Abstract
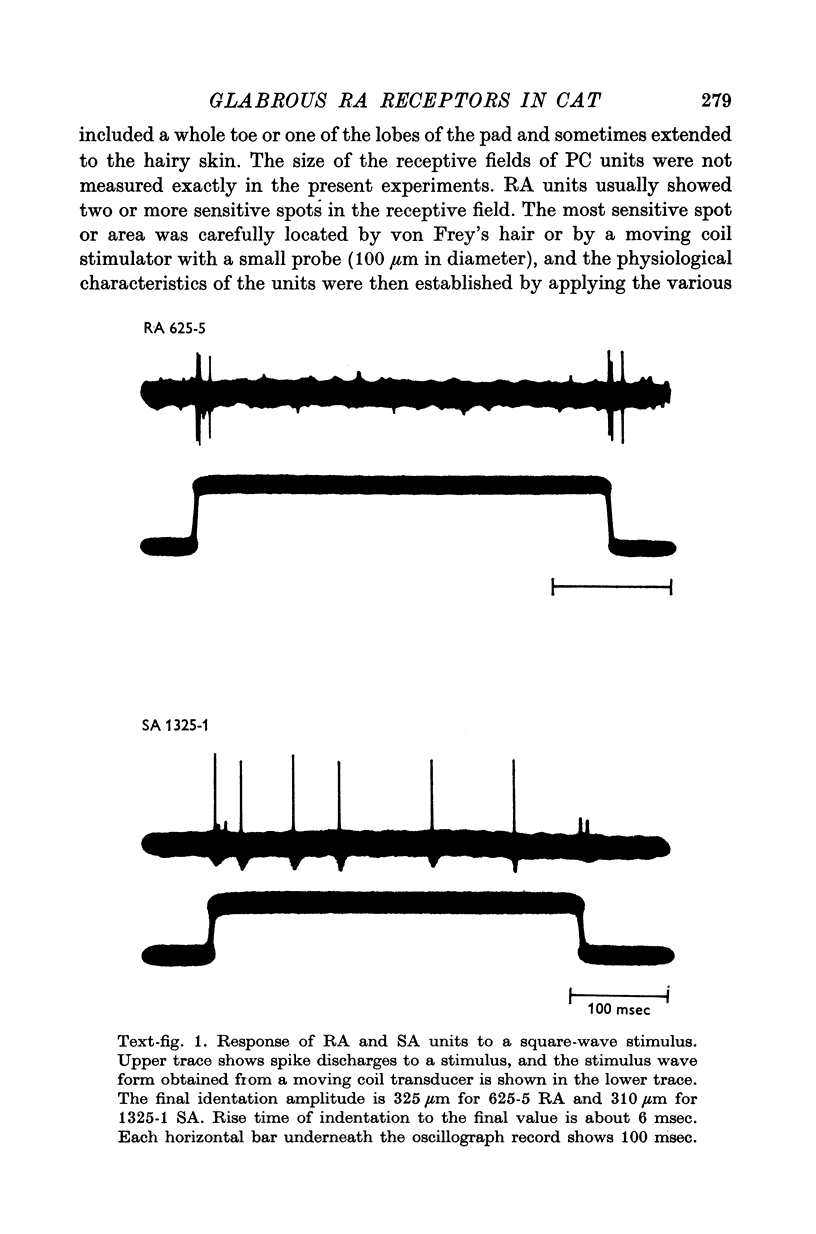
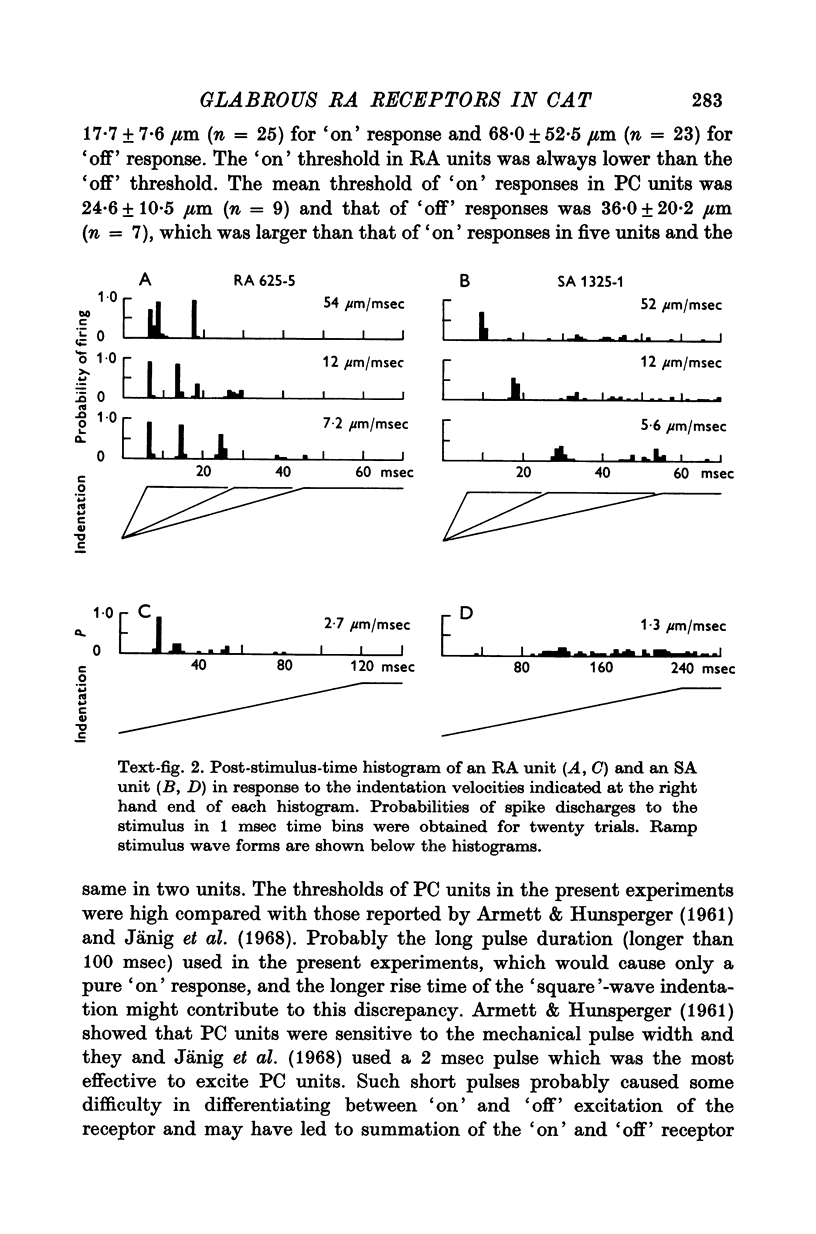
1. A total of fifty-four mechanoreceptor afferent units with fast conducting axons in the tibial nerve innervating the glabrous skin of the hind leg were isolated in anaesthetized cats. 2. Twenty-six rapidly adapting units (RA), eighteen slowly adapting units (SA) and ten Pacinian corpuscle units (PC) were differentiated from each other mainly on the presence of the off response in RA and PC units to a ramp stimulation, the persistence of discharges of the SA units during steady pressure on the receptive field and the classical tuning curve seen in the PC units. A few PC units in the hairy skin were also studied for comparison. 3. Lamellated corpuscles were found histologically in the skin of the receptive field of RA units and identified as Krause's corpuscle of cylindrical type by their superficial location in the cutaneous tissue and their structure revealed by electron microscopy. 4. Physiological characteristics of RA units to various forms of mechanical stimulation were studied and compared with those of the other two kinds of units. SA units had the lowest critical slope among three groups and PC units the highest. 5. The discharge pattern of RA and PC units to a ramp stimulation was found to be time-locked, whereas with SA unites only the first spike appeared at a fixed latency from the start of stimulation. 6. Some RA units showed a tuning curve which was flat from 10 to 200 Hz. Those with narrowly tuned curves had a best turning frequency at around 20 Hz. They were easily differentiated from the SA and PC units. SA units were tuned best at 5 HZ or less, and PC units at around 200 HZ. 7. The relation between the indentation velocity and amplitude of the ramp and the spike discharges was analysed in eleven RA units. In most cases the relation between identation velocity and maximum instataneous frequency was found to be best fit with a power function although other kinds of functions (linear, logarithmic, and logarithmic hyperbolic tangent) could also fit the relation at the 1% significance level. The instantaneous impuse frequency in RA units in response to various indentation amplitudes showed a step function. 8. The "off" responses to a ramp stimulation in RA units were also analysed in detail.
Full text
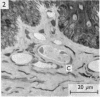
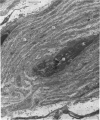
PDF






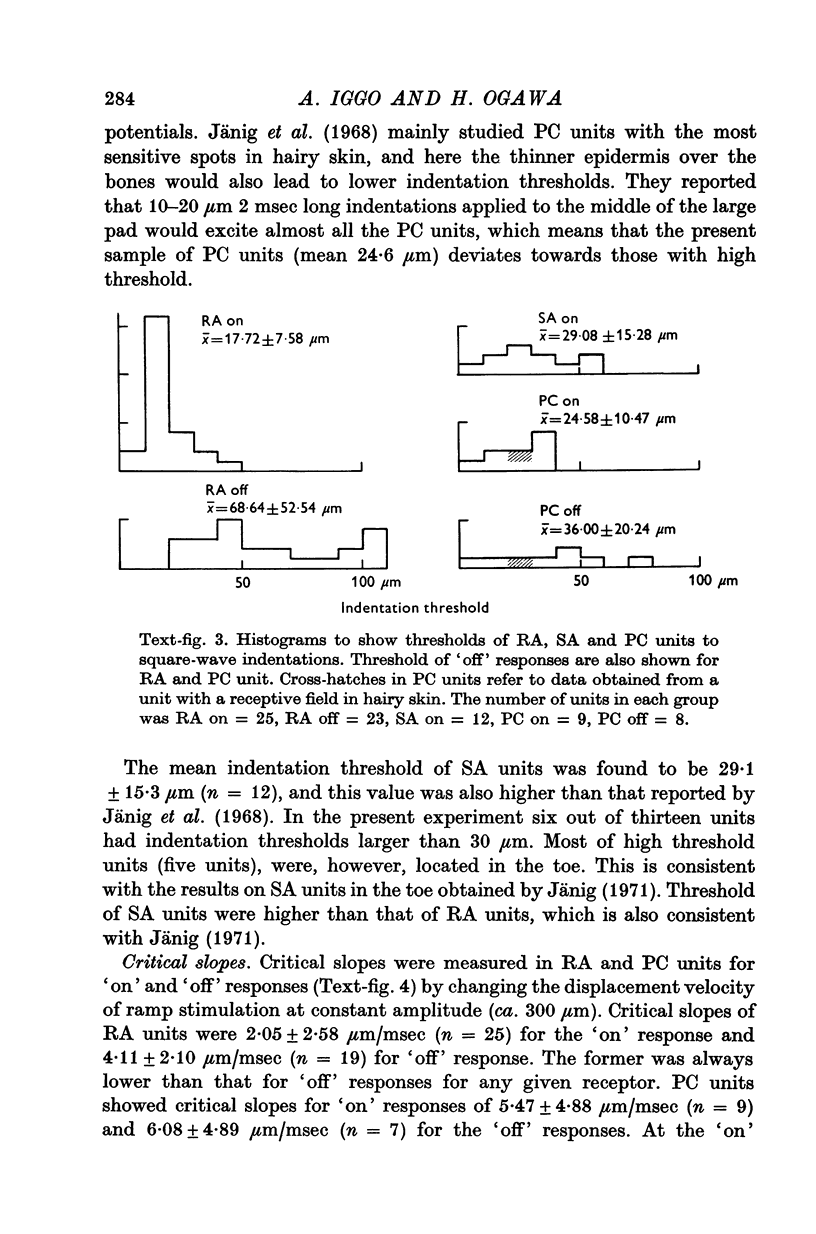

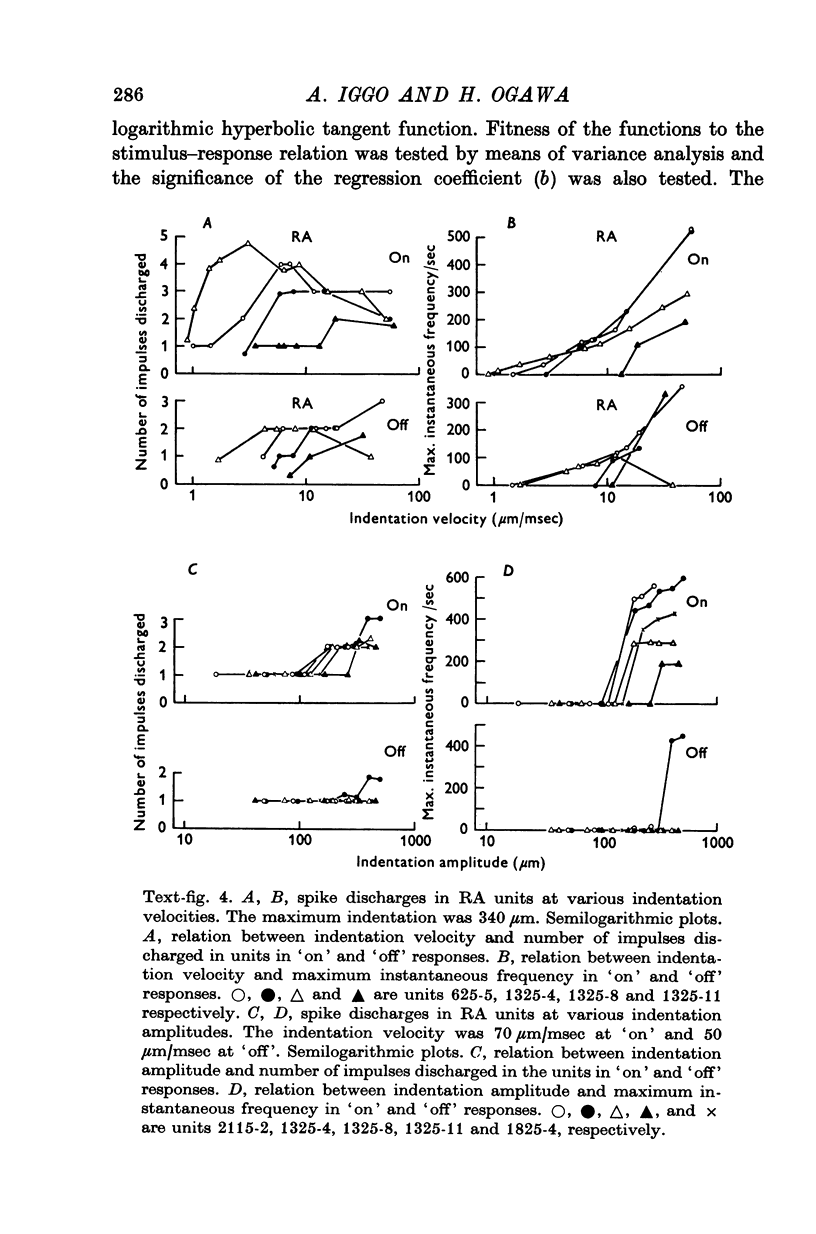
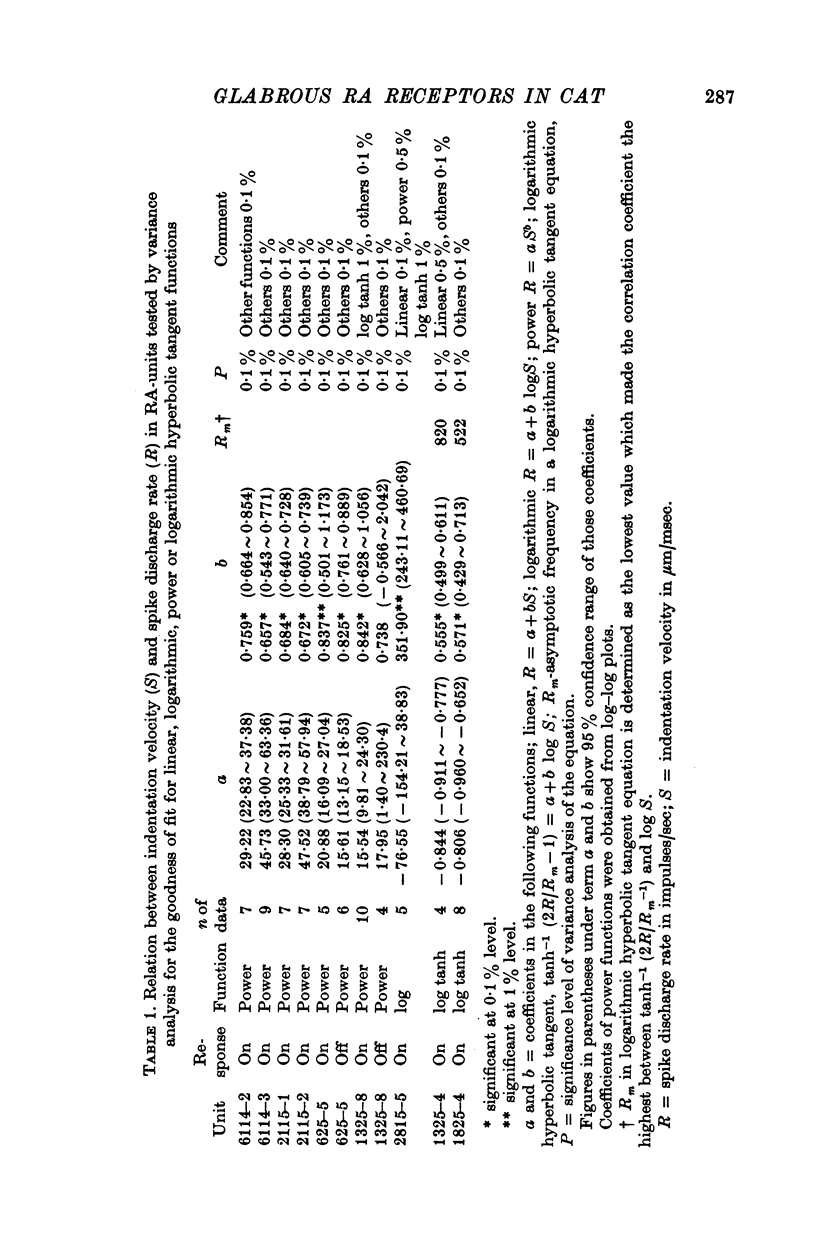

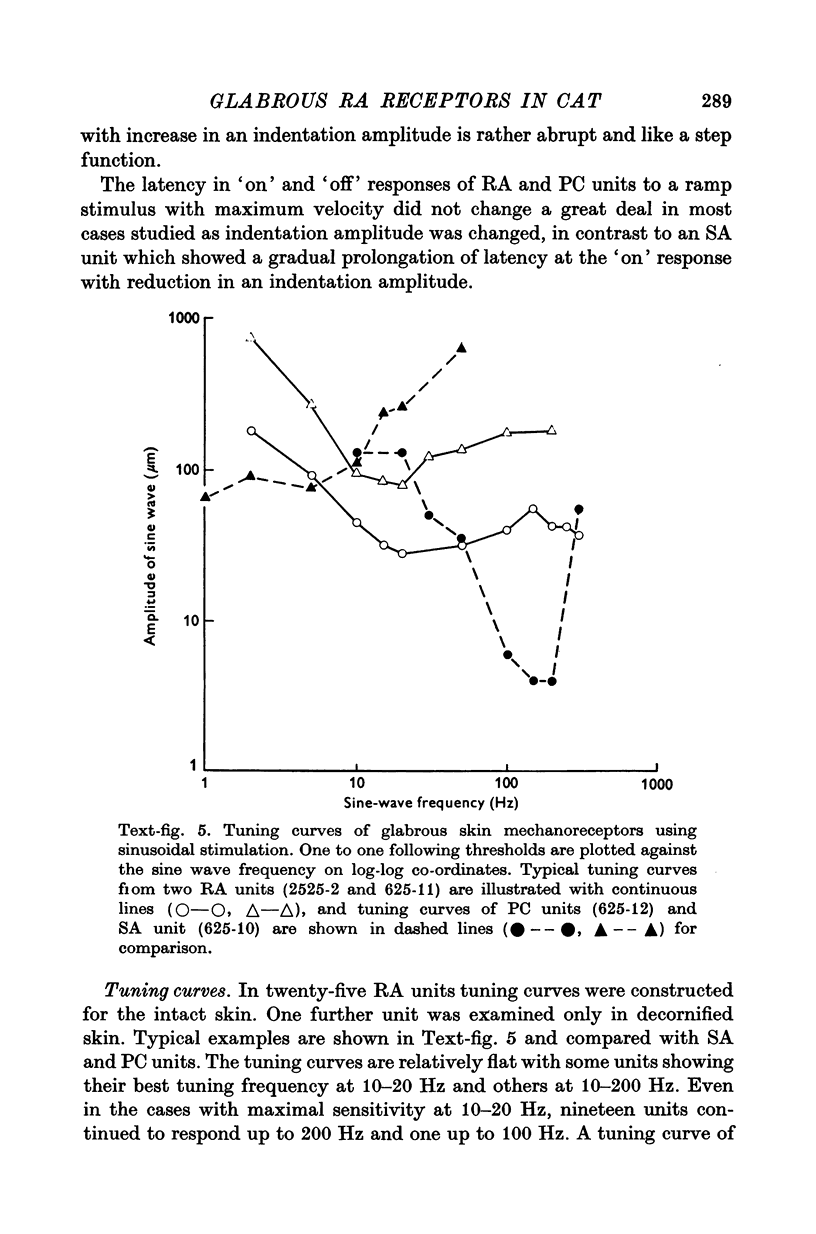



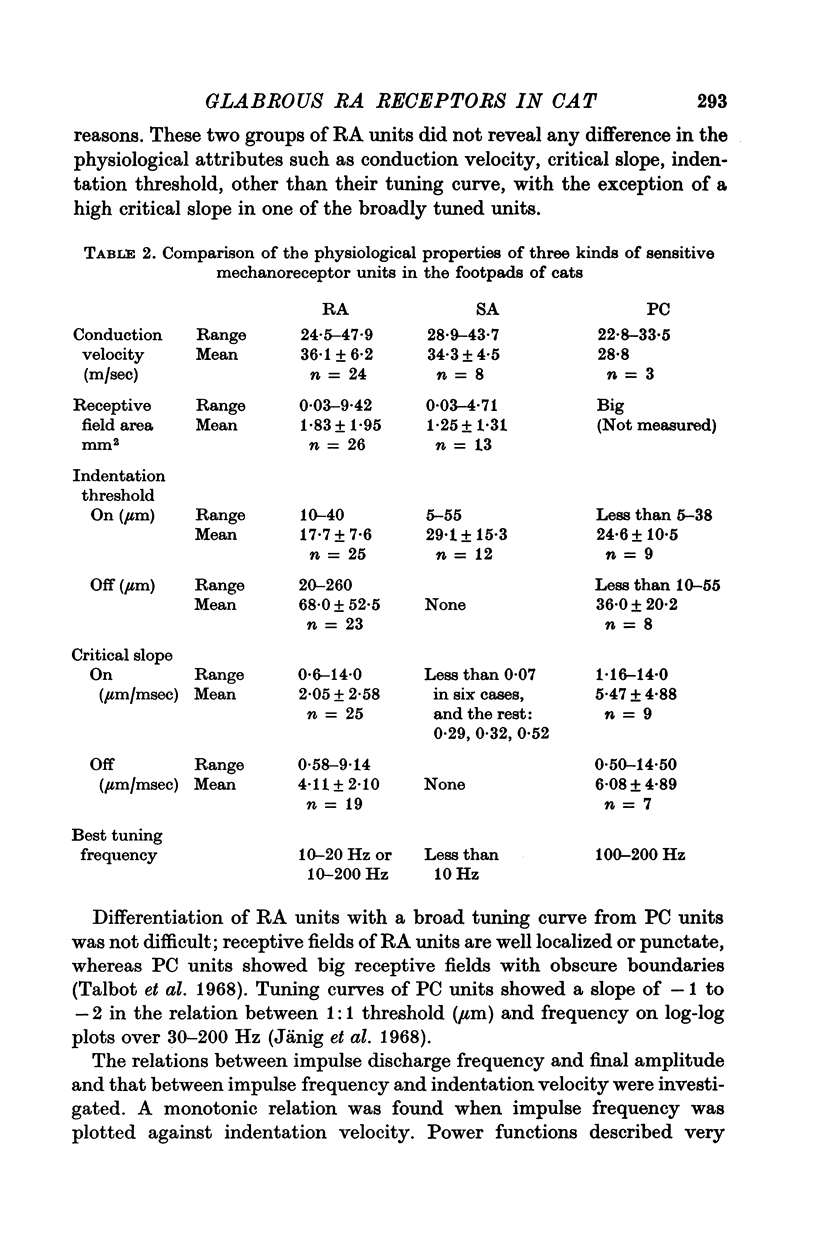



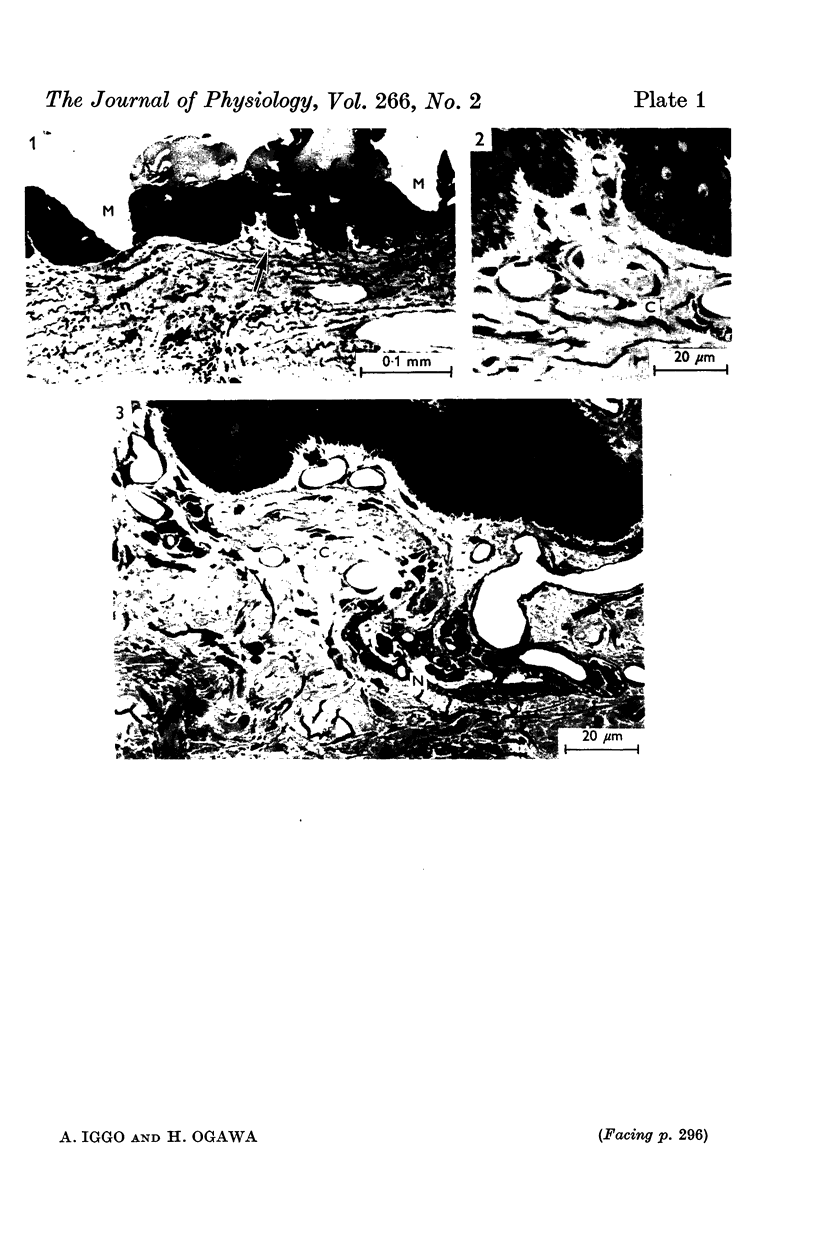
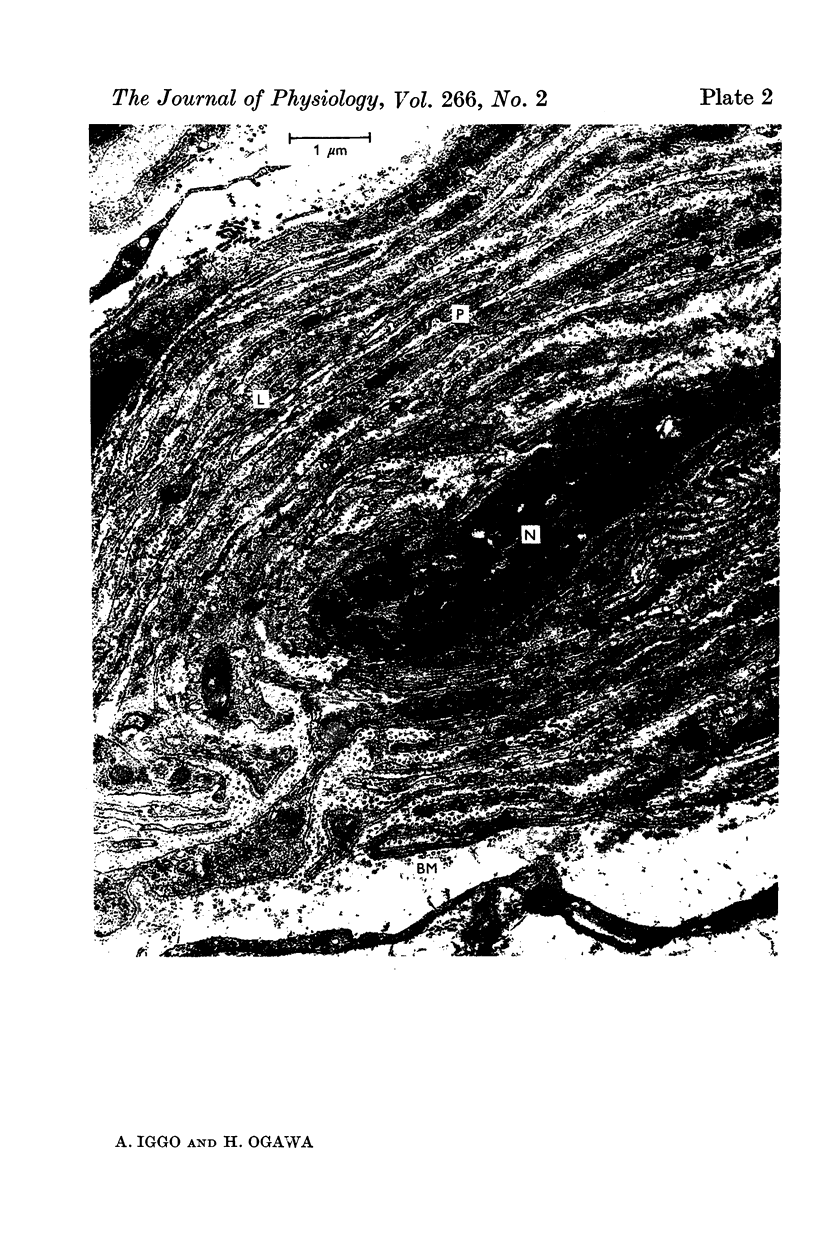
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMETT C. J., HUNSPERGER R. W. Excitation of receptors in the pad of the cat by single and double mechanical pulses. J Physiol. 1961 Sep;158:15–38. doi: 10.1113/jphysiol.1961.sp006751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armett C. J., Gray J. A., Hunsperger R. W., Lal S. The transmission of information in primary receptor neurones and second-order neurones of a phasic system. J Physiol. 1962 Dec;164(3):395–421. doi: 10.1113/jphysiol.1962.sp007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers M. R., Andres K. H., von Duering M., Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- Gottschaldt K. M., Iggo A., Young D. W. Functional characteristics of mechanoreceptors in sinus hair follicles of the cat. J Physiol. 1973 Dec;235(2):287–315. doi: 10.1113/jphysiol.1973.sp010388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. An analysis of fibre diameter and receptor characteristics of myelinated cutaneous afferent fibres in cat. J Physiol. 1960 Aug;153:99–112. doi: 10.1113/jphysiol.1960.sp006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel H., Andres K. H., von Düring M. Structure and function of cold receptors. Pflugers Arch. 1974;352(1):1–10. doi: 10.1007/BF01061945. [DOI] [PubMed] [Google Scholar]
- ITO S., WINCHESTER R. J. The fine structure of the gastric mucosa in the bat. J Cell Biol. 1963 Mar;16:541–577. doi: 10.1083/jcb.16.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W. Morphology of rapidly and slowly adapting mechanoreceptors in the hairless skin of the cat's hind foot. Brain Res. 1971 May 7;28(2):217–231. doi: 10.1016/0006-8993(71)90656-1. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- Jänig W. The afferent innervation of the central pad of the cat's hind foot. Brain Res. 1971 May 7;28(2):203–216. doi: 10.1016/0006-8993(71)90655-x. [DOI] [PubMed] [Google Scholar]
- Knibestöl M. Stimulus-response functions of rapidly adapting mechanoreceptors in human glabrous skin area. J Physiol. 1973 Aug;232(3):427–452. doi: 10.1113/jphysiol.1973.sp010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom U., Lund L. The discharge from vibration-sensitive receptors in the monkey foot. Exp Neurol. 1966 Aug;15(4):401–417. doi: 10.1016/0014-4886(66)90138-5. [DOI] [PubMed] [Google Scholar]
- Lynn B. The form and distribution of the receptive fields of Pacinian corpuscles found in and around the cat's large foot pad. J Physiol. 1971 Sep;217(3):755–771. doi: 10.1113/jphysiol.1971.sp009598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B. The nature and location of certain phasic mechanoreceptors in the cat's foot. J Physiol. 1969 May;201(3):765–773. doi: 10.1113/jphysiol.1969.sp008786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger B. L., Pubols L. M. The sensorineural organization of the digital skin of the raccoon. Brain Behav Evol. 1972;5(4):367–393. doi: 10.1159/000123757. [DOI] [PubMed] [Google Scholar]
- Polácek P., Halata Z. Development of simple encapsulated corpuscles in the nasolabial region of the cat. Ultrastructural study. Folia Morphol (Praha) 1970;18(4):359–passim. [PubMed] [Google Scholar]
- Pubols L. M., Pubols B. H., Jr, Munger B. L. Functional properties of mechanoreceptors in glabrous skin of the raccoon's forepaw. Exp Neurol. 1971 May;31(2):165–182. doi: 10.1016/0014-4886(71)90185-3. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO M. Response of Pacinian corpuscles to sinusoidal vibration. J Physiol. 1961 Dec;159:391–409. doi: 10.1113/jphysiol.1961.sp006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- WINKELMANN R. K. The sensory endings in the skin of the cat. J Comp Neurol. 1958 Apr;109(2):221–232. doi: 10.1002/cne.901090206. [DOI] [PubMed] [Google Scholar]