Abstract
Cholera toxin (CT) and heat-labile enterotoxin (LT) are powerful mucosal adjuvants whose cellular targets and mechanism of action are unknown. There is emerging evidence that dendritic cells (DC) are one of the principal cell types that mediate the adjuvant effects of these toxins in vivo. Here we investigate the effects of CT and LT on the maturation of human monocyte-derived DC (MDDC) in vitro. We found that an enzymatically active A domain is necessary for both CT and LT to induce the maturation of MDDC and that this activation is strictly cyclic AMP (cAMP) dependent. ADP-ribosylation-defective derivatives of these toxins failed to induce maturation of MDDC, whereas dibutyryl-cyclic-3′,5′-AMP and Forskolin mimic the maturation of MDDC induced by CT and LT. In addition, an inhibitor of cAMP-dependent kinases, Rp-8-Br-cAMPs, blocked the ability of CT, LT, and Forskolin to activate MDDC. CT, LT, dibutyryl-cyclic-3′,5′-AMP, and Forskolin also dominantly inhibit interleukin 12 and tumor necrosis factor alpha production by MDDC in the presence of saturating concentrations of lipopolysaccharide. Taken together, these results show that the effects of CT and LT on MDDC are mediated by cAMP.
Cholera toxin (CT) and heat-labile enterotoxin (LT) are AB5 enterotoxins produced by Vibrio cholerae and enteropathic Escherichia coli, the primary causative agents of cholera and traveler's diarrhea, respectively. CT and LT consist of a 27-kDa catalytic A domain anchored in a ring of five identical, 11.7-kDa B subunits (18). The B pentamers of these toxins bind to gangliosides on cell membranes (12). The B pentamer of CT (CTB) binds exclusively to GM1 gangliosides, while the B pentamer of LT (LTB) binds to other gangliosides in addition to GM1 (9). These toxins exploit the host protein retention and degradation pathways to gain access to the cytoplasm, reviewed in reference 11. In the cytosol, their A1 subunits catalyze the transfer of an ADP-ribose from NAD to stimulatory α-subunits of G proteins (Gsα). After ADP-ribosylation, Gsα binds to adenylate cyclase and constitutively activates it, leading to a sustained increase in intracellular cyclic AMP (cAMP) concentration (4).
CT and LT are also powerful mucosal immunogens and adjuvants, reviewed in reference 14. In mice, antibody responses to CT and bystander antigens can last up to 2 years (15, 26). CT has been shown to induce primarily Th2 responses, characterized by CD4+ T cells producing interleukin 4 (IL-4), IL-5, IL-6, and IL-10 and by the production of immunoglobulin G1 (IgG1), IgA, and IgE antibodies (17, 29). By contrast, LT has been reported to induce mixed Th1 and Th2 responses (24). It has been proposed that differences in the ganglioside binding specificities of their B pentamers contribute to their discordant Th1/Th2 patterns (30, 31). Although these toxins are extensively used as adjuvants in animal models, their toxicity makes them unsuitable for human use. For this reason, a number of investigators have attempted to identify nontoxic derivatives of CT and LT that retain adjuvanticity by either removing the A domain (25) or by rendering it enzymatically inactive by site-directed mutagenesis (6, 8, 32). Studies addressing the adjuvant effects of recombinant B subunits of CT and LT have generated conflicting results. Several studies reported an adjuvant effect for recombinant CTB (rCTB) or recombinant LTB (rLTB) (5, 13, 28), while others have reported no adjuvant effect (1, 16, 26). A number of mutants of CT and LT have been generated that have abrogated or reduced enzymatic activity. For example, CT and LT that are rendered enzymatically inactive by the insertion of a lysine into the ADP-binding cleft of the A domain (denoted CTK63 and LTK63) have been studied (6, 8, 32). Several studies reported an adjuvant effect for these mutants (6, 8, 32); however, these responses were generally weaker than those induced by the wild-type toxins.
Of particular interest, one study compared the adjuvanticity of LTK63, which lacks enzymatic activity, with that of LTR72, which has reduced enzymatic activity. The rank order of adjuvanticity was LT ≈ LTR72 > LTK63 ≫ LTB, showing that CT and LT derive their adjuvanticity from both enzymatic activity and the intrinsic structure of the AB complex itself, regardless of enzymatic activity (21). Although these in vivo studies are informative, the cellular loci at which these molecules exert their action were not addressed. There is emerging data suggesting that dendritic cells (DC) are one of the major cellular targets for CT in vivo (19, 27). It was reported that CT activates monocyte-derived DC (MDDC) in vitro as defined by increased surface marker expression and enhanced alloantigen presentation in an allogeneic T-cell response (10). CT was also shown to inhibit IL-12 production by MDDC stimulated with strong IL-12-inducing stimuli, such as lipopolysaccharide (LPS) and CD40L (2, 10). Given the Th1-polarizing nature of IL-12, it has been proposed that the ability of CT to inhibit IL-12 production is responsible for driving immune responses toward a Th2 phenotype (2).
In the studies described below, we tested the hypothesis that the ability of CT and LT to activate human MDDC in vitro is due to contributions of both the enzymatic activity of the toxin and the intrinsic structure of the AB complex itself. Results will be presented showing that both CT and LT are potent activators of MDDC in vitro and that this activation is strictly dependent on the activation of adenylate cyclase. These results strongly suggest that there is little or no contribution of the intrinsic structure of the AB complex to this step in the immune response and that the reported differences in the adjuvanticity of CT and LT (24) are not due to differences in the measured effects of these toxins on MDDC.
MATERIALS AND METHODS
DC culture medium.
DC culture medium consisted of RPMI 1640 (Life Technologies, Carlsbad, Calif.) supplemented with 2 mM l-glutamine (Sigma, St. Louis, Mo.), 1% nonessential amino acids (Life Technologies), 1% sodium pyruvate (Life Technologies), 50 μM 2-mercaptoethanol (Sigma), 50 μg of gentamicin (Life Technologies)/ml, and 10% fetal calf serum (Life Technologies).
T-cell proliferation medium.
T-cell proliferation medium consisted of α-minimal essential medium without ribonucleosides and deoxyribonucleosides (Life Technologies) supplemented with 10% human AB serum (Sigma), 1% sodium pyruvate, 4 mM l-glutamine, 20 mM HEPES buffer (Life Technologies), 100 μg of streptomycin (Life Technologies)/ml, 100 U of penicillin G (Life Technologies)/ml, and 50 μM 2-mercaptoethanol.
DC preparations.
All human specimens were obtained under informed consent as approved by the University of Maryland Baltimore Institutional Review Board. MDDC were generated as described previously with minor modifications (22). Briefly, human peripheral blood mononuclear cells were isolated by Ficoll-Hypaque density gradient centrifugation and were enriched for CD14+ monocytes by negative selection using a cocktail of monoclonal antibodies from StemCell Technologies (Vancouver, Canada) according to the manufacturer's instructions. The isolated monocytes were made to adhere to plastic by plating at 106 cells per ml in RPMI medium for 2 h. Adherent monocytes were washed with RPMI medium and were then cultured at 106 cells per ml in DC culture medium supplemented with 50 ng of recombinant granulocyte-macrophage colony-stimulating factor/ml and 1,000 U of recombinant IL-4 (R & D Systems, Minneapolis, Minn.)/ml.
Cell treatments.
CT, rCTB (List Biological Laboratories, Campbell, Calif.), LPS, LT, dibutyryl-cyclic-3′,5′-AMP (d-cAMP), Forskolin, n-butyric acid (Sigma), Rp-8-Br-cAMPs (Biolog Life Science Institute, Bremen, Germany), CTK63, LTK63, and rLTB (the latter three preparations were provided generously by Rino Rappouli, Chiron Institute, Siena, Italy) were added directly to MDDC cultures in individual wells at the concentrations indicated below. At the times indicated below, the cells were harvested, washed, and stained for phenotypic analysis by flow cytometry.
Flow cytometry.
Cells were incubated for 30 min at 4°C with the following murine monoclonal antibodies, singly or in combination, specific for CD80: CD83, CD86, and HLA-DR (BD Pharmingen, San Diego, Calif.). They were washed and then fixed with 2% paraformaldehyde for analysis using a FACScalibur flow cytometer (BD, San Jose, Calif.). Data analysis was carried out using FlowJo software (Tree Star Inc., San Carlos, Calif.).
Endotoxin quantification.
LPS concentrations were monitored in the toxin preparations using the Limulus assay (Bio-Whittaker, Walkersville, Md.). LPS concentrations were maximally 40 pg/ml in the final dilutions of CT and LT or their derivatives used in the studies.
Allogeneic T-cell response.
MDDC for the allogeneic T-cell response were prepared as described above and washed three times with T-cell proliferation medium before addition to naïve, allogeneic CD4+ T cells. Naïve CD4+ T cells were enriched from peripheral blood mononuclear cells by negative selection using a cocktail of monoclonal antibodies (StemCell Technologies) according to the manufacturer's instructions. Naïve CD4+ T cells (≥93% pure as determined by the expression of CD45R0 and CD62L using flow cytometry) were cultured in triplicates at 105 cells/well with the indicated numbers of allogeneic MDDC in 96-well U-bottom plates. The cells were pulsed with 1 μCi of [3H]thymidine (Perkin-Elmer Life Sciences, Boston, Mass.)/well for the last 18 h of culture before measuring thymidine incorporation using a Wallac 1450 Microbetta Trilux liquid scintillation spectrometer (Wallac, Turku, Finland) on the 4th or 5th day. The standard deviation of triplicate wells in the same experiment was less than 10%.
Cytokine ELISA.
Tumor necrosis factor alpha (TNF-α) and IL-12 p70 concentrations were determined by enzyme-linked immunosorbent assay (ELISA) (R & D Systems) according to the manufacturer's instructions.
RESULTS
An enzymatically active A domain is necessary for CT and LT to activate MDDC.
In a series of preliminary experiments, we found that day 4 MDDC incubated with 1 μg of CT, LT, or LPS per ml for 20 h yielded optimal activation. Therefore, we used this protocol as our standard for the activation of MDDC by CT, LT, and LPS. It should be noted that there was donor-specific variability in the fraction of MDDC responding to the agonists. We found that the fraction of MDDC responding to 1 μg of CT/ml, as judged by CD83 upregulation, varied from 5.0 to 83.5%. The average percentage of cells that upregulated CD83 among 50 different individuals was 38.5% (standard error of the mean = 2.62; data not shown). CD80, CD86, and HLA-DR also varied to a similar extent among donors (data not shown). This effect was donor specific and not stimulus specific, as cells showing a greater response to CT also showed a greater response to LPS. This variability was not a result of contaminating monocytes, because fewer than 1% of the day 4 MDDC are CD14+. In addition, at least 90% of these MDDC displayed dendritic morphology and >90% become CD83+ when incubated for 20 h with saturating concentrations of LPS (data not shown). To avoid the influence of donor variability, MDDC derived from the same donor preparation were used to compare the magnitude of activation induced by different agonists.
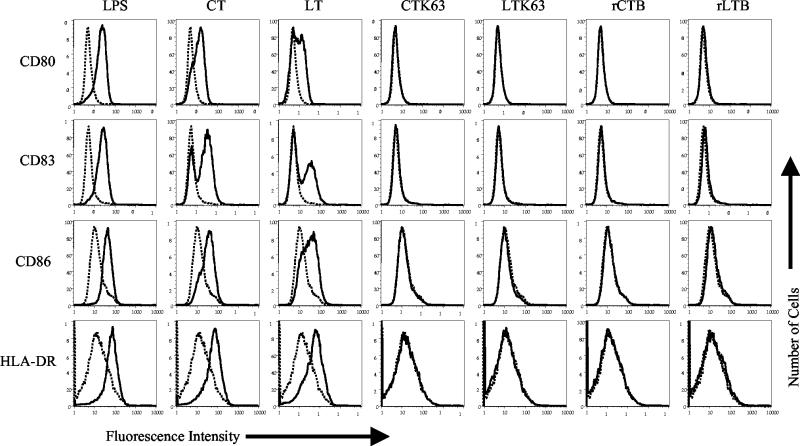
Next, we compared the effects of wild-type CT and LT with those of the enzymatically inactive toxins CTK63 and LTK63 and their recombinant B pentamers on the expression of activation markers on MDDC. Day 4 MDDC were incubated with CT, LT, CTK63, LTK63, rCTB, or rLTB at a final concentration of 1 μg/ml for a total of 20 h. As shown in Fig. 1, CD80, CD83, CD86, and HLA-DR were upregulated on MDDC incubated with CT or LT, although LT was less potent than CT. Titration experiments showed that CT is approximately three times more potent than LT in this assay (data not shown). This may be due to differences in the cleavage sites of the A domains of these toxins, which are thought to make CT more toxic than LT (20). In contrast to the wild-type toxins, none of the enzymatically inactive derivatives (CTK63, LTK63, rCTB, and rLTB) upregulated these markers on MDDC (Fig. 1). The failure of these molecules to activate MDDC is unlikely to be due to an insufficient concentration of the CT or LT or incubation time, as 1 μg of CT/ml is approximately 160-fold greater than the minimal concentration necessary to induce the maturation of these cells from most donors (data not shown) and as the level of expression of the activation markers did not increase at later time points (48 and 72 h; data not shown). In addition, although 10 μg of CTB/ml was shown to inhibit the proinflammatory responsiveness of macrophages (3), this concentration of CTB had no effect on the activation of MDDC or the production of inflammatory cytokines from these cells (10). Taken together, these results show that an enzymatically active A domain is required for CT and LT to activate MDDC, as judged by the upregulation of activation markers.
FIG. 1.
Enzymatic activity is required for CT and LT to activate MDDC. Cell surface expression of the indicated markers on untreated MDDC (dashed histograms) or on MDDC treated with the indicated stimulus (solid histograms) is shown. Day 4 MDDC were incubated with 1 μg of CT, LT, CTK63, LTK63, rCTB, or rLTB per ml for 20 h. The cells were harvested and stained for four-color flow cytometry with phycoerythrin anti-CD80, fluorescein isothiocyanate anti-CD83, Cy-Chrome anti-CD86, or allophycocyanin anti-HLA-DR. The data shown are representative of four experiments performed.
Elevated cAMP levels are responsible for the activation of MDDC by CT and LT.
The principal biochemical consequence of the enzymatic activity of CT and LT is the sustained increase in intracellular cAMP, due to the constitutive activation of adenylate cyclase. Therefore, a strong prediction is that the ability of these toxins to activate MDDC is mediated by cAMP. In this case, pharmacological agents that increase intracellular cAMP levels should mimic their effects on MDDC. In addition, the effects of these toxins and these pharmacological agents should be blocked by antagonists of the adenylate cyclase signaling pathway. This prediction was tested using two pharmacological mediators, d-cAMP and Forskolin, that elevate intracellular cAMP and an inhibitor, Rp-8-Br-cAMPs, that blocks cAMP-dependent protein kinase A (PKA).
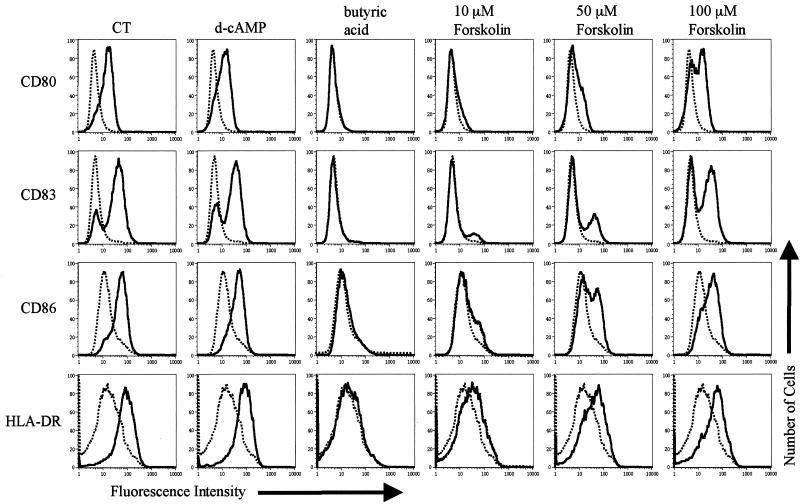
Day 4 MDDC were incubated with 1 μg of LPS or CT (as positive controls) per ml, 1 mM d-cAMP, 2 mM butyric acid, or Forskolin (at escalating concentrations of 10, 50, and 100 μM) for 20 h and were analyzed by flow cytometry for the upregulation of activation markers. As shown in Fig. 2, activation was observed for each of these agonists except for butyric acid and Forskolin at the 10 μM dose. However, activation was obtained with the higher doses of Forskolin. It should be noted that 10 μM was the only dose of Forskolin used for reference 10, where no activation was observed. Based on that observation, it was concluded that adenylate cyclase-independent signaling pathways must contribute to the effects of CT on MDDC (10). Our data contradict this conclusion. In addition, the central role of adenylate cyclase activation is supported by the ability of 1 mM d-cAMP to activate MDDC to levels similar to those elicited by 1 μg of CT/ml (Fig. 2).
FIG. 2.
The elevation of intracellular cAMP activates MDDC. Cell surface expression of the indicated markers on untreated MDDC (dashed histograms) or on MDDC treated with the indicated stimulus (solid histograms) is shown. Day 4 MDDC were incubated with 1 μg of CT/ml, 1 mM d-cAMP, 2 mM butyric acid, or the indicated concentration of Forskolin for 20 h. The cells were then harvested and stained for four-color flow cytometry with phycoerythrin anti-CD80, fluorescein isothiocyanate anti-CD83, Cy-Chrome anti-CD86, or allophycocyanin anti-HLA-DR. The data shown are representative of five experiments performed.
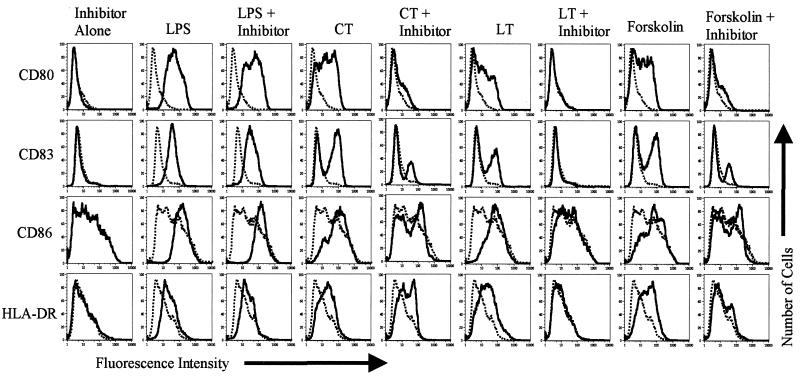
This hypothesis was tested further using the membrane-permeable, metabolically stable, competitive inhibitor of cAMP-dependent protein kinases, Rp-8-Br-cAMPs (7), to block the activation of MDDC by CT, LT, and Forskolin. Day 4 MDDC were incubated with 0.5 μg of CT, LT, or LPS per ml or 100 μM Forskolin with or without a prior 1-h incubation with 1 mM Rp-8-Br-cAMPs. This dose of Rp-8-Br-cAMPs and this concentration of CT and LT were determined to be optimal in preliminary blocking experiments (data not shown). Twenty hours later, the cells were harvested and analyzed for activation by flow cytometry. As shown in Fig. 3, preincubating MDDC for 1 h with Rp-8-Br-cAMPs inhibited the activation elicited by CT, LT, and Forskolin but not by LPS. In a separate series of experiments, we also found that Rp-8-Br-cAMPs inhibits activation induced by d-cAMP (data not shown). Taken together, these data strongly support the hypothesis that elevation of intracellular cAMP is the principal pathway by which CT and LT activate MDDC. These data also indicate that LPS activates MDDC through cAMP-independent signaling pathways; however, it cannot be ruled out that this concentration of the inhibitor may not be sufficient to inhibit the activation of these cells by LPS. Titration studies to determine this were not undertaken.
FIG. 3.
Inhibition of cAMP-dependent kinases inhibits the ability of CT and LT to activate MDDC. Cell surface expression of the indicated markers on untreated MDDC (dashed histograms) or MDDC treated with the indicated stimulus (solid histograms) is shown. Day 4 MDDC were incubated for 1 h with or without 1 mM Rp-8-Br-cAMPs before the addition of 0.5 μg of CT, LT, or LPS per ml or 100 μM Forskolin. Twenty hours later, the cells were harvested and stained for single-color flow cytometry with phycoerythrin anti-CD80, fluorescein isothiocyanate anti-CD83, phycoerythrin anti-CD86, or phycoerythrin anti-HLA-DR. The data shown are representative of four experiments performed.
cAMP-elevating agents increase the ability of MDDC to stimulate allogeneic naïve CD4+ T cells.
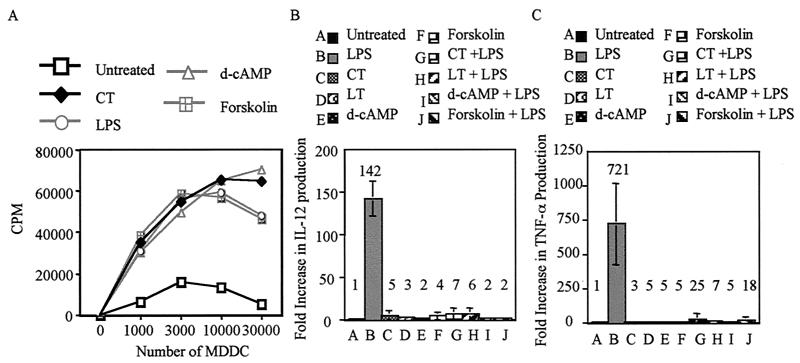
It was important to determine whether the activation of MDDC by CT and LT and the pharmacological agents that elevate intracellular cAMP (as determined by surface marker upregulation) correlates with an increased ability to stimulate proliferation of allogeneic naïve CD4+ T cells. For these experiments, untreated day 5 MDDC or MDDC activated by a 20-h incubation with 1 μg of LPS or CT per ml, 1 mM d-cAMP, or 100 μM Forskolin were used to stimulate allogeneic naïve CD4+ T cells. Figure 4A shows that MDDC activated by LPS, CT, d-cAMP, or Forskolin increased the proliferation of naïve CD4+ T cells at least sevenfold over that induced by untreated MDDC. Together these results show that phenotypic maturity as determined by surface marker upregulation correlates with functional maturity.
FIG. 4.
Activation of MDDC by increased intracellular cAMP enhances their ability to present alloantigen in the allogeneic T-cell response and dominantly inhibits LPS-induced IL-12 and TNF-α secretion. (A) Naïve CD4+ T cells were plated at 105 cells/well in 96-well U-bottom plates in T-cell medium. MDDC were activated by a 20-h incubation with 1 μg of CT or LPS per ml, 1 mM d-cAMP, or 100 μM Forskolin. Untreated and activated MDDC were washed, and then the indicated numbers were added to naïve T cells. Proliferation was determined at day 5 by pulsing the cells with 1 μCi of [3H]thymidine per well for the last 18 h of culture. Thymidine incorporation was measured with a Wallac 1450 Microbetta Trilux liquid scintillation counter. Data are representative of two independent experiments performed. Day 4 MDDC were left untreated or were treated with 1 μg of CT/ml, 1 μg of LT/ml, 1 mM d-cAMP, or 100 μM Forskolin in the presence or absence of 1 μg of LPS/ml. After 12 h of culture, the levels of IL-12 (B) and TNF-α (C) in the supernatants were determined by ELISA. The graphs show the average increase (n-fold) in IL-12 and TNF-α production over that by untreated MDDC. Data are the average of at least three separate experiments on MDDC generated from different donors.
Elevation of intracellular cAMP levels in MDDC dominantly inhibits IL-12 and TNF-α production.
Previously, it was shown that CT inhibits the production of inflammatory cytokines and chemokines by LPS-stimulated MDDC (10). To determine if this phenomenon depends on the elevation of intracellular cAMP, day 4 MDDC were left untreated or were treated with 1 μg of CT/ml, 1 μg of LT/ml, 1 mM d-cAMP, or 100 μM Forskolin in the presence or absence of 1 μg of LPS/ml. After 12 h of culture, the levels of IL-12 and TNF-α were determined by ELISA. Figure 4B shows that MDDC activated with LPS produce high levels of IL-12, approximately 140-fold greater than those produced by untreated MDDC. Likewise, Fig. 4C shows that MDDC activated with LPS produce high levels of TNF-α, approximately 720-fold greater than those produced by untreated MDDC. By contrast, as shown in Fig. 4B and C, MDDC activated by CT, LT, d-cAMP, or Forskolin in the presence or absence of LPS produce no more than 25 times the IL-12 or TNF-α of untreated MDDC. This corresponds to 20- and 28-fold reductions in the levels of IL-12 and TNF-α, respectively, compared to those found in MDDC activated by LPS alone. Collectively, these results strongly suggest that CT and LT dominantly inhibit cytokine production by MDDC via a cAMP-dependent mechanism.
DISCUSSION
In the studies described above, six observations strongly support the hypothesis that CT and LT activate MDDC predominantly, if not solely, by the elevation of intracellular cAMP. First, CT and LT, which elevate intracellular cAMP, induce maturation of MDDC as judged by changes in surface phenotype. Second, none of the enzymatically inactive derivatives of CT or LT tested in this study activated MDDC. Third, the activation of MDDC by CT or LT can be mimicked consistently by the pharmacological agonists d-cAMP and Forskolin. Fourth, the activation of MDDC by CT, LT, d-cAMP, and Forskolin but not by LPS can be inhibited by Rp-8-Br-cAMPs, a type II selective inhibitor of cAMP-dependent PKA. Fifth, MDDC activated by CT, d-cAMP, or Forskolin are more potent inducers of T-cell proliferation than are untreated MDDC in an allogeneic T-cell response. Sixth, as shown for CT (10), our studies show that LT, d-cAMP, and Forskolin also dominantly inhibit IL-12 and TNF-α production by LPS-activated MDDC. In summary, these results show that an enzymatically active A domain is required for the activation of MDDC by CT or LT and that this effect is mediated by cAMP. Several aspects of this work deserve additional comment.
In an earlier study (10), it was reported that 10 μg of rCTB/ml did not activate MDDC, suggesting a requirement for an enzymatically active A domain in the activation of MDDC by CT and LT. While suggestive, that study was not conclusive, because enzymatically inactive CT and LT (CTK63 and LTK63) exhibit adjuvant activity exceeding that of rCTB and rLTB (6, 8, 32) (data not shown). Since CTK63 and LTK63 have an A domain, albeit enzymatically inactive, we considered the possibility that the AB structure itself might be responsible for the activation of MDDC by CT and LT, as suggested by others (10). This is plausible in that these toxins traffic through a retrograde pathway from the cell surface to the cytoplasm via the ERAD protein quality control pathway (11). This could potentially generate signals that activate MDDC. If this were so, CTK63 and LTK63 should behave like wild-type toxins. It is clear from the studies described above that they do not. We have never seen any activity of CTK63 or LTK63 on MDDC. This provides compelling evidence that the biological properties of these molecules in MDDC are strictly dependent upon the presence of an enzymatically active A1 domain.
The requirement for an enzymatically active A domain in the activation of MDDC by CT and LT is supported further by the ability to replace the toxins with agonists that elevate intracellular cAMP. A previous study (10) reported that Forskolin, unlike CT, did not activate MDDC, suggesting the involvement of cAMP-independent pathways in CT-mediated activation. By contrast, our titration studies showed that Forskolin activates MDDC at doses higher than 10 μM, which was the only dose evaluated in reference 10. Furthermore, a central role for elevated cAMP in the activation of MDDC was shown by our ability to specifically activate MDDC with d-cAMP. This result confirms a previous study in which d-cAMP and Forskolin activated MDDC, as judged by CD83 upregulation and the ability to present antigen (23).
Our ability to mimic the effects of CT and LT on MDDC by d-cAMP and Forskolin are consistent with a central role of cAMP in the activation of MDDC by these proteins, but they do not formally establish causality. For this reason, we sought specific inhibitors of cAMP-dependent pathways to determine whether they could block the activation of MDDC by CT. We chose Rp-8-Br-cAMPs for these studies, because it is a specific inhibitor of PKA that is cAMP dependent. This inhibitor specifically blocked the activation of MDDC by all of the cAMP-elevating agents studied above. This inhibitor did not affect activation induced by LPS. This strongly supports a central role for elevated cAMP in the activation of MDDC by CT and LT. These results also suggest that the adjuvanticity of CTK63, LTK63, rCTB, and rLTB relies on signaling pathways that lie elsewhere.
As shown above, CT and Forskolin inhibit inflammatory cytokine production by LPS-stimulated DC (see also references 2 and 10). We have extended those findings by showing that LT and d-cAMP also dominantly inhibit IL-12 and TNF-α production. This lends further credence to the hypothesis that this effect is cAMP dependent. Since IL-12 plays a key role in the differentiation of CD4+ T cells toward a Th1 phenotype, it is reasonable to speculate that the inhibition of IL-12 production by CT results in the polarization of CD4+ T cells toward a Th2 phenotype in vivo (17, 29). In support of this hypothesis, MDDC activated by CT have been shown to polarize IL-2-expanded T-cell lines toward Th2 cytokine production (10). By contrast, LT has been reported to induce mixed Th1/Th2 responses in vivo (24). It is possible that, while CT inhibits inflammatory cytokine production from MDDC, LT might induce moderate levels of cytokine production somewhere between those of LPS-stimulated and CT-stimulated MDDC. Our studies argue against this hypothesis in that only low levels of IL-12 and TNF-α were produced by MDDC in the presence of CT and LT. For this reason, we speculate that any differences in the ability of CT and LT to polarize CD4+ T-cell responses are not the result of the effects of these toxins on MDDC measured in this study. In this regard, it is interesting that CT was reported to directly inhibit Th1 responses from isolated CD4 T cells stimulated directly via the T-cell receptor-CD3 complex, whereas LT did not (31; reviewed in reference 32). In addition, it should be noted that this study examined only one DC lineage, MDDC. It is possible that CT and LT or their enzymatically inactive derivatives display different effects on other DC lineages, such as plasmacytoid DC.
In summary, the results of this study strongly support the requirement for an enzymatically active A domain in the ability of CT and LT to activate MDDC and suggest that there is little or no contribution of the intrinsic structure of the AB complex to this step in the immune response. These results also show that this activation is highly likely to be a direct result of elevated levels of cAMP in the MDDC and that cAMP-independent pathways are not involved in this phenomenon. Finally, these studies suggest that the reported differences in the adjuvanticity of CT and LT (24) lie in pathways other than those studied here.
Acknowledgments
This work is supported by NIH grants AI38192 and AI43046 to G.K.L.
We thank Rino Rappouli, The Chiron Immunological Research Institute, Siena, Italy, for the generous supply of CTK63, LTK63, and LTB.
Editor: J. D. Clements
REFERENCES
- 1.Blanchard, T. G., N. Lycke, S. J. Czinn, and J. G. Nedrud. 1998. Recombinant cholera toxin B subunit is not an effective mucosal adjuvant for oral immunization of mice against Helicobacter felis. Immunology 94:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun, M. C., J. He, C. Y. Wu, and B. L. Kelsall. 1999. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor beta1 and beta2 chain expression. J. Exp. Med. 189:541-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkart, V., Y. E. Kim, B. Hartmann, I. Ghiea, U. Syldath, M. Kauer, W. Fingberg, P. Hanifi-Moghaddam, S. Muller, and H. Kolb. 2002. Cholera toxin B pretreatment of macrophages and monocytes diminishes their proinflammatory responsiveness to lipopolysaccharide. J. Immunol. 168:1730-1737. [DOI] [PubMed] [Google Scholar]
- 4.Cassel, D., and Z. Selinger. 1977. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. USA 74:3307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Haan, L., W. R. Verweij, I. K. Feil, M. Holtrop, W. G. Hol, E. Agsteribbe, and J. Wilschut. 1998. Role of GM1 binding in the mucosal immunogenicity and adjuvant activity of the Escherichia coli heat-labile enterotoxin and its B subunit. Immunology 94:424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickinson, B. L., and J. D. Clements. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 63:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dostmann, W. R., S. S. Taylor, H. G. Genieser, B. Jastorff, S. O. Doskeland, and D. Ogreid. 1990. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3′,5′-cyclic phosphorothioates. J. Biol. Chem. 265:10484-10491. [PubMed] [Google Scholar]
- 8.Douce, G., C. Turcotte, I. Cropley, M. Roberts, M. Pizza, M. Domenghini, R. Rappuoli, and G. Dougan. 1995. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc. Natl. Acad. Sci. USA 92:1644-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuta, S., J. L. Magnani, E. M. Twiddy, R. K. Holmes, and V. Ginsburg. 1988. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect. Immun. 56:1748-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 11.Hazes, B., and R. J. Read. 1997. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry 36:11051-11054. [DOI] [PubMed] [Google Scholar]
- 12.Heyningen, S. V. 1974. Cholera toxin: interaction of subunits with ganglioside GM1. Science 183:656-657. [DOI] [PubMed] [Google Scholar]
- 13.Isaka, M., Y. Yasuda, S. Kozuka, Y. Miura, T. Taniguchi, K. Matano, N. Goto, and K. Tochikubo. 1998. Systemic and mucosal immune responses of mice to aluminium-adsorbed or aluminium-non-adsorbed tetanus toxoid administered intranasally with recombinant cholera toxin B subunit. Vaccine 16:1620-1626. [DOI] [PubMed] [Google Scholar]
- 14.Lycke, N. 1997. The mechanism of cholera toxin adjuvanticity. Res. Immunol. 148:504-520. [DOI] [PubMed] [Google Scholar]
- 15.Lycke, N., and J. Holmgren. 1987. Long-term cholera antitoxin memory in the gut can be triggered to antibody formation associated with protection within hours of an oral challenge immunization. Scand. J. Immunol. 25:407-412. [DOI] [PubMed] [Google Scholar]
- 16.Lycke, N., and J. Holmgren. 1986. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology 59:301-308. [PMC free article] [PubMed] [Google Scholar]
- 17.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, H. Bluethmann, et al. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 18.Ohtomo, N., T. Muraoka, A. Tashiro, Y. Zinnaka, and K. Amako. 1976. Size and structure of the cholera toxin molecule and its subunits. J. Infect. Dis. 133(Suppl.):31-40. [DOI] [PubMed] [Google Scholar]
- 19.Porgador, A., H. F. Staats, Y. Itoh, and B. L. Kelsall. 1998. Intranasal immunization with cytotoxic T-lymphocyte epitope peptide and mucosal adjuvant cholera toxin: selective augmentation of peptide-presenting dendritic cells in nasal mucosa-associated lymphoid tissue. Infect. Immun. 66:5876-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodighiero, C., A. T. Aman, M. J. Kenny, J. Moss, W. I. Lencer, and T. R. Hirst. 1999. Structural basis for the differential toxicity of cholera toxin and Escherichia coli heat-labile enterotoxin. Construction of hybrid toxins identifies the A2-domain as the determinant of differential toxicity. J. Biol. Chem. 274:3962-3969. [DOI] [PubMed] [Google Scholar]
- 21.Ryan, E. J., E. McNeela, M. Pizza, R. Rappuoli, L. O'Neill, and K. H. Mills. 2000. Modulation of innate and acquired immune responses by Escherichia coli heat-labile toxin: distinct pro- and anti-inflammatory effects of the nontoxic AB complex and the enzyme activity. J. Immunol. 165:5750-5759. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinbrink, K., L. Paragnik, H. Jonuleit, T. Tuting, J. Knop, and A. H. Enk. 2000. Induction of dendritic cell maturation and modulation of dendritic cell-induced immune responses by prostaglandins. Arch. Dermatol. Res. 292:437-445. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi, I., M. Marinaro, H. Kiyono, R. J. Jackson, I. Nakagawa, K. Fujihashi, S. Hamada, J. D. Clements, K. L. Bost, and J. R. McGhee. 1996. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J. Infect. Dis. 173:627-635. [DOI] [PubMed] [Google Scholar]
- 25.Tamura, S., H. Funato, T. Nagamine, C. Aizawa, and T. Kurata. 1989. Effectiveness of cholera toxin B subunit as an adjuvant for nasal influenza vaccination despite pre-existing immunity to CTB. Vaccine 7:503-505. [DOI] [PubMed] [Google Scholar]
- 26.Vajdy, M., and N. Y. Lycke. 1992. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology 75:488-492. [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson, E., G. M. Westrich, and J. L. Viney. 1999. Modulating dendritic cells to optimize mucosal immunization protocols. J. Immunol. 163:3668-3675. [PubMed] [Google Scholar]
- 28.Wu, H. Y., and M. W. Russell. 1998. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine 16:286-292. [DOI] [PubMed] [Google Scholar]
- 29.Xu-Amano, J., R. J. Jackson, K. Fujihashi, H. Kiyono, H. F. Staats, and J. R. McGhee. 1994. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine 12:903-911. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto, M., H. Kiyono, M. N. Kweon, S. Yamamoto, K. Fujihashi, H. Kurazono, K. Imaoka, H. Bluethmann, I. Takahashi, Y. Takeda, M. Azuma, and J. R. McGhee. 2000. Enterotoxin adjuvants have direct effects on T cells and antigen-presenting cells that result in either interleukin-4-dependent or -independent immune responses. J. Infect. Dis. 182:180-190. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto, M., J. R. McGhee, Y. Hagiwara, S. Otake, and H. Kiyono. 2001. Genetically manipulated bacterial toxin as a new generation mucosal adjuvant. Scand. J. Immunol. 53:211-217. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto, S., Y. Takeda, M. Yamamoto, H. Kurazono, K. Imaoka, K. Fujihashi, M. Noda, H. Kiyono, and J. R. McGhee. 1997. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 185:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]