Andbjer, B., Mathe, A. A. & Fuxe, K. (2002) Neurosci. Lett. 317, 101-105. [DOI] [PubMed] [Google Scholar]
- 17.Kehr, J., Yoshitake, T., Wang, F.-H., Razani, H., Gimenez-Llort, L., Jansson, A., Yamaguchi, M. & Ögren, S. O. (2002) Neuropsychopharmacology 27, 341-356. [DOI] [PubMed] [Google Scholar]
- 18.Misane, I., Razani, H., Wang, F. H., Jansson, A., Fuxe, K. & Ögren, S. O. (1998) Ann. N.Y. Acad. Sci. 863, 442-444. [DOI] [PubMed] [Google Scholar]
- 19.Razani, H., Diaz-Cabiale, Z., Misane, I., Wang, F., H., Fuxe, K. & Ögren, S. O. (2001) Neurosci. Lett. 299, 145-149. [DOI] [PubMed] [Google Scholar]
- 20.Holmes, P. V., Koprivica, V., Chough, E. & Crawley, J. N. (1994) Peptides (Tarrytown, NY) 15, 1303-1308. [DOI] [PubMed] [Google Scholar]
- 21.Lu, X., Lundström, L., Langel, U. & Bartfai, T. (2005) Neuropeptides 39, 143-146. [DOI] [PubMed] [Google Scholar]
- 22.Borowsky, B., Walker, M. W., Huang, L.-Y., Jones, K. A., Smith, K. E., Bard, J., Branchek, T. A. & Gerald, C. (1998) Peptides (Tarrytown, NY) 19, 1771-1781. [DOI] [PubMed] [Google Scholar]
- 23.Haddjeri, N., Lavoie, N. & Blier, P. (2004) Neuropsychopharmacology 29, 1800-1806. [DOI] [PubMed] [Google Scholar]
- 24.Xu, Z.-Q. D., Zhang, X., Pieribone, V. A., Grillner, S. & Hökfelt, T. (1998) Neuroscience 87, 79-94. [DOI] [PubMed] [Google Scholar]
- 25.Blackburn, T. P., Konkel, M. J., Boteju, L. W., Talisman, I. J., Wetzel, J. M., Packiarajan, M., Chen, H. & Jimenez, H. (2003) U.S. Patent Appl. 2,003,007,827; Chem. Abstr. no. 303149-14-6.
- 26.Konkel, M. J., Packiarajan, M., Chen, H., Topiwala, U. P., Jimenez, H., Talisman, I. J., Coate, H. & Walker, M. W. (2005) Med. Chem. Lett., in press. [DOI] [PubMed]
- 27.Amat, J., Matus-Amat, P., Watkins, L. R. & Maier, S. F. (1998) Brain Res. 812, 113-120. [DOI] [PubMed] [Google Scholar]
- 28.Yoshitake, T., Reenila, I., Ögren, S. O., Hökfelt, T. & Kehr, J. (2003) Neurosci. Lett. 339, 239-242. [DOI] [PubMed] [Google Scholar]
- 29.Blier, P., Pineyro, G., el Mansari, M., Bergeron, R. & de Montigny, C. (1998) Ann. N.Y. Acad. Sci. 861, 204-216. [DOI] [PubMed] [Google Scholar]
- 30.Kuteeva, E., Hökfelt, T. & Ögren, S. O. (2005) Neuropeptides 39, 299-304. [DOI] [PubMed] [Google Scholar]
- 31.Yoshitake, T., Wang, F. H., Kuteeva., E., Holmberg, K., Yamaguchi, M., Crawley, J., N., Steiner, R., Bartfai, T., Ögren, S. O., Hökfelt, T. & Kehr, J. (2004) Proc. Natl. Acad. Sci. USA 101, 354-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods, J. H., Katz, J. L. & Winger, G. (1992) Pharmacol. Rev. 44, 151-347. [PubMed] [Google Scholar]
- 33.Schmitt, U., Luddens, H. & Hiemke, C. (2001) Prog. Neuropsychopharmacol. Biol. Psychiatry 25, 1145-1160. [DOI] [PubMed] [Google Scholar]
- 34.Blier, P. & de Montigny, C. (1998) Biol. Psychiatry 44, 313-323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.