Abstract
Following infection of susceptible BALB/c mice with Leishmania major, early production of interleukin-4 (IL-4) is associated with the development of a nonprotective Th2 response and the development of progressive disease. Treatment of mice with IL-12 at the time of infection can promote the activation of a protective Th1 response; however, IL-12 treatment of mice with established infections has little effect on the progress of lesion development. This may be due to a down-regulation of the IL-12 receptor β2 chain (IL-12Rβ2) that accompanies the expansion of IL-4-producing Th2 cells. We have examined whether prostaglandins function to regulate in vivo responsiveness to IL-12. Mice treated with indomethacin are responsive to treatment with exogenous IL-12 through at least the first 2 weeks of infection and, unlike control mice treated with IL-12, develop an enhanced Th1-type response associated with increased enhanced resistance to infection. Cells from indomethacin-treated mice also exhibit enhanced production of gamma interferon (IFN-γ) following in vitro stimulation with IL-12. Although in vivo indomethacin treatment did not appear to influence IL-12 production in infected mice, cells from indomethacin-treated mice did express higher levels of IL-12Rβ2, suggesting that prostaglandins may play a role in the loss of IL-12 responsiveness observed during nonhealing L. major infections.
Although a majority of inbred strains of mice develop small, self-healing lesions following infection with the protozoan parasite Leishmania major, a few mouse strains, such as BALB/c, develop large, nonhealing cutaneous lesions and ultimately succumb to disseminated disease (7, 16). Mice that spontaneously resolve their infections develop Th1-type responses characterized by heightened production of the macrophage-activating cytokine, IFN-γ, while nonhealing BALB/c mice develop Th2-type responses in which interleukin-4 (IL-4) is the dominant cytokine produced by a CD4+ effector cells (11, 22, 32). This differential development of Th1- versus Th2-type responses in infected mice has provided an excellent model for the study of factors that regulate the in vivo development of CD4+ subsets, and various immunological manipulations have been shown to alter the disease phenotype in susceptible mice. For example, BALB/c mice lacking the gene for IL-10 can control infection with L. major (18). In addition, treatment with recombinant IL-12 or with antibodies to IL-4, IL-2, CD4, or transforming growth factor β (TGF-β) will enable BALB/c mice to resolve L. major or Leishmania amazonenis infection, provided the treatments are administered at the time of parasite inoculation (2, 4, 10, 13, 30, 31, 35, 36). Presumably, these immunological interventions influence the initial pathway of CD4+ differentiation into Th1 or Th2 effector cells. In contrast, treatment of infected mice with IL-12 or anti-IL-4 antibody after they have developed dominant Th2-type responses does not promote a dominant Th1-type response or healing, suggesting that established effector cell populations are less amenable to immunological manipulation. However, we have shown that treatment with IL-12 or IFN-γ or with anti-IL-4 antibody, when combined with conventional antileishmanial drug therapy, can induce healing in chronically infected BALB/c mice and, importantly, that combined treatment results in a switch from a dominant Th2- to a Th1-type immune response (21, 24, 25).
How drug treatment of infected mice enables IL-12 or anti-IL-4 therapy to promote a Th2-to-Th1 shift in cytokine production is currently unclear. Drug treatment does lead to a marked reduction in parasite numbers that may alter the in vivo antigenic stimulus driving the expansion of a parasite-specific Th2 cell population. In addition, by reducing parasite numbers, drug treatment reduces the accumulation of inflammatory cells at the cutaneous site of infection and adjacent lymphoid tissues. Inflammatory cells, including macrophages, the host cells for L. major, are capable of producing multiple factors, such as IL-10, TGF-β, and prostaglandin E2 (PGE2), which may modify an immunological response. We have recently shown that treatment of L. major-infected CB6F1 mice with anti-TGF-β antibody leads to an increase in nitric oxide (NO) production and a decrease in parasite numbers within cutaneous lesions (20). In addition, combined treatment with IL-12 and anti-TGF-β antibody treatment significantly shifted the balance from Th2- to Th1-type cytokine production, showing that TGF-β may influence the in vivo response to IL-12 treatment (20).
Prostaglandins, which may be produced by multiple cell types, including macrophages, also modify immunological responses (28). PGE2 has been shown to inhibit T-cell proliferation as well as cytokine production by Th1-type cells (1, 3, 6, 13, 19, 23, 34). In addition, it may influence the differentiation of naive T cells to Th1 cells by suppressing IL-12 production and IL-12 receptor (IL-12R) expression (12, 13, 17, 37, 38). We have previously shown that PGE2 production is elevated during L. major infection in BALB/c mice and that in vivo treatment with the cyclooxygenase inhibitor indomethacin can significantly slow the growth of parasitized lesions, although it does not, by itself, induce healing (8, 33). More recently, it has been shown that indomethacin treatment can enhance the production of Th1-type cytokines in L. major-infected mice, although the treated mice again did not resolve their infections (5). In addition, in vitro addition of indomethacin to cultures of cells from mice infected with Leishmania mexicana upregulates the production of IL-12 and IFN-γ (27).
In this study, we have examined the effects of combined treatment with indomethacin plus IL-12 on L. major infections in BALB/c mice. We show that mice treated with this combination therapy can control their infections, while treatment with either indomethacin or IL-12 alone fails to halt disease progression. In addition, we demonstrate that mRNA levels for IL-12R β2 chain (IL-12Rβ2) in lymph nodes (LNs) of indomethacin-treated mice are elevated over those in nontreated mice and that cells from indomethacin-treated mice produce elevated levels of IFN-γ following in vitro stimulation with IL-12. Together, these results suggest that heightened prostaglandin production may play a role in the rapid loss of IL-12 responsiveness that accompanies L. major infection in BALB/c mice.
MATERIALS AND METHODS
Parasites and animals.
Female BALB/c mice were purchased from The Jackson Laboratories (Bar Harbor, Maine) and were 5 to 7 weeks of age at the time of infection. L. major (WHO MHOM/IL/80/Friedlin) was maintained in Grace's insect cell culture medium (GIBCO, Grand Island, N.Y.) containing 20% fetal bovine serum (FBS), 2 mM l-glutamine, 100 μg of streptomycin, and 100 U of penicillin G-potassium per ml. Metacyclic promastigotes were selected from stationary-phase cultures with Arachis hypogaea agglutinin as previously described (29). Soluble leishmanial antigen (SLA) was prepared from promastigotes (25).
Infections and treatment protocols.
Mice were inoculated into one hind footpad with 5 × 105 metacyclic promastigotes. Lesion size was measured with a dial caliper (L. S.Starrett Co., Athol, Mass.) and expressed as the difference in thickness between the infected and the uninfected contralateral footpads. Parasites were enumerated by a limiting dilution assay as previously described in which homogenates of infected lesions were serially diluted in Grace's insect culture medium (Life Technologies, Grand Island, N.Y.) and observed 5 to 7 days later for growth of promastigotes. Parasite numbers are expressed as the negative log10 dilution at which promastigote growth was observed. One day prior to infection, groups of mice were treated with indomethacin (Sigma, Saint Louis, Mo.) administered in drinking water (20 μg/ml in 0.4% ethanol). Control mice received 0.4% ethanol in drinking water. Indomethacin-treated and untreated mice were inoculated with murine IL-12 (a gift from Genetics Institute, Cambridge, Mass.) delivered directly into the parasitized footpad. IL-12-treated mice received a total of six intralesional injections with 0.2 μg of IL-12 given every 3rd day starting on day 15 of infection. Control mice received 0.4% ethanol in drinking water or were inoculated with phosphate-buffered saline (PBS) delivered into the parasitized footpad.
ELISA and ELISPOT assays.
For determination of total cytokine production, draining LN cells were cultured at 5 × 106/ml with SLA (50 μg/ml) for 72 h. IFN-γ and IL-4 levels in supernatants were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (25). Recombinant mIFN-γ and mIL-4 used as standards were generously provided by Genentech (South San Francisco, Calif.) and DNAX (Palo Alto, Calif.), respectively. NO production from the same supernatants harvested at 72 h was assessed with the Greiss reagent (9). Levels of PGE2 production in cell supernatants were assayed by ELISA with a commercial kit purchased from Cayman Chemical Company (Ann Arbor, Mich.). The number of IL-12p40-secreting cells in LN and spleen cell suspensions was determined with an enzyme-linked immunospot (ELISPOT) assay. The monoclonal antibodies C17.8 and biotinylated C15.6 were generously provided by Christopher Hunter, University of Pennsylvania, Philadelphia. IL-12-secreting cells were determined in cell cultures following overnight stimulation with SLA (50 μg/ml).
RPA.
Total RNA was isolated from popliteal draining LNs with RNA STAT-60 (Tel-Test B, Friendswood, Tex.) as directed by the manufacturer. mRNA was quantified by RNase protection assay (RPA) with a Riboquant kit (PharMingen, San Diego, Calif.) as directed. A custom probe from PharMingen was prepared with [32P]UTP and hybridized to 15 μg of each sample RNA. The protected probe was purified and resolved on 5% denaturing polyacrylamide gel by using Ultra Pure Sequagel reagents (National Diagnostics, Atlanta, Ga.). Dried gels were exposed to a phosphorimaging screen, and protected fragments were visualized with a Phospho-Imager GS-525 Molecular Imager system (Bio-Rad, Richmond, Calif.). Cytokine mRNA levels were normalized to level L32, and the results are expressed as the increase in message level in experimental mice compared to the level in control mice.
Statistical analysis.
Data were analyzed for statistical significance with Student's t test. Results were considered significant at P < 0.05.
RESULTS
Indomethacin-treated mice control infection following in vivo treatment with IL-12.
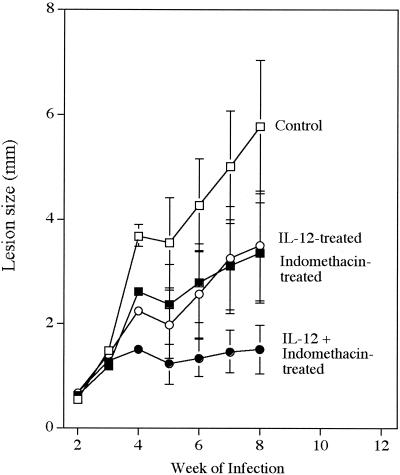
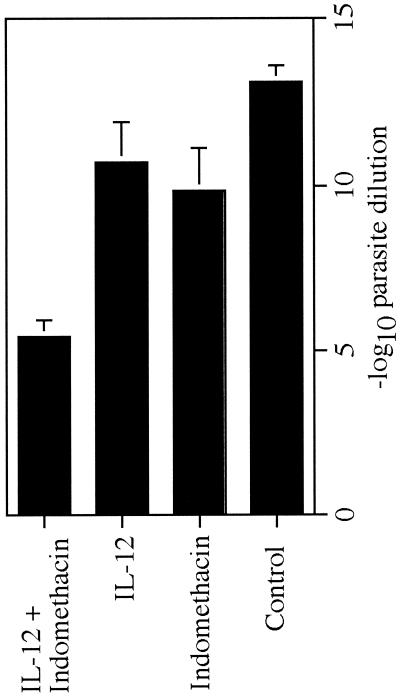
We have previously shown that combined treatment with IL-12 plus the antileishmanial drug sodium stibogluconate, initiated during the 3rd week of infection with L. major, induces healing and a switch from a Th2- to Th1-type response in BALB/c mice. Here, we have used a similar treatment protocol to test whether substitution of indomethacin for sodium stibogluconate could, when combined with IL-12, induce the same protective effect. The results shown in Fig. 1 demonstrate that it does. Although mice treated with IL-12 or indomethacin alone exhibited slowed lesion development, neither independent treatment dramatically altered the overall course of disease. However, when indomethacin and IL-12 treatment were combined, mice appeared capable of controlling disease through at least week 8 of infection. Analysis of parasite numbers at 8 weeks showed that only the group of mice treated with both IL-12 and indomethacin had significantly reduced levels (P < 0.05) of infection compared to those in control mice (Fig. 2).
FIG. 1.
Effect of indomethacin and/or IL-12 treatment on infection. BALB/c mice were given either indomethacin (20 μg/ml in 0.4% ethanol) or 0.4% ethanol in drinking water starting 1 day before infection with 2 × 105 L. major metacyclic promastigotes. Treated and control mice received a total of six intralesional injections of 0.2 μg of either IL-12 or PBS given every 3rd day starting on day 15 of infection. Lesion size was measured weekly for 8 weeks and expressed as the difference in thickness between the infected and the uninfected contralateral footpads. Values are the mean ± standard deviation of four to five mice per group and are representative of results of two separate experiments.
FIG. 2.
Parasite burden in infected mice. Numbers of lesion parasites at week 8 of infection were determined by limiting dilution assay and expressed as the negative log10 dilution at which promastigote growth was observed. Error bars represent the mean ± standard deviation of four to five mice per group.
Treatment with indomethacin and/or IL-12 alters the immunological response.
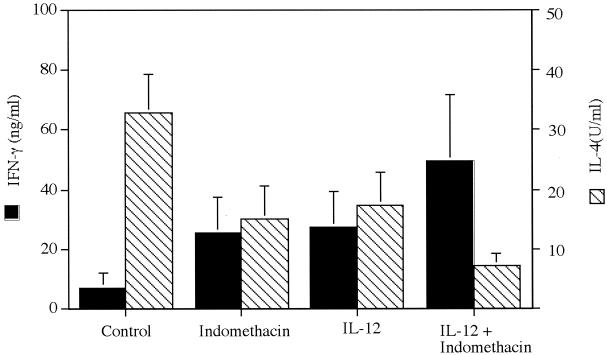
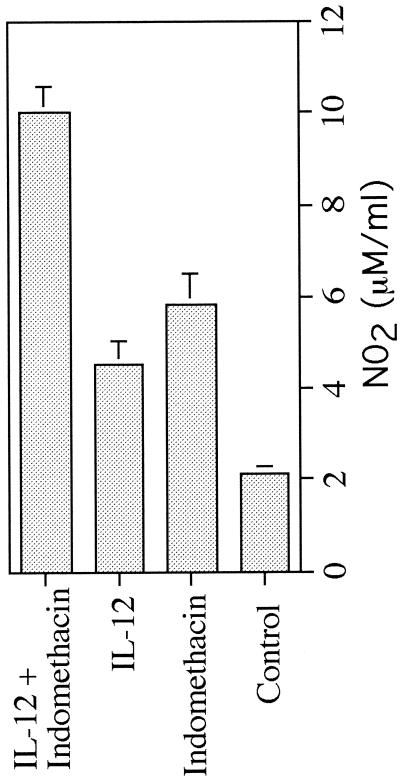
Since treatment with IL-12 and indomethacin, either individually or in combination, slowed lesion development, we examined whether these treatments also altered the profile of Th1- or Th2-type cytokine production. As would be expected, draining LN cells from nonhealing control mice produced low levels of IFN-γ and high levels of IL-4, characteristic of a dominant Th2-type response (Fig. 3). Cells from mice treated individually with IL-12 or indomethacin produced higher levels of IFN-γ and lower levels of IL-4, consistent with their reduced lesion size, although these alterations in cytokine production were not sufficient to promote healing. The most dramatic shift in cytokine production was noted in cultures of cells from mice receiving the combined treatment. LN cells from mice treated with indomethacin and IL-12 produced high levels of IFN-γ and low levels of IL-4 compatible with the development of a dominant Th1-type response (Fig. 3). In addition, cells from these healing mice produced elevated levels of NO2, indicative of enhanced NO production required for macrophage microbicidal activity against intracellular parasites (Fig. 4).
FIG. 3.
Cytokine production in LN cells. LN cells from the mice in Fig. 1 were assayed for cytokine production at week 8 of infection. IL-4 and IFN-γ levels were measured in cultures by ELISA following in vitro stimulation of cells with SLA. Error bars represent the mean ± standard deviation of four to five mice per group and are representative of results from two studies.
FIG. 4.
NO2 production by LN cells from mice at week 8 of infection as determined by the Greiss assay.
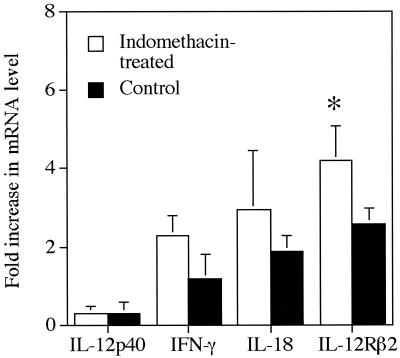
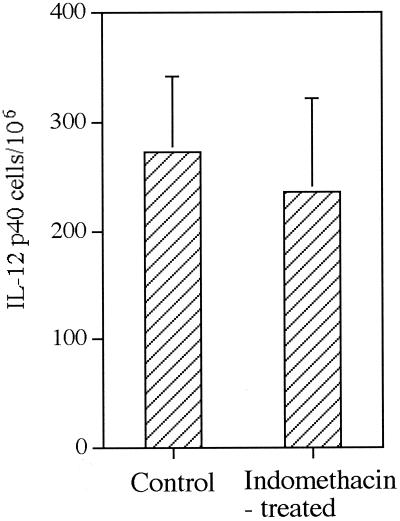
We also examined cytokine message levels by RPA in draining LNs at weeks 2 to 3 of infection in an effort to understand how in vivo indomethacin treatment altered responsiveness to IL-12. Although we noted a general trend of increased message levels for inducible NO synthase (iNOS), IL-18, and IFN-γ, the results were variable, and none of the differences noted in treated and untreated mice were significant (Fig. 5). It should also be noted that IL-18 message levels do not reflect levels of active IL-18 protein production. No increases in message levels for IFN-γ or IL-12p40 over those in naïve mice were detected in either group of infected mice. However, a small, but significant, increase in mRNA levels for IL-12Rβ2 was noted in mice receiving indomethacin (Fig. 5). We also enumerated IL-12-producing cells in LNs of infected mice. In keeping with our observation on IL-12p40 mRNA levels, indomethacin treatment did not appear to increase the number of IL-12-producing cells in infected mice (Fig. 6).
FIG. 5.
Cytokine mRNA levels in LNs at week 3 of infection. Message levels were determined by RPA analysis and are expressed as the fold increase over mRNA levels in LNs of naïve mice. Values represent the mean ± standard deviation of three mice. *, significantly different from the value in control mice (P < 0.05).
FIG. 6.
IL-12p40 production in LNs from control and indomethacin-treated mice. LN cells from control and indomethacin-treated mice were assayed at week 4 of infection for IL-12p40 production by direct ELISPOT assay. The data are expressed as mean frequency per 106 LN cells ± standard deviation of three or more mice per group.
Cells from infected, indomethacin-treated mice produce more IFN-γ in response to in vitro stimulation with IL-12.
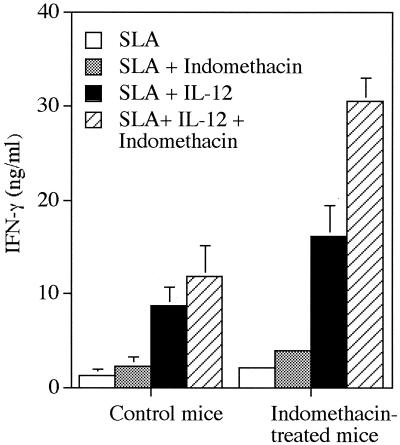
Since IL-12 therapy promoted resistance in indomethacin-treated mice, we next examined whether cells from these mice were more responsive to IL-12 stimulation in vitro. LN cells from 4-week-infected mice were cultured with SLA and IL-12, indomethacin, or a combination of IL-12 plus indomethacin, and culture supernatants were assayed for levels of IFN-γ production. The results in Fig. 7 show that IL-12 stimulation induced higher levels of IFN-γ production by cells from indomethacin-treated mice. In addition, addition of indomethacin to in vitro cultures further enhanced IFN-γ production, particularly by cells from indomethacin-treated mice. The effects of in vitro indomethacin treatment on IFN-γ production, even in cultures of cells from mice receiving in vivo indomethacin therapy, are not surprising, since in vivo treatment did not totally block PGE2 production. At week 3 of infection, levels of PGE2 in cultures of draining LN cells from three untreated, infected mice were 820 ± 80 pg/ml, compared to 448 ± 72 pg/ml in cultures of cells from three indomethacin-treated mice. Addition of indomethacin to in vitro cultures reduced PGE2 levels to approximately 200 pg/ml in all cultures. The ability of IL-12 to promote enhance IFN-γ production by cells from indomethacin-treated mice was noted in cultures of cells from mice infected for 2 weeks; however, by week 6 of infection, cells from both control and indomethacin-treated mice responded poorly to IL-12, and no differences in IFN-γ production were noted in cultures of treated versus nontreated animals (data not shown). These results suggest that the positive effects of in vivo indomethacin treatment were eventually lost with increased time of infection.
FIG. 7.
Effects of IL-12 and/or indomethacin on in vitro IFN-γ production by cells from control and indomethacin-treated mice at week 4 of infection. Cells were stimulated with SLA in the presence or absence of indomethacin (2 μg/ml) and/or IL-12 (1 ng/ml) for 72 h. Values represent the mean ± standard deviation of three to four mice per group.
DISCUSSION
The events that influence the activation of a Th1- versus Th2-type response during L. major infection are complex. BALB/c mice infected with L. major develop a dominant Th2-type response in which the population of antigen-specific CD4+ effector cells produces high levels of IL-4 and low levels of IFN-γ. Message levels for IL-4 are rapidly elevated after infection in BALB/c mice, and compared to resistant strains of mice, cells from infected BALB/c mice produce small amounts of IFN-γ, presumably because early production of IL-4 suppresses the expression of the IL-12Rβ2 (14, 15). Loss of expression of the IL-12R, in turn, could block IL-12 signaling required for development of a population of Th1-type cells (14). Still, mice engineered to express the transgene for IL-12Rβ2 still fail to control infection and develop a Th2-type response, suggesting that the loss of IL-12Rβ2 expression by T cells is not the reason, per se, that infected mice fail to develop a protective Th1-type response (26). However, this study is complicated by the observation that continued expression of the IL-12R on Th2 cells may actually promote enhanced IL-4 production (26). Thus, loss of response to IL-12 still provides a plausible explanation as to why IL-12 treatment fails to promote healing if given to mice with established infections.
Although IL-4 production during early stages of infection appears to be central to the down-regulation of IL-12Rβ2 expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12, other factors, such as PGE2, may play a role in this process. PGE2 is known to suppress multiple responses, including T-cell proliferation and Th1 cell differentiation (28). It can also suppress the production of IL-12 by human monocytes and dendritic cells and inhibit the expression of IL-12R by human monocytes (37, 38). We have previously shown that cells from BALB/c mice infected with L. major produce large amounts of PGE2 that can suppress in vitro proliferation by parasite-specific T cells, an effect that could be reversed by culturing cells with the cyclooxygenase inhibitor indomethacin (33). Addition of indomethacin to cultures of antigen-stimulated cells from L. mexicana-infected mice has been shown to promote increased production of both IL-12 and IFN-γ (27). Importantly, in vivo administration of indomethacin to L. major-infected mice can moderate the course of lesion development and promote an enhanced Th1-type response, although indomethacin treatment by itself does not induce BALB/c mice to control infection (5, 8).
In this study, we confirm our previous observation that indomethacin treatment during L. major infection can slow the expansion of parasitized lesions and also confirm the observation of others that cells from treated mice produce increased amounts of IFN-γ and NO and reduced amounts of IL-4. We also show that IL-12 treatment initiated during the 3rd week of infection can promote enhanced resistance and the development of a dominant Th1-type response in mice given indomethacin, but not in untreated control mice. The capacity of in vivo IL-12 treatment to promote a more vigorous Th1-type response in indomethacin-treated mice is mirrored by the fact that cells from indomethacin-treated mice produce increased amounts of IFN-γ following in vitro stimulation with parasite antigen and IL-12 compared to cells from untreated mice. Enhanced in vitro responses to IL-12 were observed in cultures of cells from mice infected for 2 or 4 weeks. However, the differential capacities of cells from treated and untreated mice to respond to IL-12 in vitro were lost by week 6 of infection, suggesting that indomethacin treatment may slow, but not prevent, the loss of responsiveness to IL-12.
The reason that in IL-12 promotes resistance in indomethacin-treated mice or that cells from indomethacin-treated mice exhibit enhanced responsiveness to IL-12 in vitro is unclear. Although PGE2 can suppress IL-12 production, we could find no evidence that either the number of IL-12p40-producing cells or the level of mRNA for IL-12p40 was increased in mice following indomethacin treatment. However, cells from treated mice are clearly more responsive to exogenous IL-12, and we did observe a small, but significant, increase in message levels for IL-12Rβ2 following indomethacin treatment. As noted above, IL-12R expression during infection is presumably maintained on IFN-γ-producing Th1 cells, but lost in Th2 cells by a mechanism that appears to involve IL-4 signaling. Since PGE2 can directly suppress IL-12Rβ2 expression on T cells, it is possible that inhibition of PGE2 production by indomethacin directly enhances IL-12R on antigen-activated cells. Alternatively, inhibition of PGE2 production, by promoting a modest increase in the Th1-type response, may help maintain IL-12 responsiveness during infection by altering the balance of positive (IFN-γ) and negative (IL-4) regulators of the IL-12R expression. Increased production of IFN-γ following IL-12 stimulation may thus reflect increased numbers of Th1-type cells in cultures of cells from indomethacin-treated mice rather than any direct effect of indomethacin on IL-12Rβ2 expression. Either way, this study demonstrates that PGE2 plays a role in the activation and maintenance of a Th2-type response and, importantly, illustrates that inhibition of endogenous PGE2 production may have beneficial effects on immunotherapeutic treatments for leishmaniasis.
Acknowledgments
This work was supported by National Institutes of Health grants AI-27828 (to J.P.F.) and AI-35914 (to P.S.)
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anastassiou, E. D., F. Poliogianni, J. P. Balow, H. Yamada, and D. T. Boumpas. 1992. Prostaglandin E2 and other cyclic AMP-elevating agents modulate IL-2 and IL-2R gene expression at multiple levels. J. Immunol. 148:2845-2852. [PubMed] [Google Scholar]
- 2.Barral-Netto, M., A. Barral, C. E. Brownell, Y. A. Skeiky, L. R. Ellingsworth, D. R. Twardzik, and S. G. Reed. 1992. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science 257:545-548. [DOI] [PubMed] [Google Scholar]
- 3.Betz, M., and B. S. Fox. 1991. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 146:108-113. [PubMed] [Google Scholar]
- 4.Chatelain, R., K. Varkila, and R. L. Coffman. 1992. IL-4 induces a Th2 response in Leishmania major-infected mice. J. Immunol. 148:1182-1187. [PubMed] [Google Scholar]
- 5.De Freitas, L. A., L. M. Mbow, M. Estay, J. A. Bleyenberg, and R. G. Titus. 1999. Indomethacin treatment slows disease progression and enhances a Th1 response in susceptible BALB/c mice infected with Leishmania major. Parasite Immunol. 21:273-277. [DOI] [PubMed] [Google Scholar]
- 6.Demeure, C. E., L. P. Yang, C. Desjardins, P. Raynauld, and G. Delespesse. 1997. Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur. J. Immunol. 27:3526-3531. [DOI] [PubMed] [Google Scholar]
- 7.Detolla, L. J., Jr., P. A. Scott, and J. P. Farrell. 1981. Single gene control of resistance to cutaneous leishmaniasis in mice. Immunogenetics 14:29-39. [DOI] [PubMed] [Google Scholar]
- 8.Farrell, J. P., and C. E. Kirkpatrick. 1987. Experimental cutaneous leishmaniasis. II. A possible role for prostaglandins in exacerbation of disease in Leishmania major-infected BALB/c mice. J. Immunol. 138:902-907. [PubMed] [Google Scholar]
- 9.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 10.Heinzel, F. P., R. M. Rerko, F. Hatam, and R. M. Locksley. 1993. Interleukin 2 is necessary for progression of leishmaniasis in susceptible murine hosts. J. Immunol. 150:3924-3931. [PubMed] [Google Scholar]
- 11.Heinzel, F. P., M. D. Sadick, B. J. Holaday, R. L. Coffman, and R. M. Locksley. 1989. Reciprocal expression of interferon γ for interleukin 4 during the resolution or progression of murine leishmaniasis. J. Exp. Med. 169:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinzel, F. P., D. S. Schoenhaut, R. M. Rerko, L. E. Rosser, and M. K. Gately. 1993. Recombinant interleukin 12 cures mice infected with Leishmania major. J. Exp. Med. 177:1505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilkens, C. M., A. Snijders, H. Vermeulen, P. H. van der Meide, E. A. Wierenga, and M. L. Kapsenberg. 1996. Accessory cell-derived IL-12 and prostaglandin E2 determine the IFN-γ level of activated human CD4+ T cells. J. Immunol. 156:1722-1727. [PubMed] [Google Scholar]
- 14.Himmelrich, H., C. Parra-Lopez, F. Tacchini-Cottier, J. A. Louis, and P. Launois. 1998. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor beta 2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J. Immunol. 161:6156-6163. [PubMed] [Google Scholar]
- 15.Himmelrich, H., P. Launois, I. Maillard, T. Biedermann, F. Tacchini-Cottier, R. M. Locksley, M. Rocken, and J. A. Louis. 2000. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J. Immunol. 164:4819-4825. [DOI] [PubMed] [Google Scholar]
- 16.Howard, J. G., C. Hale, and W. L. Chan-Liew. 1980. Immunological regulation of experimental cutaneous leishmaniasis. I. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 2:303-314. [DOI] [PubMed] [Google Scholar]
- 17.Kalinski, P., P. L. Vieira, J. H. Schuitemaker, E. C. de Jong, and M. L. Kapsenberg. 2001. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 97:3466-3469. [DOI] [PubMed] [Google Scholar]
- 18.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 19.Katamura, K., N. Shintaku, Y. Yamauchi, T. Fukui, Y. Ohshima, M. Mayumi, and K. Furusho. 1995. Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-γ and IL-2, but not IL-4 and IL-5. J. Immunol. 155:4604.. [PubMed] [Google Scholar]
- 20.Li, J., C. A. Hunter, and J. P. Farrell. 1999. Anti-TGF-β treatment promotes rapid healing of Leishmania major infection in mice by enhancing in vivo nitric oxide production. J. Immunol. 162:974-979. [PubMed] [Google Scholar]
- 21.Li, J., S. Sutterwala, and J. P. Farrell. 1997. Successful therapy of chronic, nonhealing murine cutaneous leishmaniasis with sodium stibogluconate and gamma interferon depends on continued interleukin-12 production. Infect. Immun. 65:3225-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locksley, R. M., F. P. Heinzel, M. D. Sadick, B. J. Holaday, and K. D. Gardner, Jr. 1987. Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann. Inst. Pasteur Immunol. 138:744-749. [DOI] [PubMed] [Google Scholar]
- 23.Mary, D., C. Aussel, B. Ferrua, and M. Fehlmann. 1987. Regulation of interleukin 2 synthesis by cAMP in human T cells. J. Immunol. 139:1179-1184. [PubMed] [Google Scholar]
- 24.Nabors, G. S., L. C. C. Alfonso, J. P. Farrell, and P. Scott. 1994. Switch from a type 2 to a type1 T helper cell response and cure of established Leishmania major infection in mice is induced by combined therapy with interleukin 12 and pentostam. Proc. Natl. Acad. Sci. USA 92:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nabors, G. S., and J. P. Farrell. 1994. Depletion of interleukin-4 in BALB/c mice with established Leishmania major infections increases the efficacy of antimony therapy and promotes Th-1-like responses. Infect. Immun. 62:5498-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishikomori, R., S. Gurunathan, K. Nishikomori, and W. Strober. 2001. BALB/c mice bearing a transgenic IL-12 receptor beta 2 gene exhibit a nonhealing phenotype to Leishmania major infection despite intact IL-12 signaling. J. Immunol. 166:6776-6783. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Santos, J. L., and P. Talamas-Rohana. 2001. In vitro indomethacin administration upregulates interleukin-12 production and polarizes the immune response towards aTh1 type in susceptible BALB/c mice infected with Leishmania mexicana. Parasite Immunol. 23:599-606. [DOI] [PubMed] [Google Scholar]
- 28.Phipps, R. P., S. H. Stein, and R. L. Rope. 1991. A new view of prostaglandin E regulation of the immune response. Immunol. Today 12:349-352. [DOI] [PubMed] [Google Scholar]
- 29.Sacks, D. L., S. Heiny, and A Sher. 1985. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J. Immunol. 135:564-569. [PubMed] [Google Scholar]
- 30.Sadick, M. D., F. P. Heinzel, B. J. Holaday, R. T. Pu, R. S. Dawkins, and R. M. Locksley. 1990. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J. Exp. Med. 171:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scharton-Kersten, T., L. C. C. Afonso, M. Wysocka, G. Trinchieri, and P. Scott. 1995. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 154:5320-5330. [PubMed] [Google Scholar]
- 32.Scott, P., P. Natovitz, R. L. Coffman, E. Pearce, and A. Sher. 1988. Immunoregulation of cutaneous leishmaniasis: T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. Exp. Med. 168:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott, P. A., and J. P. Farrell. 1981. Experimental cutaneous leishmaniasis. I. Non-specific immunodepression in BALB/C mice infected with Leishmania tropica. J. Immunol. 127:2395-2400. [PubMed] [Google Scholar]
- 34.Snijdewint, F. G. M., P. Kalinski, E. A. Wierenga, J. D. Bos, and M. L. Kapsenberg. 1993. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J. Immunol. 150:5321-5329. [PubMed] [Google Scholar]
- 35.Sypek, J. P., C. L. Chung, S. E. H. Mayor, J. M. Subramanyam, S. J. Goldman, D. S. Sieburth, S. F. Wolf, and R. G. Schaub. 1993. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J. Exp. Med. 177:1797-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Titus, R. G., R. Ceredig, J. C. Cerottini, and J. A. Louis. 1985. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically susceptible BALB/c mice. J. Immunol. 135:2108-2114. [PubMed] [Google Scholar]
- 37.Van der Pouw Kraan, T. C., L. C. Boeije, R. J. Smeenk, J. Wijdenes, and L. A. Aarden. 1995. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J. Exp. Med. 181:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, C. Y., K. Wang, J. F. McDyer, and R. A. Seder. 1998. Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J. Immunol. 161:2723-2730. [PubMed] [Google Scholar]