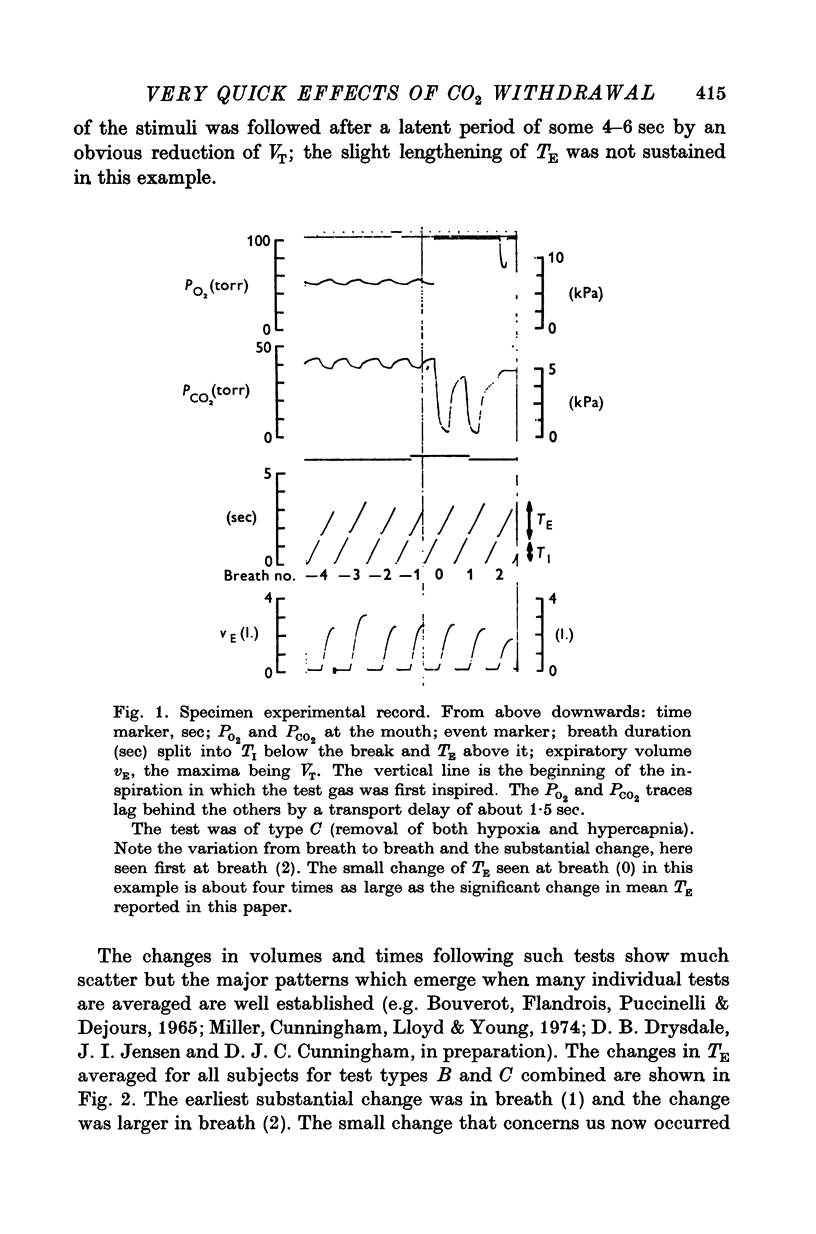
Abstract
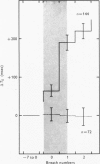
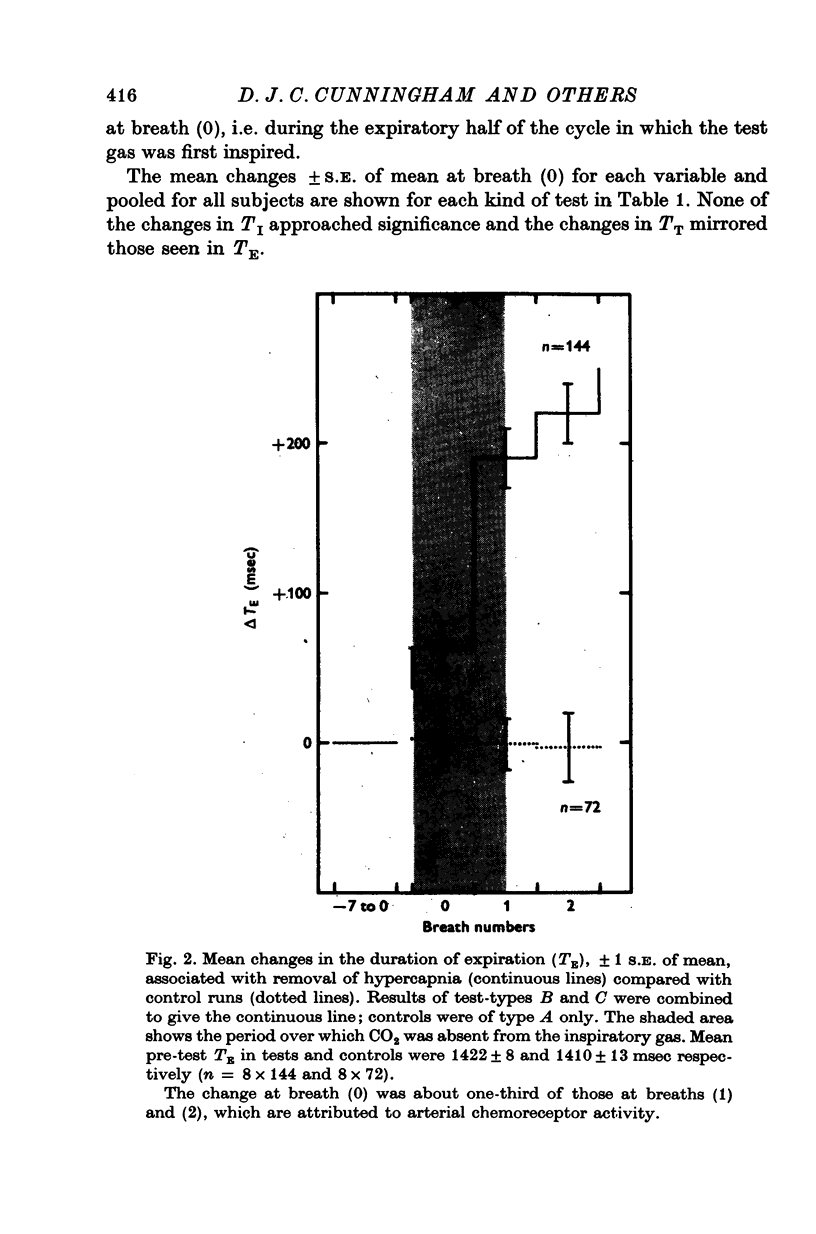
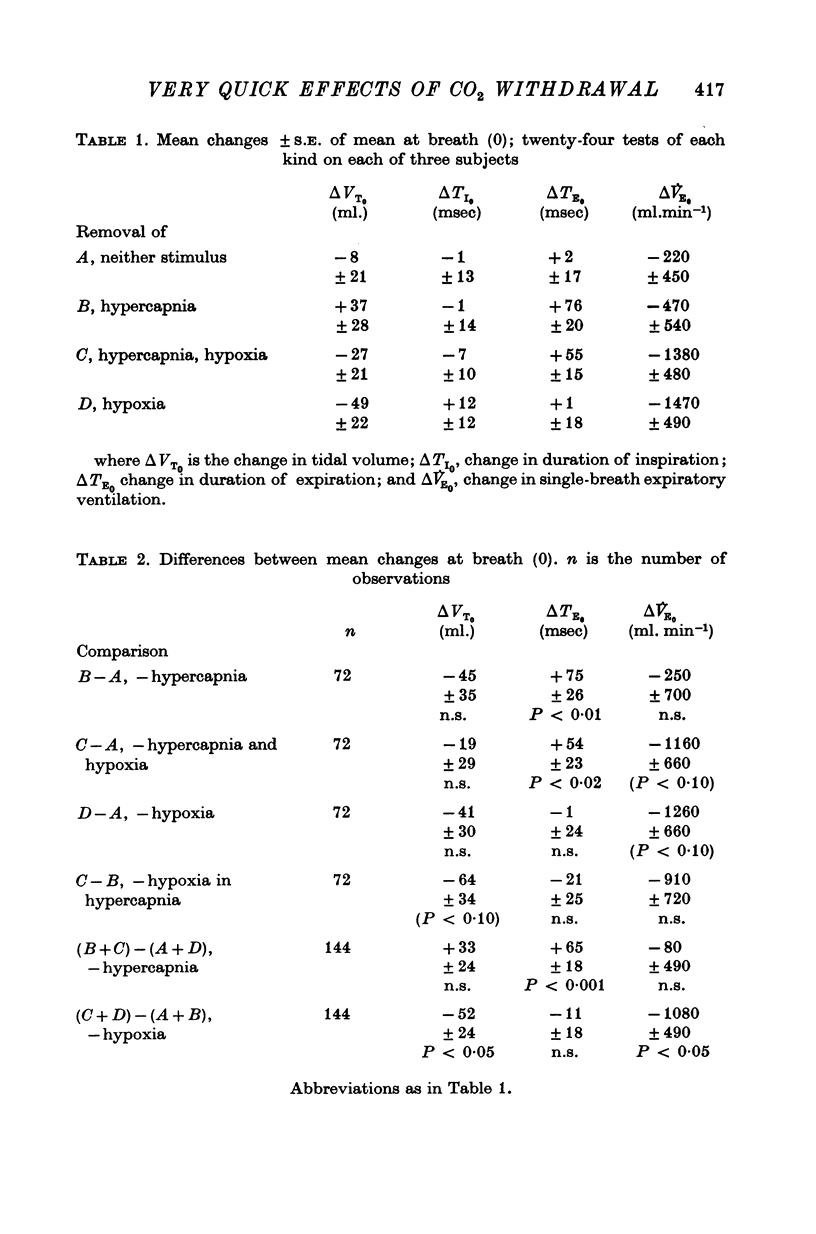
1. Three healthy young males were maintained for sessions of about 1 hr in a state of mild asphyxia (PA,O2 approximately 55, PA,CO2 approximately 45 torr), i.e. with moderately strong drives from both arterial and intracranial chemoreceptors. Tidal volume (VT), breath duration (TT) and duration of inspiration (TI) were recorded, and ventilation (VE) and duration of expiration (TE) were derived breath by breath. 2. The arterial chemoreceptor component of the drive was briefly and abruptly reduced, perhaps silenced, by three separate procedures: the inspiratory pathway was connected for two breaths to a second gas supply line containing, B, hypoxia with Pi,CO2 zero (removal of hypercapnia with maintained hypoxia); C, pure oxygen (removal of asphyxia); and D, oxygen with 40 torr added PCO2 (removal of hypoxia with maintained hypercapnia). In controls, A, the second inspiratory line contained the maintenance mixture so that the switch involved no change of inspiratory gas composition. Each type of test was repeated twenty-four times on each subject. 3. Responses attributable to silencing of arterial chemoreceptors (i.e. with 1 1/2--3 breath latencies about equal to the lung-to-ear circulation time) are reported elsewhere. 4. Very small responses, occurring only half a respiratory cycle after first inhalation of the test mixture, were detected by pooling all responses of each kind from all subjects. When hypoxia was withdrawn, with (C) or without (D) simultaneous withdrawal of hypercapnia, VT and VE were reduced by 3 and 2% respectively, probably because gas mixtures containing high oxygen concentrations are appreciably more viscous than hypoxic mixtures and so require more effort to breathe in and out. When hypercapnia was withdrawn with (C) or without (B) simultaneous withdrawal of hypoxia, TE was significantly lengthened (mean, + 65 +/- 18 msec), 5. The change of TE was discussed in relation to known effects of CO2 on airway receptors in the dog.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartoli A., Cross B. A., Guz A., Jain S. K., Noble M. I., Trenchard D. W. The effect of carbon dioxide in the airways and alveoli on ventilation; a vagal reflex studied in the dog. J Physiol. 1974 Jul;240(1):91–109. doi: 10.1113/jphysiol.1974.sp010601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushey H. A., Richardson P. S. The reflex effects of intralaryngeal carbon dioxide on the pattern of breathing. J Physiol. 1973 Jan;228(1):181–191. doi: 10.1113/jphysiol.1973.sp010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouverot P., Flandrois R., Puccinelli R., Dejours P. Etude du role des chémorécepteurs artériels dans la régulation de la respiration pulmonaire chez le chien éveillé. Arch Int Pharmacodyn Ther. 1965 Oct;157(2):253–271. [PubMed] [Google Scholar]
- Bradley G. W., Noble M. I., Trenchard D. The direct effect on pulmonary stretch receptor discharge produced by changing lung carbon dioxide concentration in dogs on cardiopulmonary bypass and its action on breathing. J Physiol. 1976 Oct;261(2):359–373. doi: 10.1113/jphysiol.1976.sp011563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G. W., von Euler C., Marttila I., Roos B. A model of the central and reflex inhibition of inspiration in the cat. Biol Cybern. 1975 Aug 8;19(2):105–116. doi: 10.1007/BF00364107. [DOI] [PubMed] [Google Scholar]
- Cunningham D. J., Howson M. G., Pearson S. B. The respiratory effects in man of altering the time profile of alveolar carbon dioxide and oxygen within each respiratory cycle. J Physiol. 1973 Oct;234(1):1–28. doi: 10.1113/jphysiol.1973.sp010331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier H., Remmers J. E., Bartlett D., Jr Control of the duration of expiration. Respir Physiol. 1973 Jul;18(2):205–221. doi: 10.1016/0034-5687(73)90051-0. [DOI] [PubMed] [Google Scholar]
- Kay J. D., Petersen E. S., Vejby-Christensen H. Mean and breath-by-breath pattern of breathing in man during steady-state exercise. J Physiol. 1975 Oct;251(3):657–669. doi: 10.1113/jphysiol.1975.sp011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K. Characteristics of inflation and deflation reflexes during expiration of the cat. J Neurophysiol. 1973 Mar;36(2):284–295. doi: 10.1152/jn.1973.36.2.284. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Cunningham D. J., Lloyd B. B., Young J. M. The transient respiratory effects in man of sudden changes in alveolar CO2 in hypoxia and in high oxygen. Respir Physiol. 1974 Feb;20(1):17–31. doi: 10.1016/0034-5687(74)90015-2. [DOI] [PubMed] [Google Scholar]
- Mustafa M. E., Purves M. J. The effect of CO 2 upon discharge from slowly adapting stretch receptors in the lungs of rabbits. Respir Physiol. 1972 Oct;16(2):197–212. doi: 10.1016/0034-5687(72)90051-5. [DOI] [PubMed] [Google Scholar]
- Wasserman K., Whipp B. J., Casaburi R., Huntsman D. J., Castagna J., Lugliani R. Regulation of arterial PCO2 during intravenous CO2 loading. J Appl Physiol. 1975 Apr;38(4):651–656. doi: 10.1152/jappl.1975.38.4.651. [DOI] [PubMed] [Google Scholar]
- Wasserman K., Whipp B. J., Castagna J. Cardiodynamic hyperpnea: hyperpnea secondary to cardiac output increase. J Appl Physiol. 1974 Apr;36(4):457–464. doi: 10.1152/jappl.1974.36.4.457. [DOI] [PubMed] [Google Scholar]