Abstract
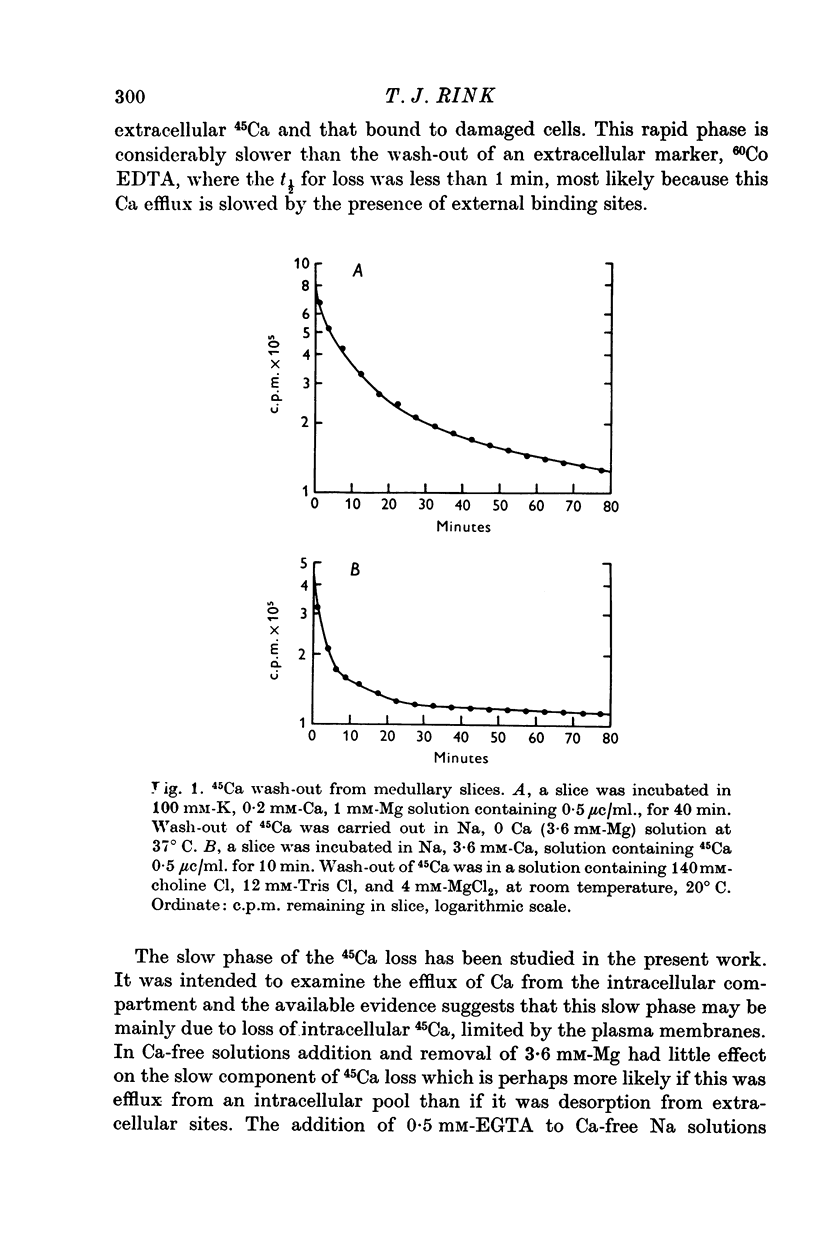
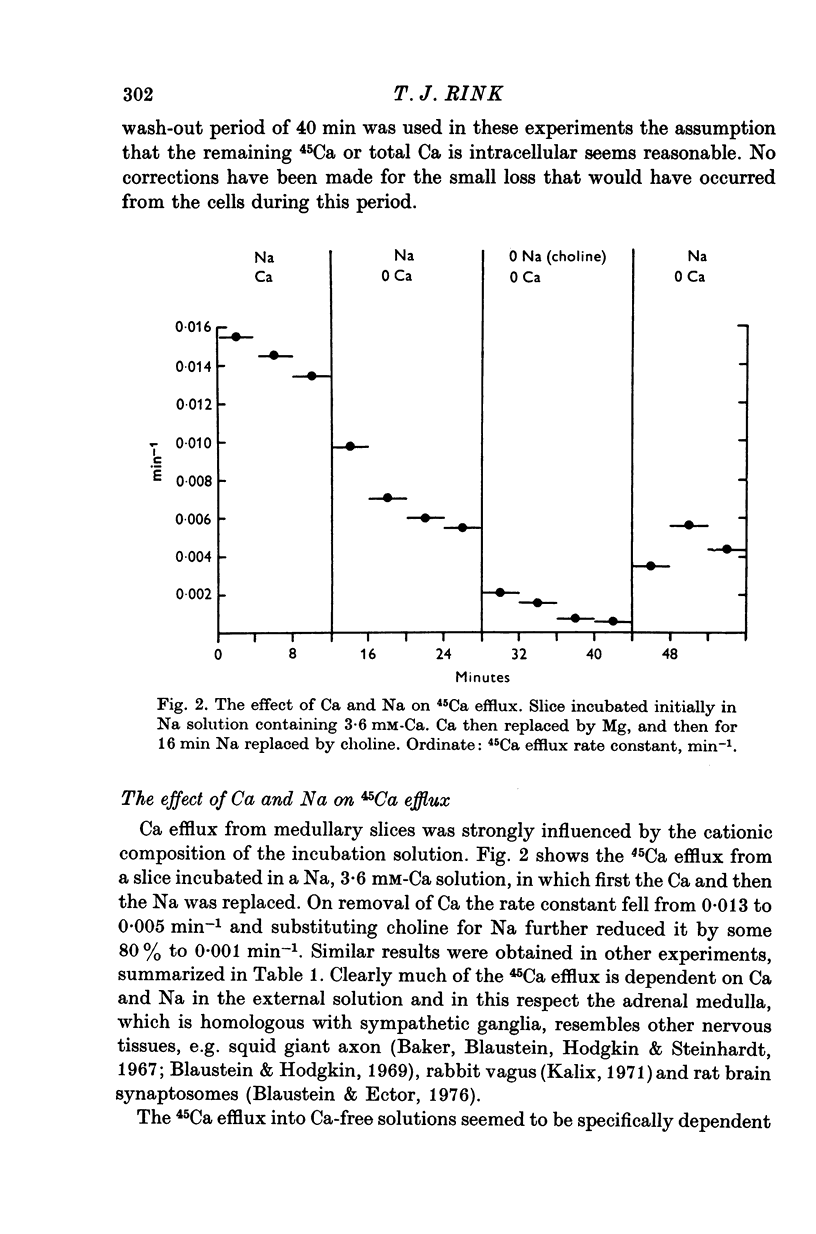
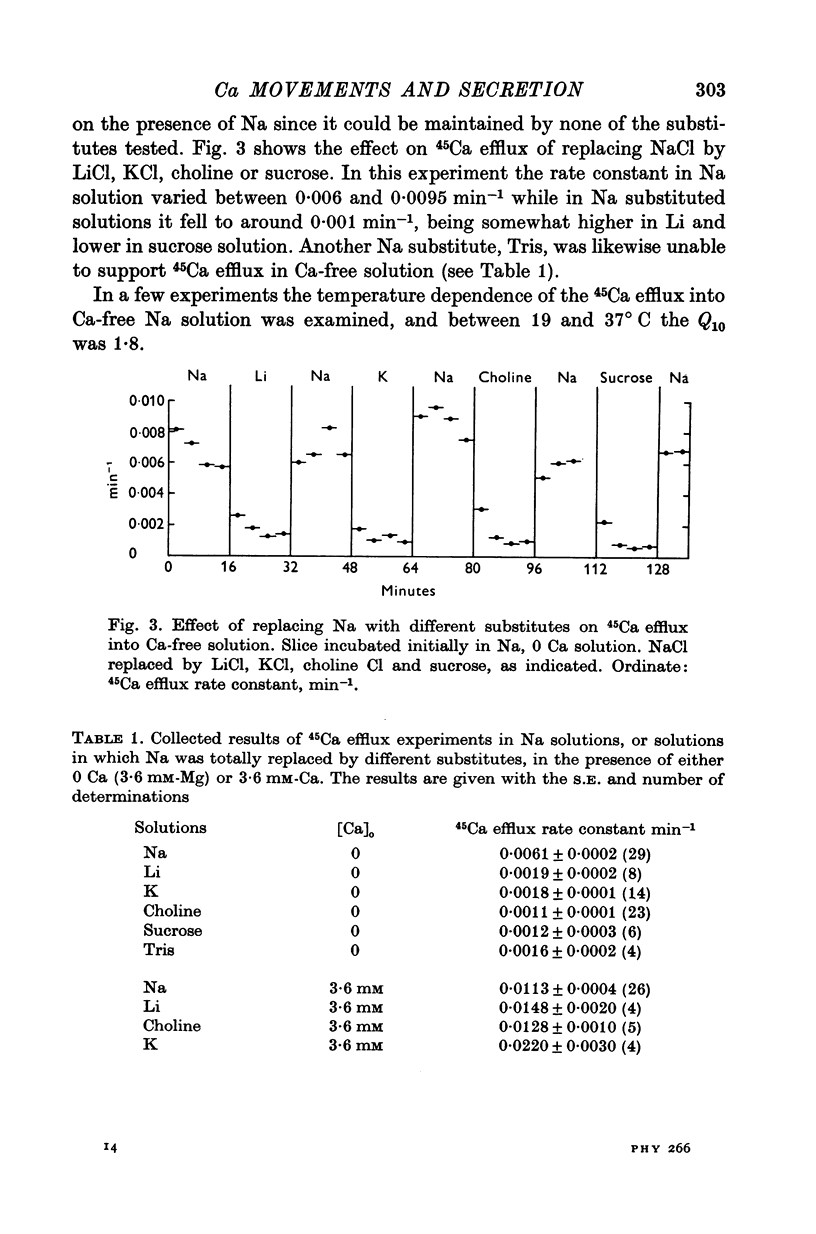
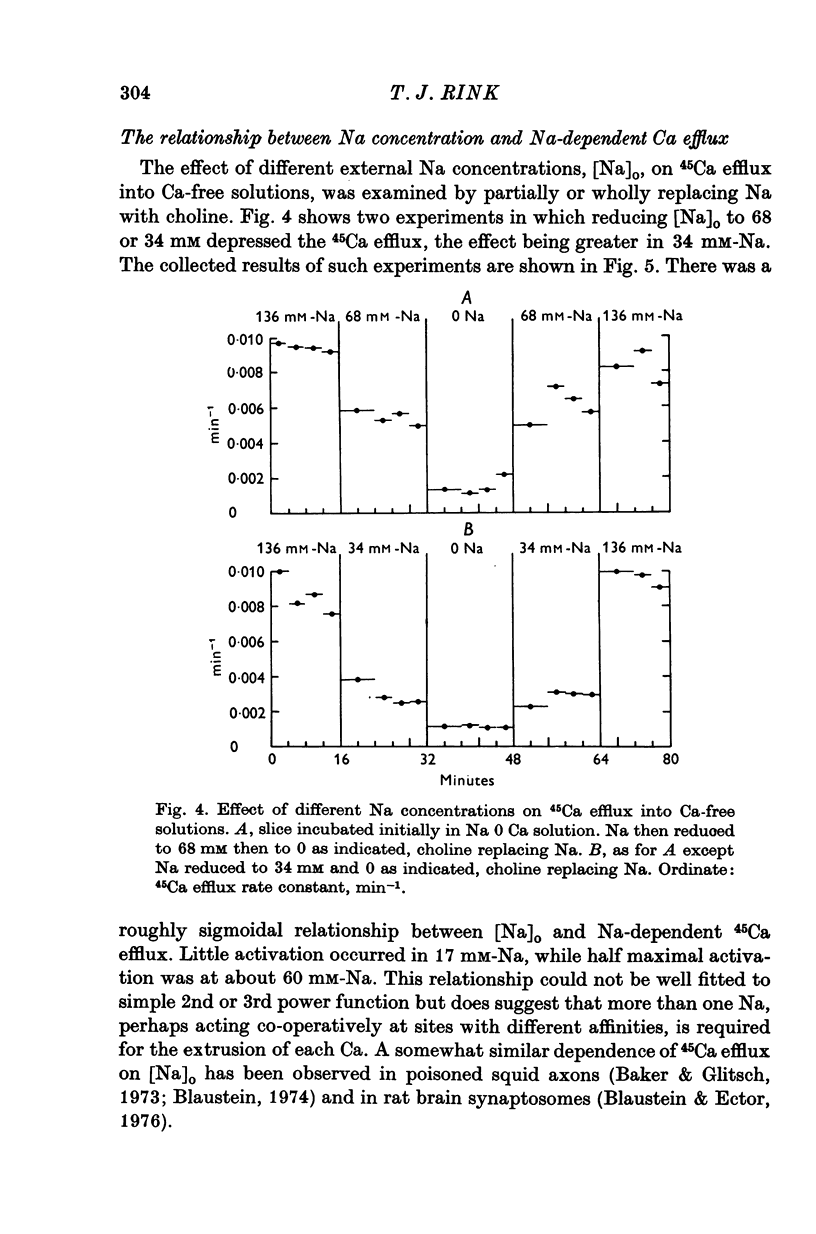
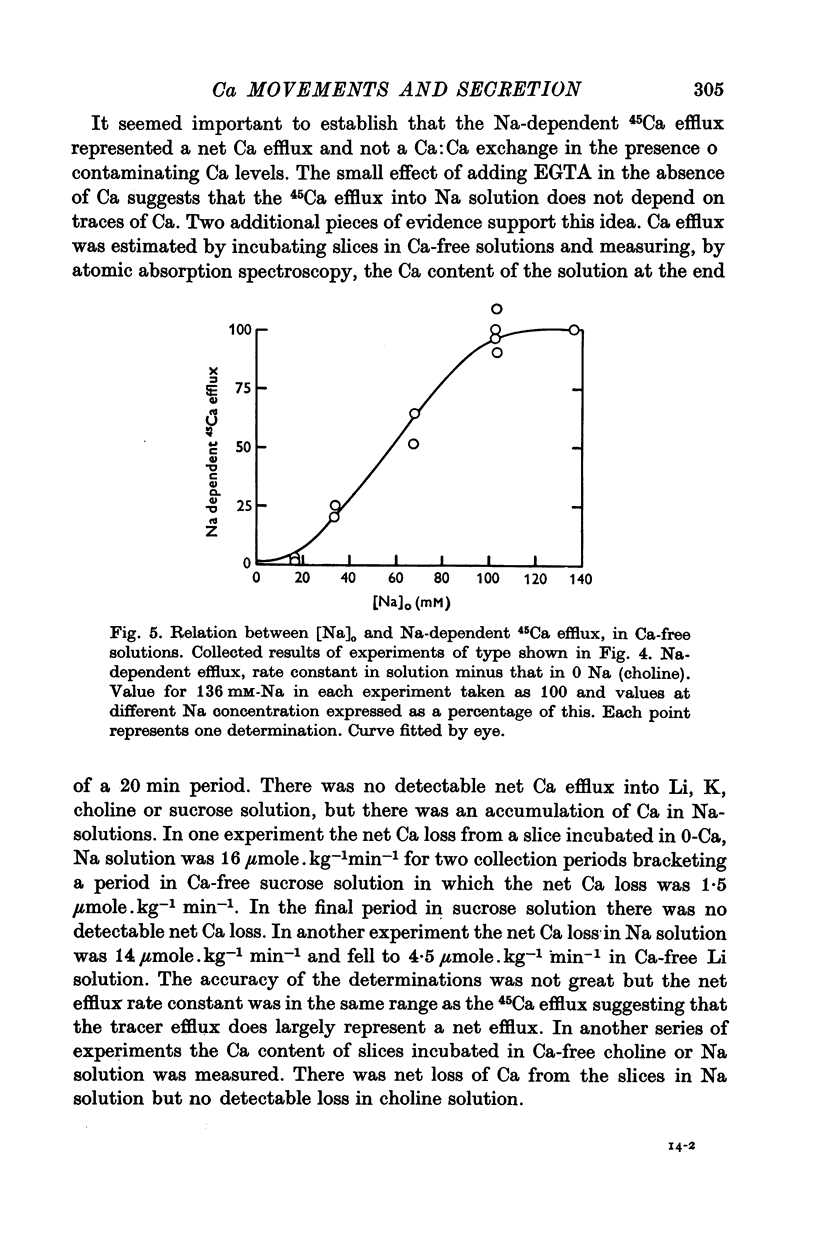
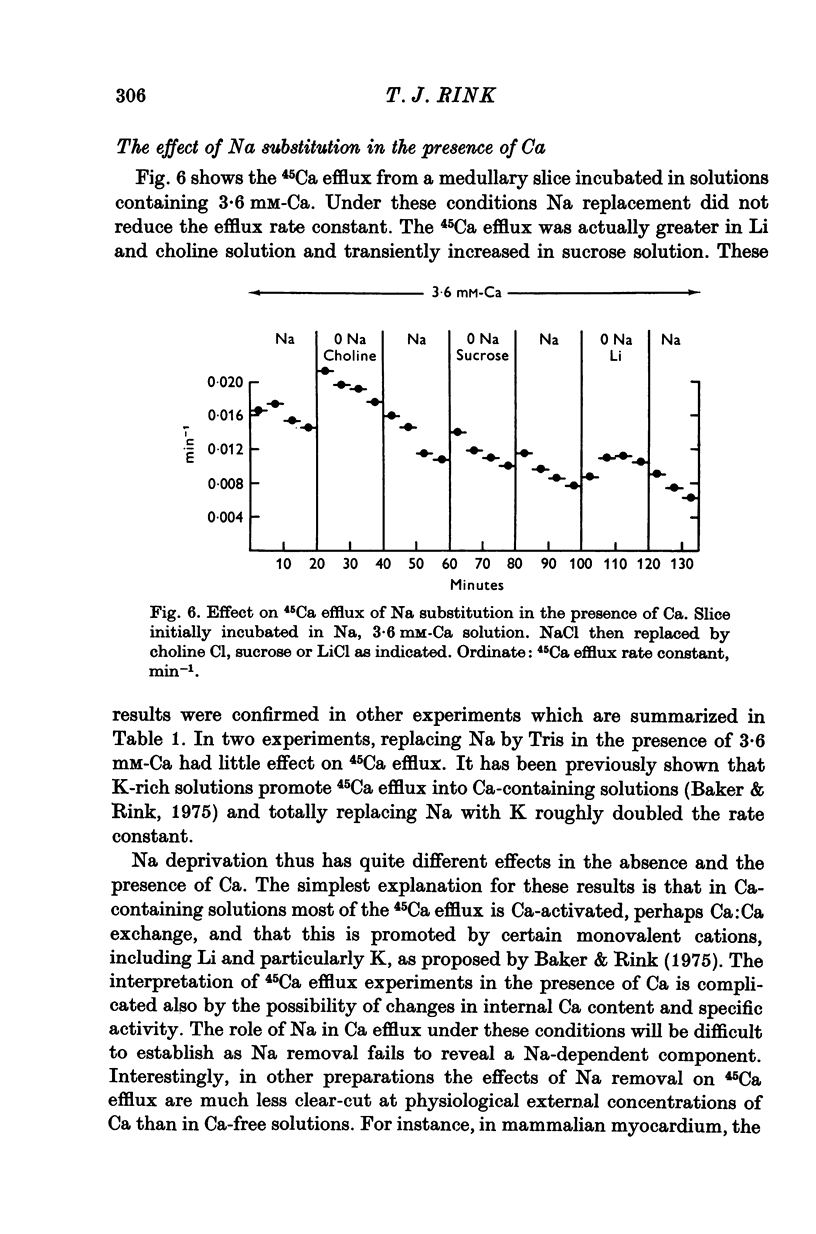
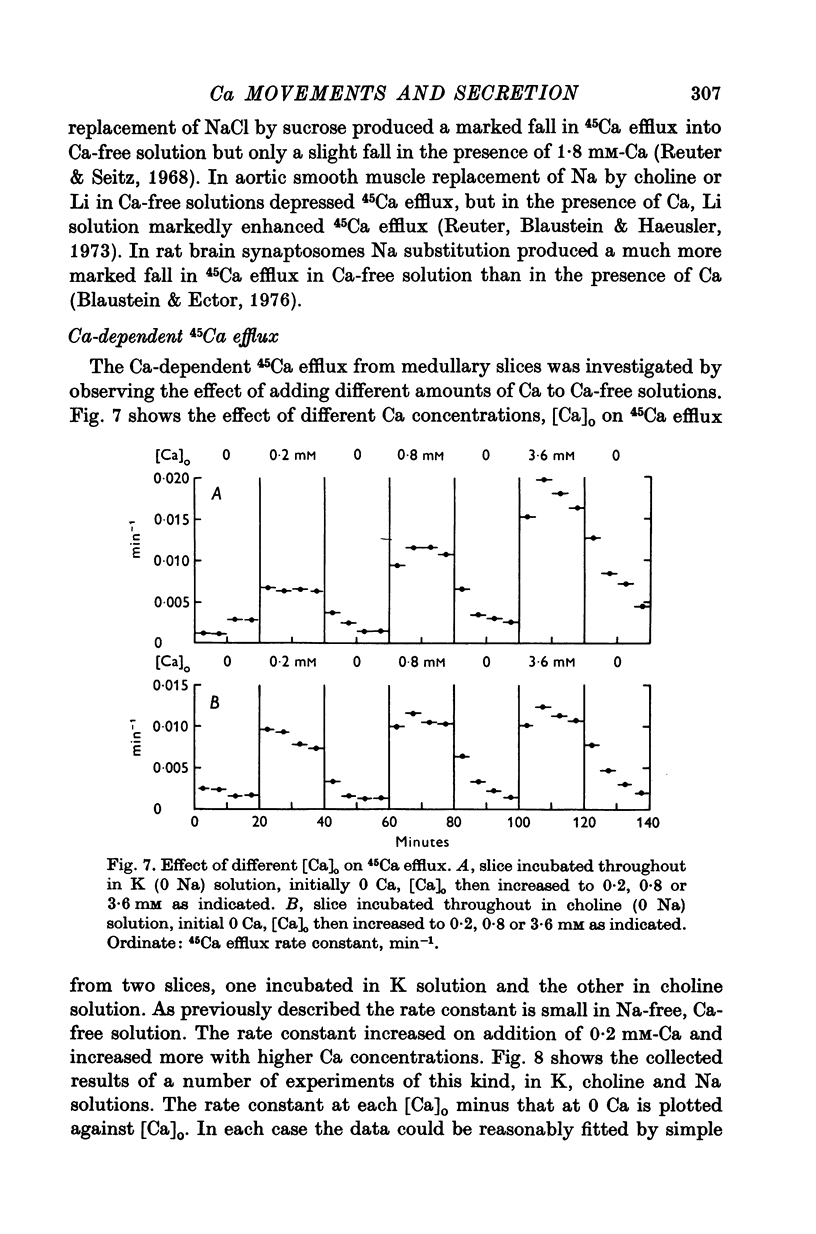
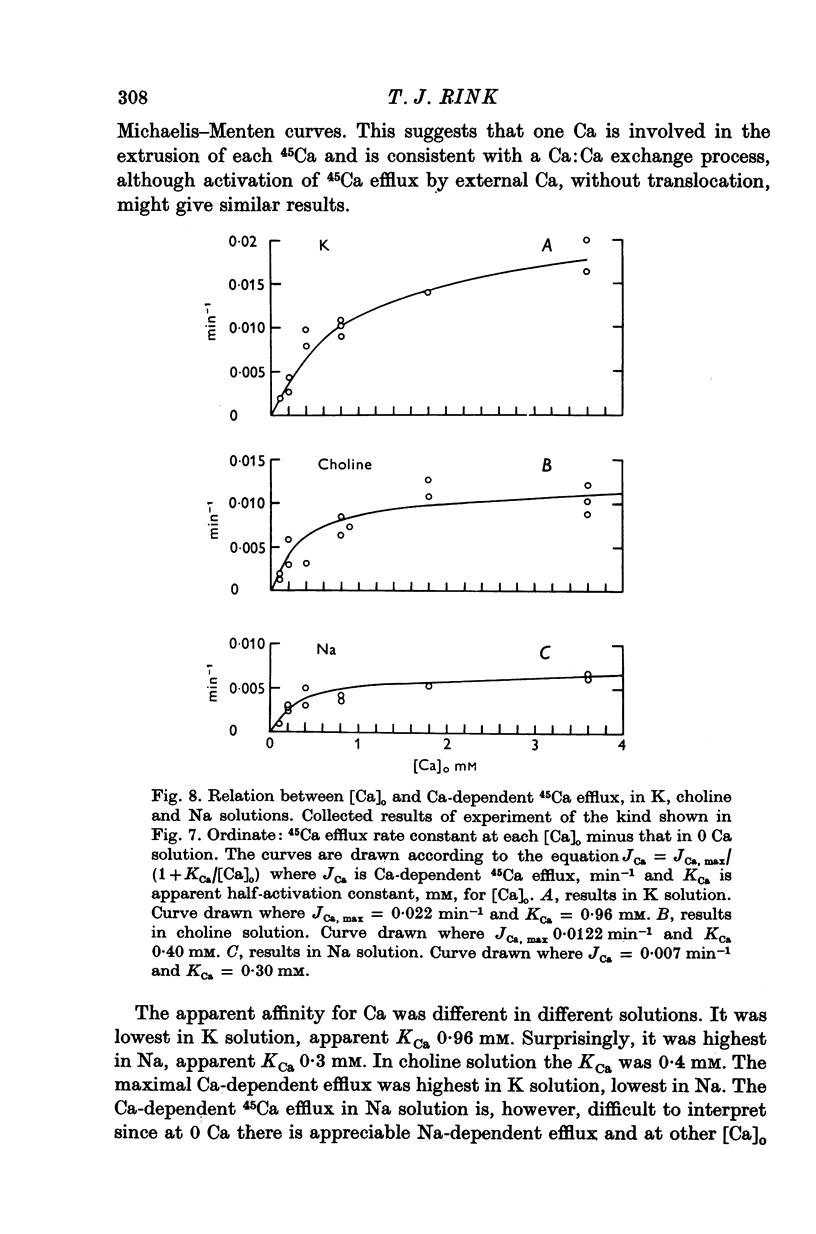
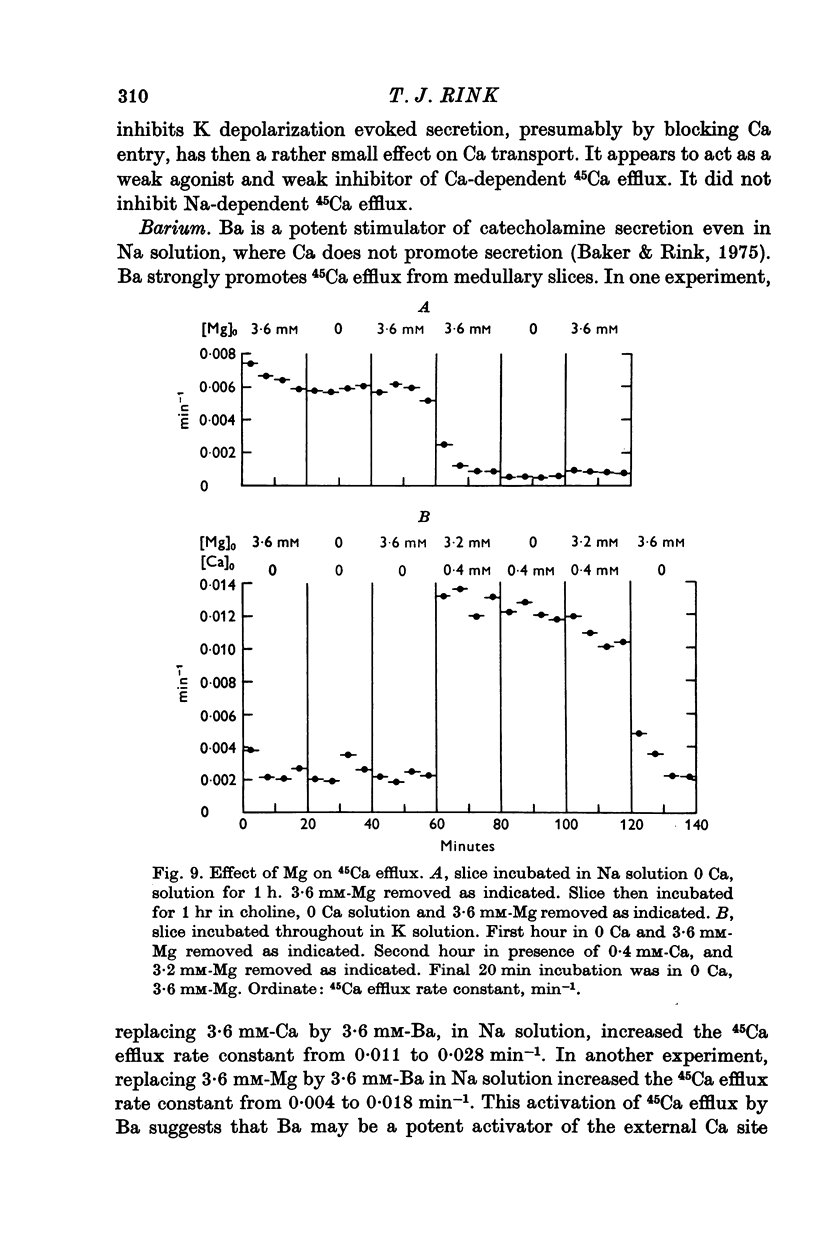
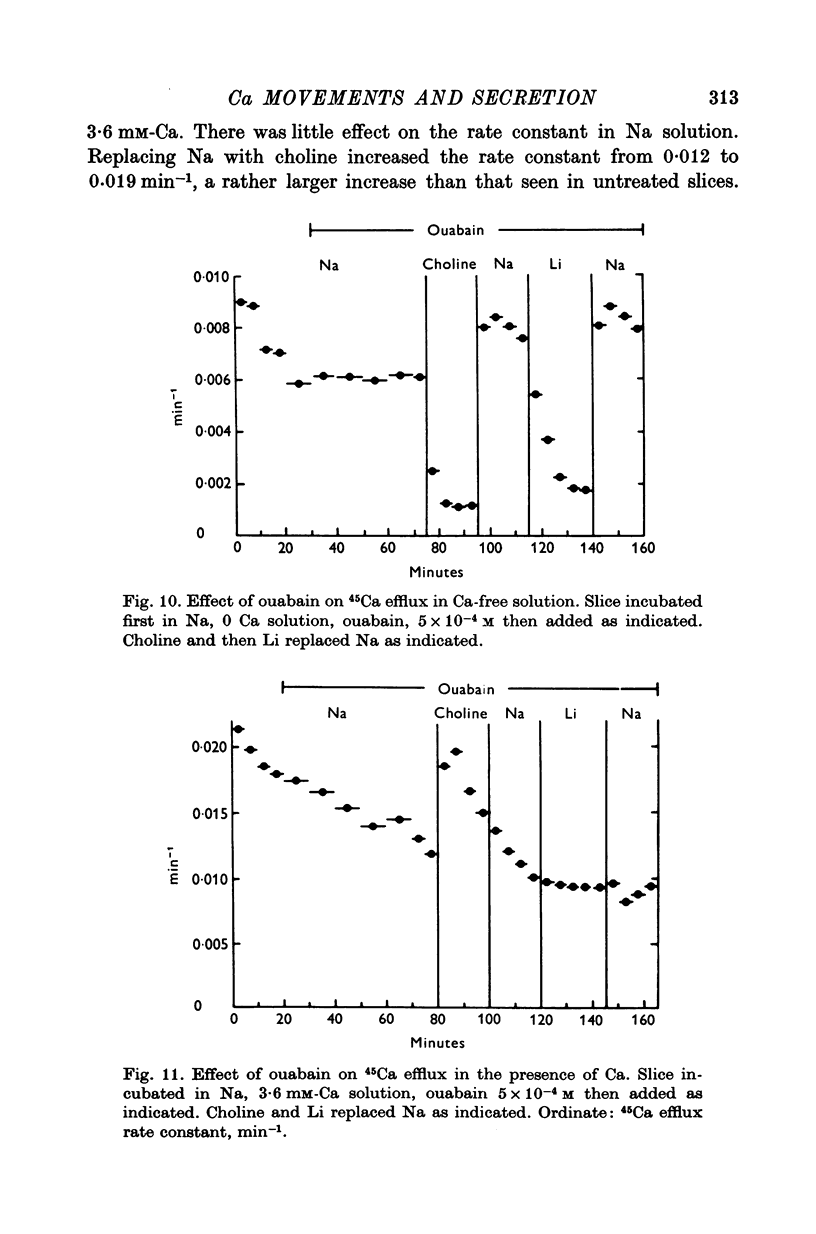
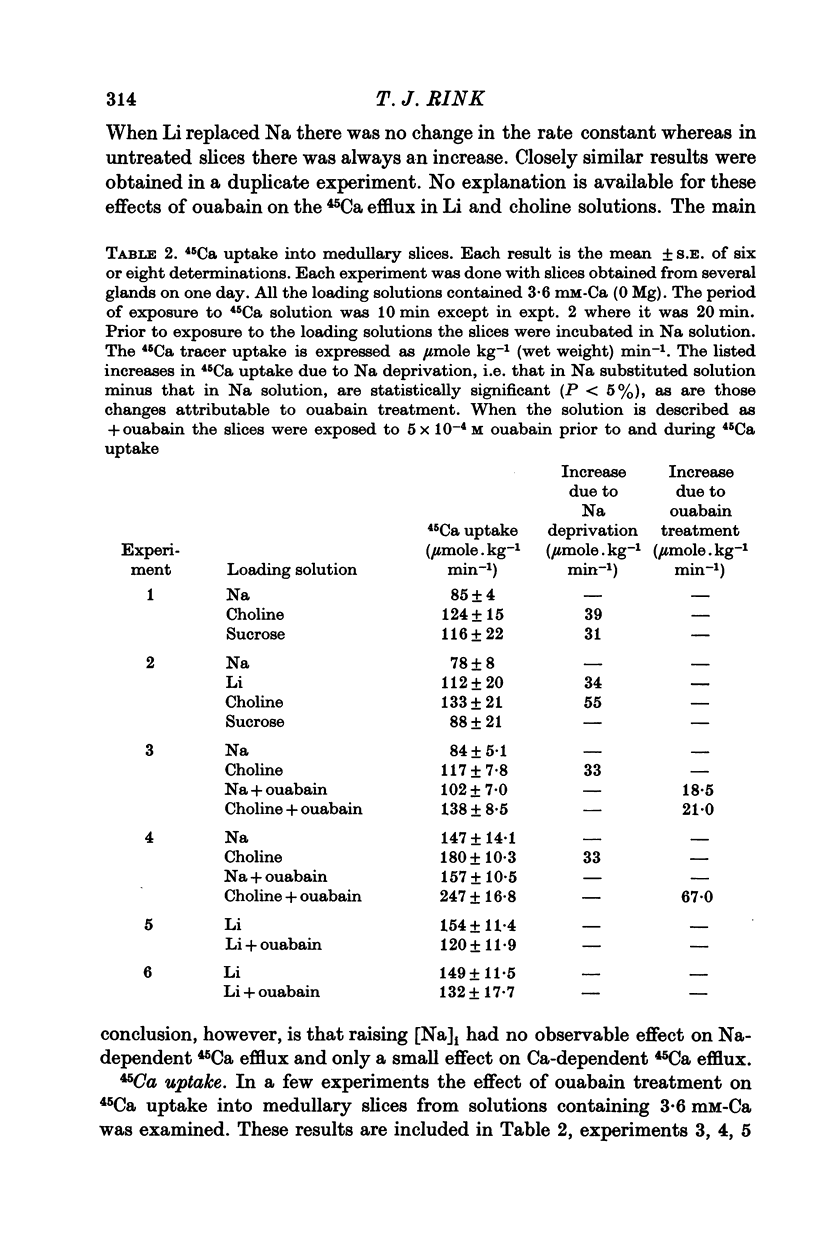
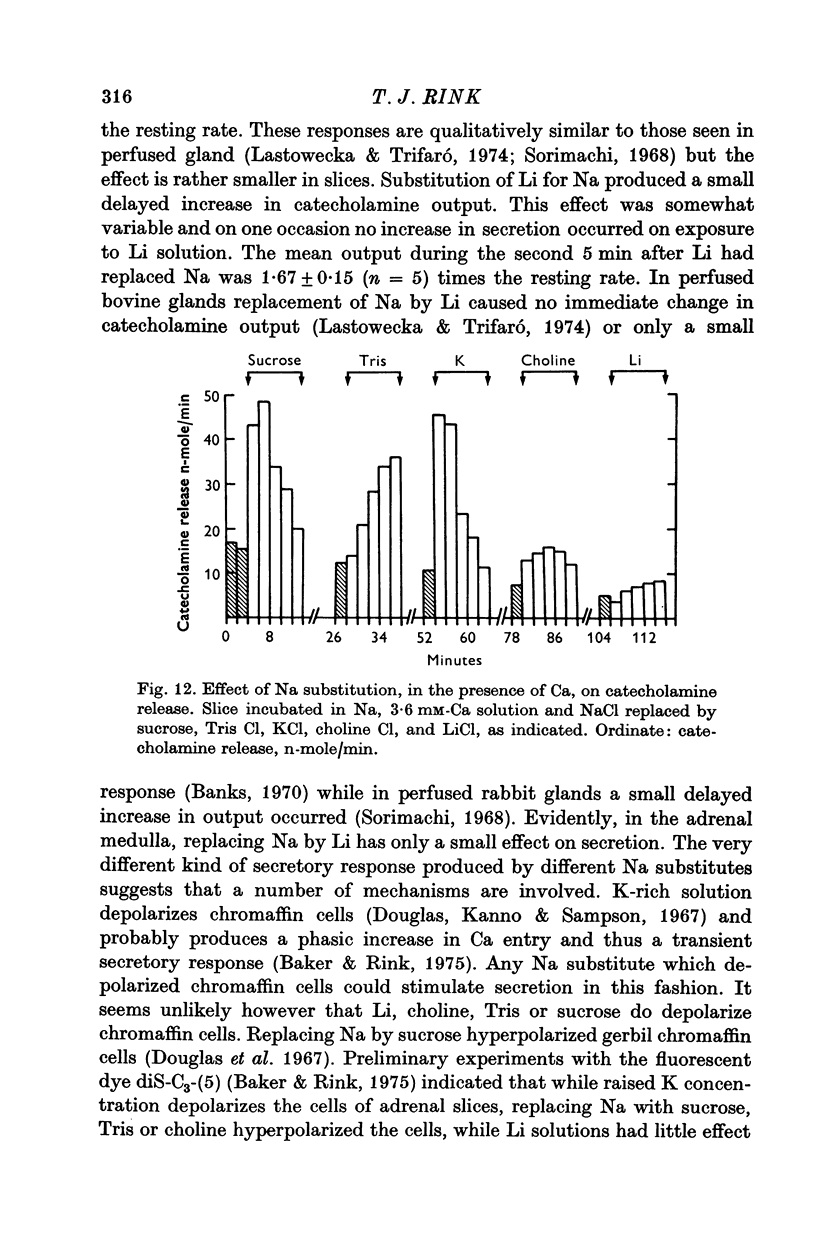
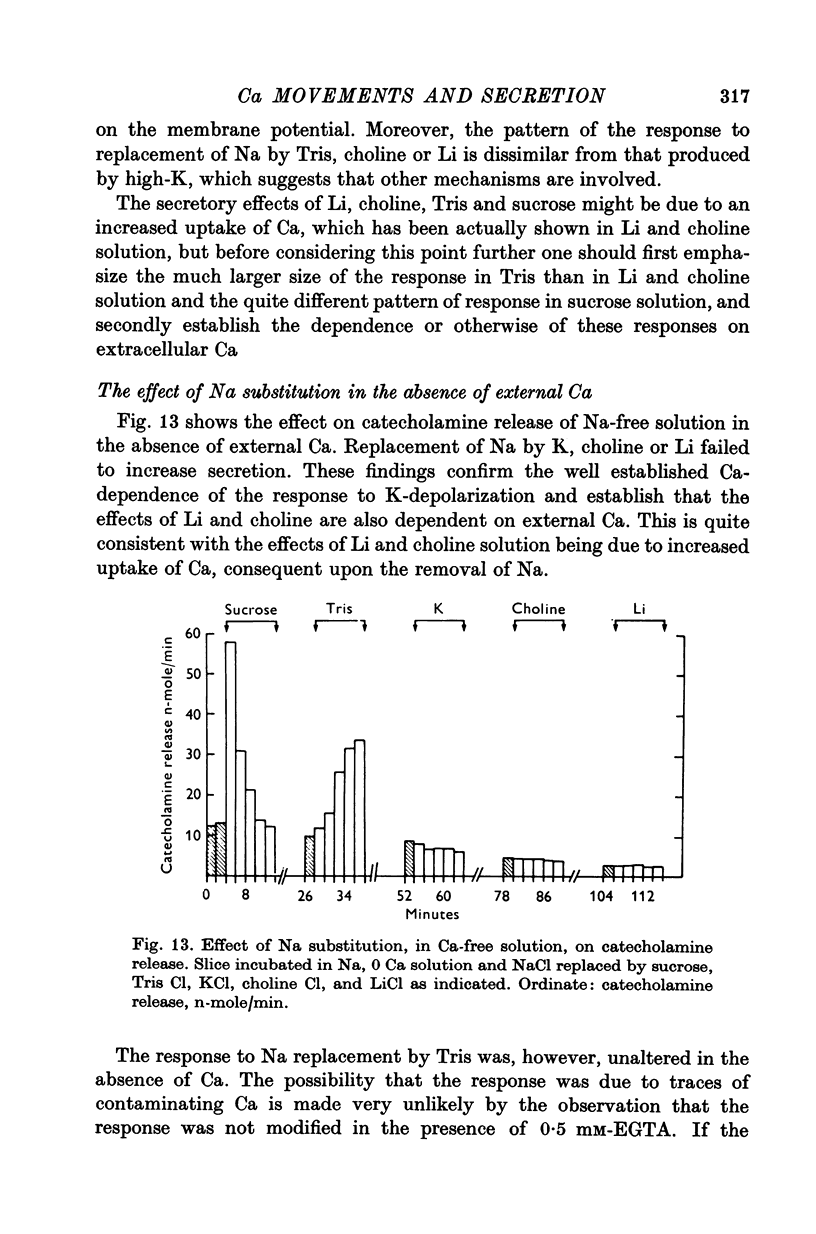
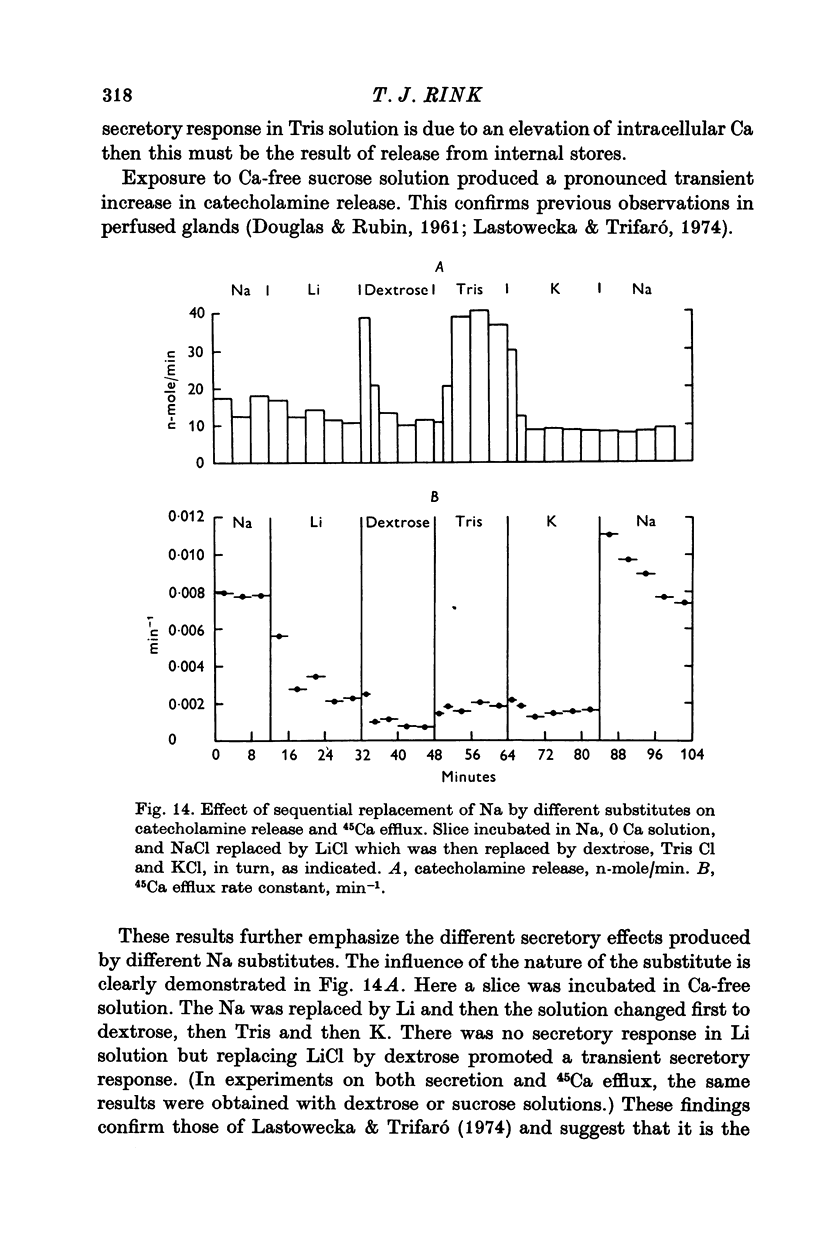
1. 45Ca efflux and uptake, net Ca movements, and catecholamine secretion were studied in thin slices of bovine adrenal medulla. 2. There was a slow component of 45Ca wash-out which is attributed to efflux to intracellular Ca. This efflux was strongly influenced by cations in the external solution, being reduced by 50% when Mg replaced Ca, and the residual efflux being reduced by 80% when choline replaced Na. 3. None of the substitutes tested, choline, K, Li, Tris or sucrose, could replace Na in maintaining 45Ca efflux into Ca-free solution. The Na-dependent Ca efflux showed sigmoidal activation by Na, indicating a requirement for the co-operative action of two or more Na ions in the extrusion of each Ca. 4. In the presence of 3-6 mM-Ca, Na deprivation failed to reduce 45Ca efflux. When K, Li and choline replaced Na the rate constant of 45Ca efflux increased. 5. Ca-dependent 45Ca efflux was studied by adding back Ca to Ca-free solutions. Its activation by Ca was hyperbolic indicating that one external Ca is involved in the extrusion of each Ca, consistent with a Ca:Ca exchange process. The apparent affinity for Ca and the maximal efflux were different in the presence of different monovalent cations. 6. 45Ca uptake was increased when Li or choline replaced Na in a solution containing 3-6 mM-Ca. Net Ca uptake also increased, but to a much smaller extent, supporting the idea of a Ca:Ca exchange process. 7. Mg had little effect in activating or inhibiting 45Ca efflux. Co appeared to act as a weak agonist and weak inhibitor of Ca-dependent 45Ca efflux. Ba strongly activated 45Ca efflux.8. Elevation of [Na]t, with ouabain treatment, did not appreciably affect Na-dependent 45Ca efflux. This may indicate that while this Ca efflux is dependent on external Na, it is not dependent on the Na gradient. Elevation of [Na]i had rather little effect on 45Ca uptake. 9 Exposure of slices to Li or choline solutions evoked a Ca-dependent increase in catecholamine output. This could be attributed to the observed increase in net Ca uptake in these conditions. Sucrose and Tris solutions produced Ca-independent secretory responses with quite different time courses. These results emphasize the importance of the Na substitute used in determining the secretory effect of Na deprivation. 10. Elevation of [Na]i did not change basal catecholamine release, nor did it greatly affect the secretory response to Na deprivation. 11. Only some of the secretory effects of NA deprivation can be attributed to the influence of Na on Ca movements.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P. Sodium-dependent uptake of calcium by crab nerve. Biochim Biophys Acta. 1968 Jan 3;150(1):167–170. doi: 10.1016/0005-2736(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Glitsch H. G. Does metabolic energy participate directly in the Na+-dependent extrusion of Ca2+ -Ca2+ ions from squid giant axons? J Physiol. 1973 Aug;233(1):44P–46P. [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol. 1975 Dec;253(2):593–620. doi: 10.1113/jphysiol.1975.sp011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Banks P. The effect of ouabain on the secretion of catecholamines and on the intracellular concentration of potassium. J Physiol. 1967 Dec;193(3):631–637. doi: 10.1113/jphysiol.1967.sp008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Ector A. C. Carrier-mediated sodium-dependent and calcium-dependent calcium efflux from pinched-off presynaptic nerve terminals (synaptosomes) in vitro. Biochim Biophys Acta. 1976 Jan 21;419(2):295–308. doi: 10.1016/0005-2736(76)90355-2. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Oborn C. J. The influence of sodium on calcium fluxes in pinched-off nerve terminals in vitro. J Physiol. 1975 Jun;247(3):657–686. doi: 10.1113/jphysiol.1975.sp010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M. Sodium-calcium exchange and calcium-calcium exchange in internally dialyzed squid giant axons. J Membr Biol. 1975 Jul 24;22(3-4):285–312. doi: 10.1007/BF01868176. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Jones A. W. Distribution and kinetics of CoEDTA in smooth muscle, and its use as an extracellular marker. J Physiol. 1969 Feb;200(2):387–401. doi: 10.1113/jphysiol.1969.sp008700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Effects of acetylcholine and other medullary secretagogues and antagonists on the membrane potential of adrenal chromaffin cells: an analysis employing techniques of tissue culture. J Physiol. 1967 Jan;188(1):107–120. doi: 10.1113/jphysiol.1967.sp008127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedynskyj N. M., Beck L. V. Tris (hydroxymethyl) aminomethane (THAM) induced stimulation of insulin release by islets of Langerhans previously isolated from rat pancreas. Diabetes. 1970 Aug;19(8):559–562. doi: 10.2337/diab.19.8.559. [DOI] [PubMed] [Google Scholar]
- Kalix P. Uptake and release of calcium in rabbit vagus nerve. Pflugers Arch. 1971;326(1):1–14. doi: 10.1007/BF00586791. [DOI] [PubMed] [Google Scholar]
- Lastowecka A., Trifaró J. M. The effect of sodium and calcium ions on the release of catecholamines from the adrenal medulla: sodium deprivation induces release by exocytosis in the absence of extracellular calcium. J Physiol. 1974 Feb;236(3):681–705. doi: 10.1113/jphysiol.1974.sp010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie S. W., Borowitz J. L. Evidence for a plasma membrane calcium pump in bovine adrenal medulla but not adrenal cortex. Biochim Biophys Acta. 1975 Jun 25;394(2):227–238. doi: 10.1016/0005-2736(75)90261-8. [DOI] [PubMed] [Google Scholar]
- Lloyd S., Pickford M. The effect of oxytocin and adrenaline on blood flow in the hind limb of the dog following chronic lumbar sympathectomy. J Physiol. 1967 Sep;192(1):43–52. doi: 10.1113/jphysiol.1967.sp008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijjar M. S., Hawthorne J. N. A plasma membrane fraction from bovine adrenal medulla: preparation, marker enzyme studies and phospholipid composition. Biochim Biophys Acta. 1974 Oct 29;367(2):190–201. doi: 10.1016/0005-2736(74)90042-x. [DOI] [PubMed] [Google Scholar]
- Reuter H., Blaustein M. P., Haeusler G. Na-Ca exchange and tension development in arterial smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):87–94. doi: 10.1098/rstb.1973.0011. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. P. The role of energy metabolism in calcium-evoked secretion from the adrenal medulla. J Physiol. 1970 Jan;206(1):181–192. doi: 10.1113/jphysiol.1970.sp009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorimachi M. Effects of alkali metal and other monovalent ions on the adrenomedullary secretion. Eur J Pharmacol. 1968 Jun;3(3):235–241. doi: 10.1016/0014-2999(68)90136-2. [DOI] [PubMed] [Google Scholar]


