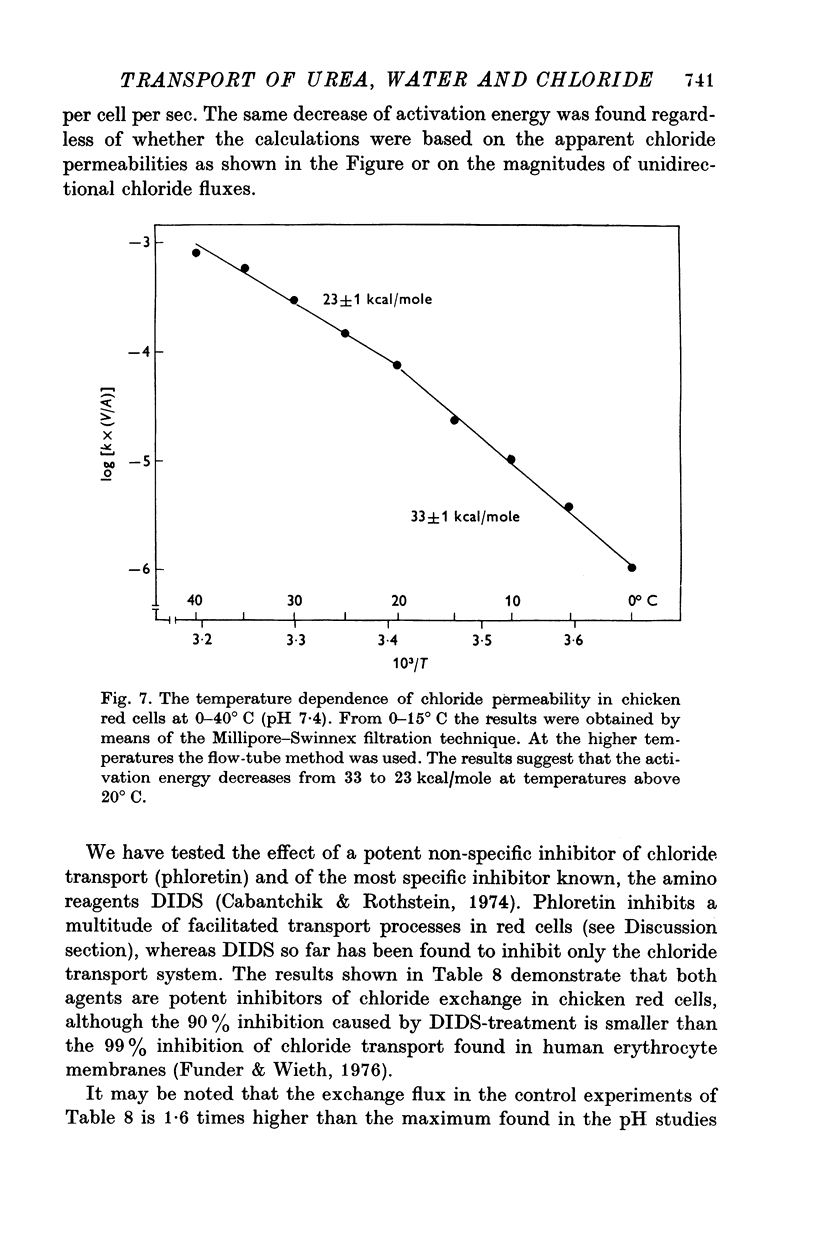
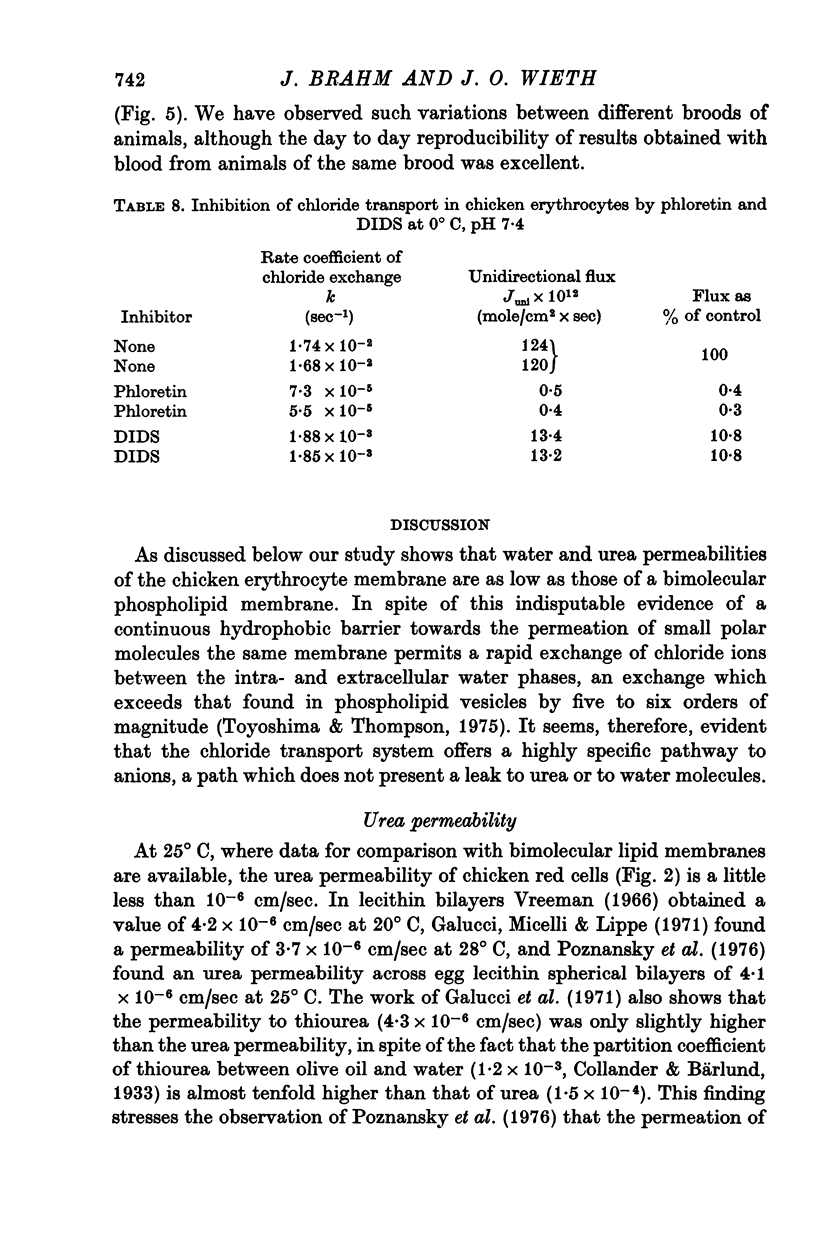
Abstract
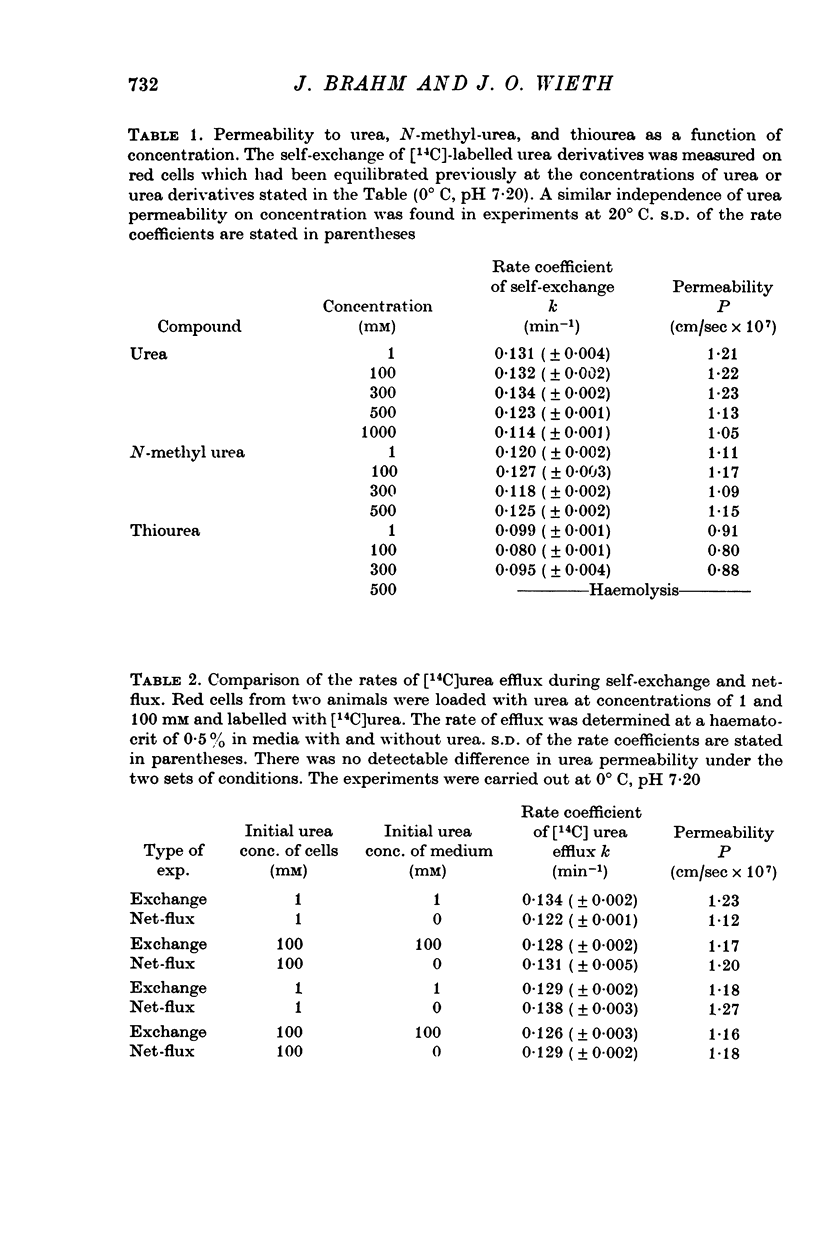
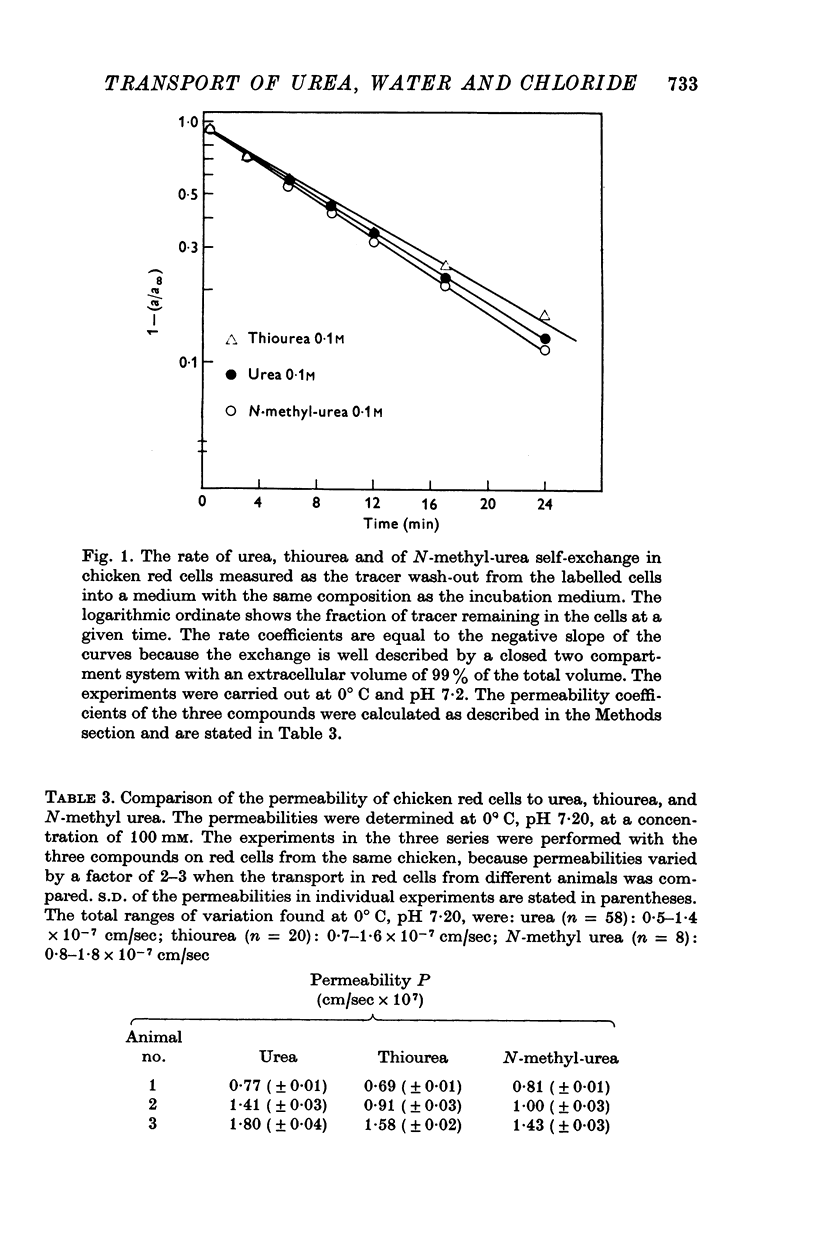
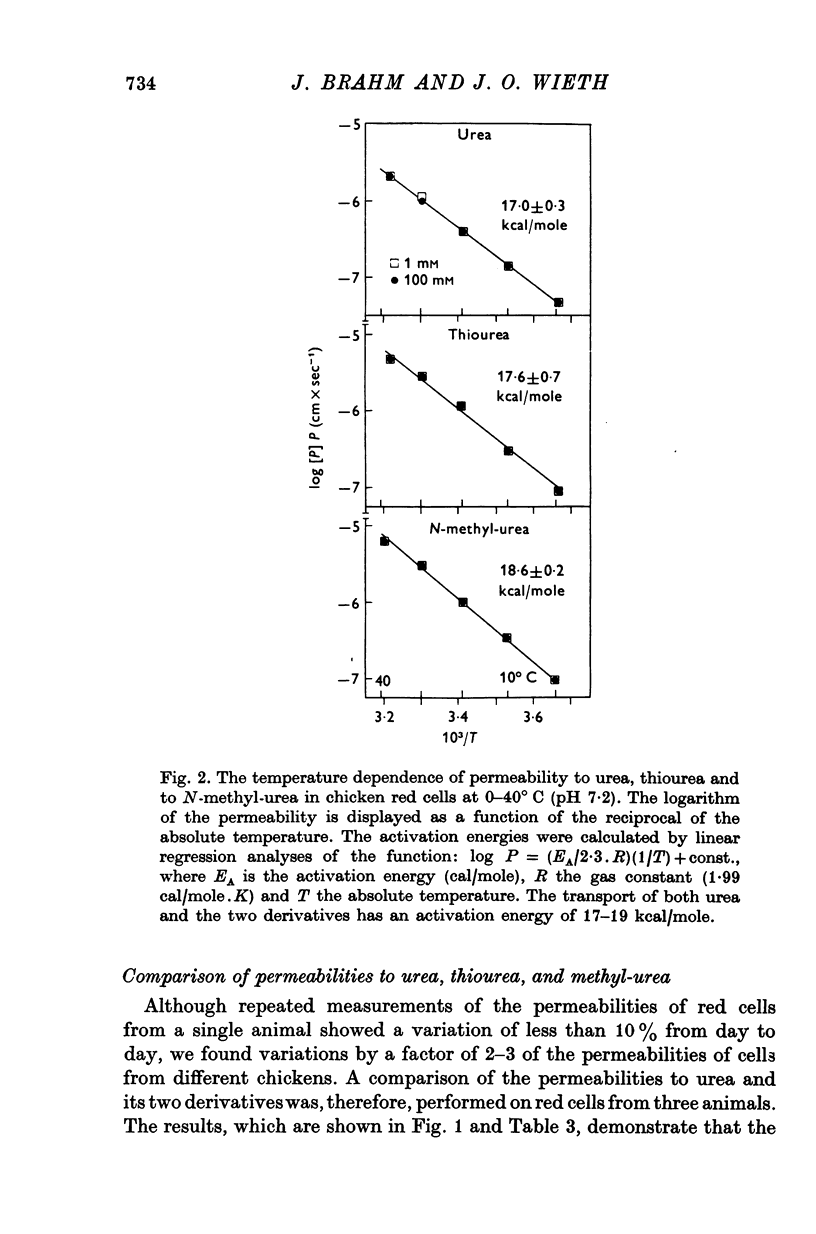
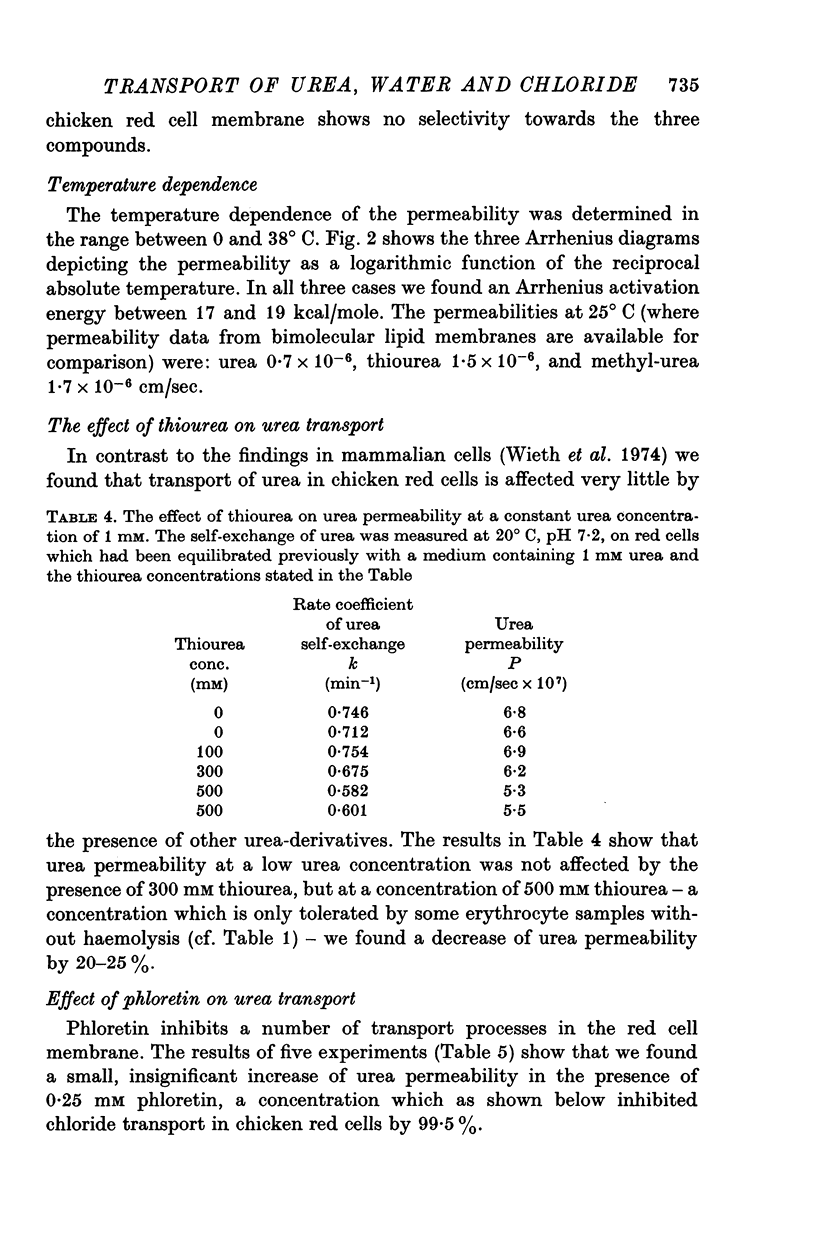
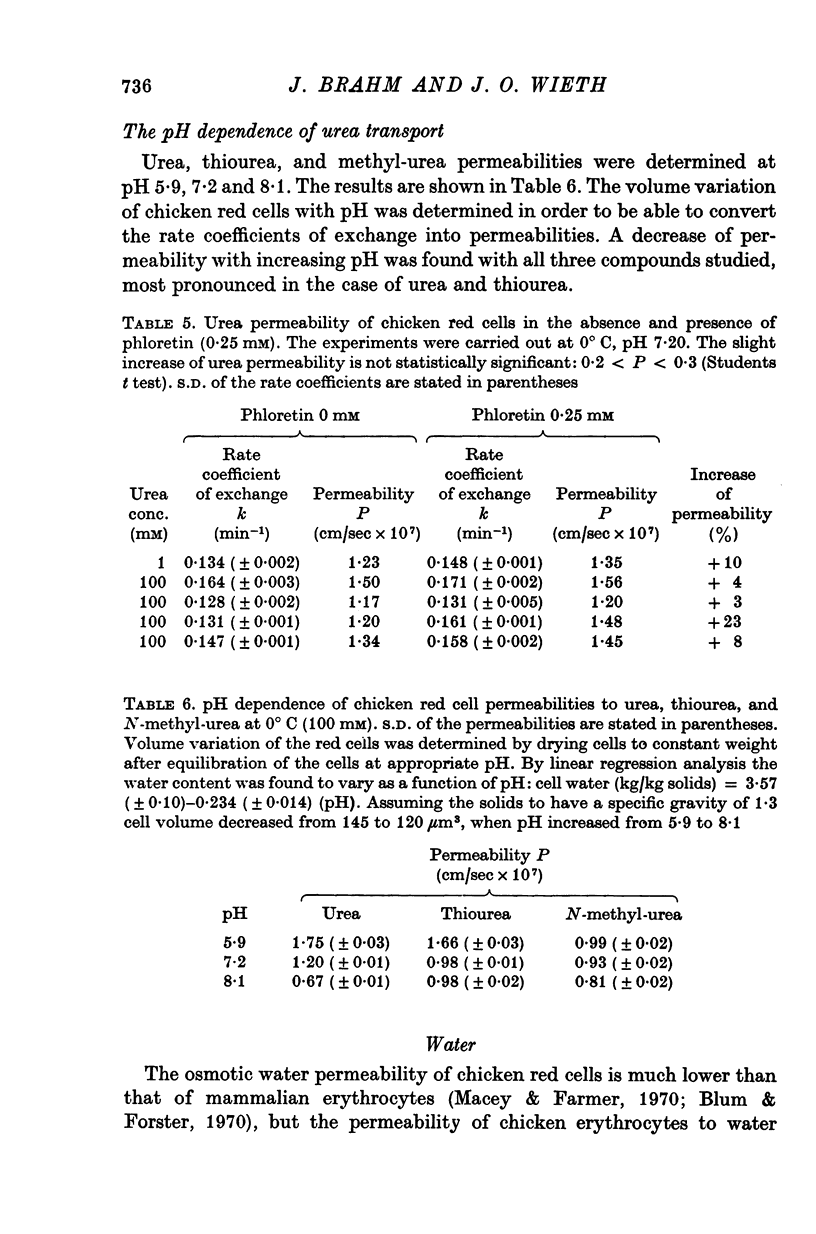
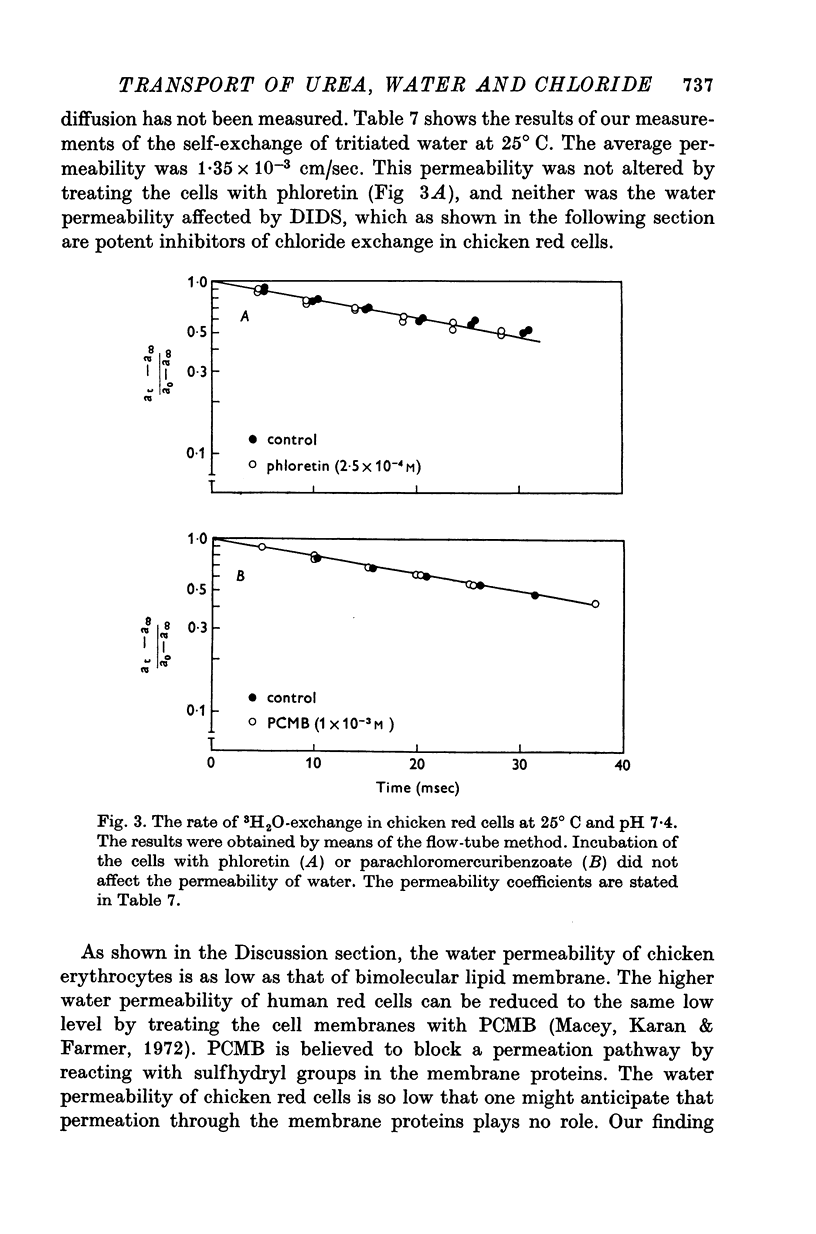
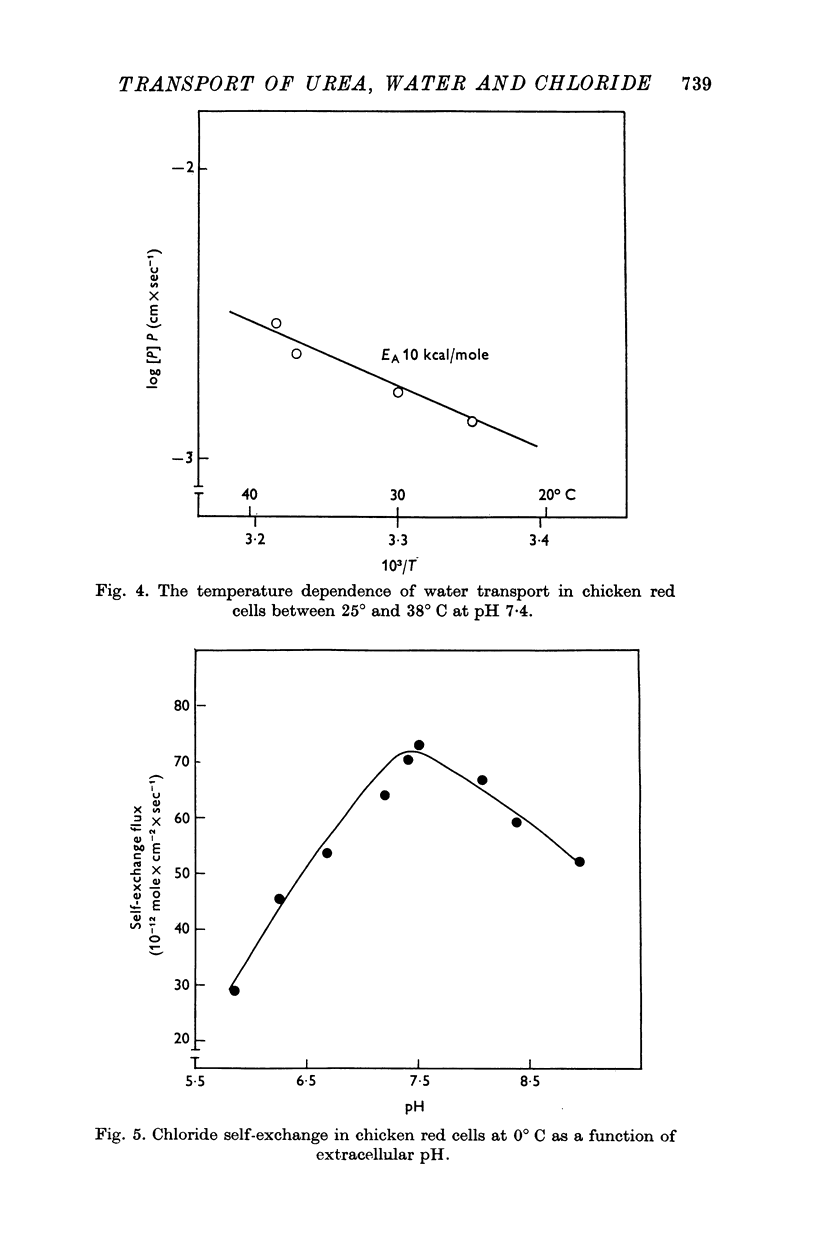
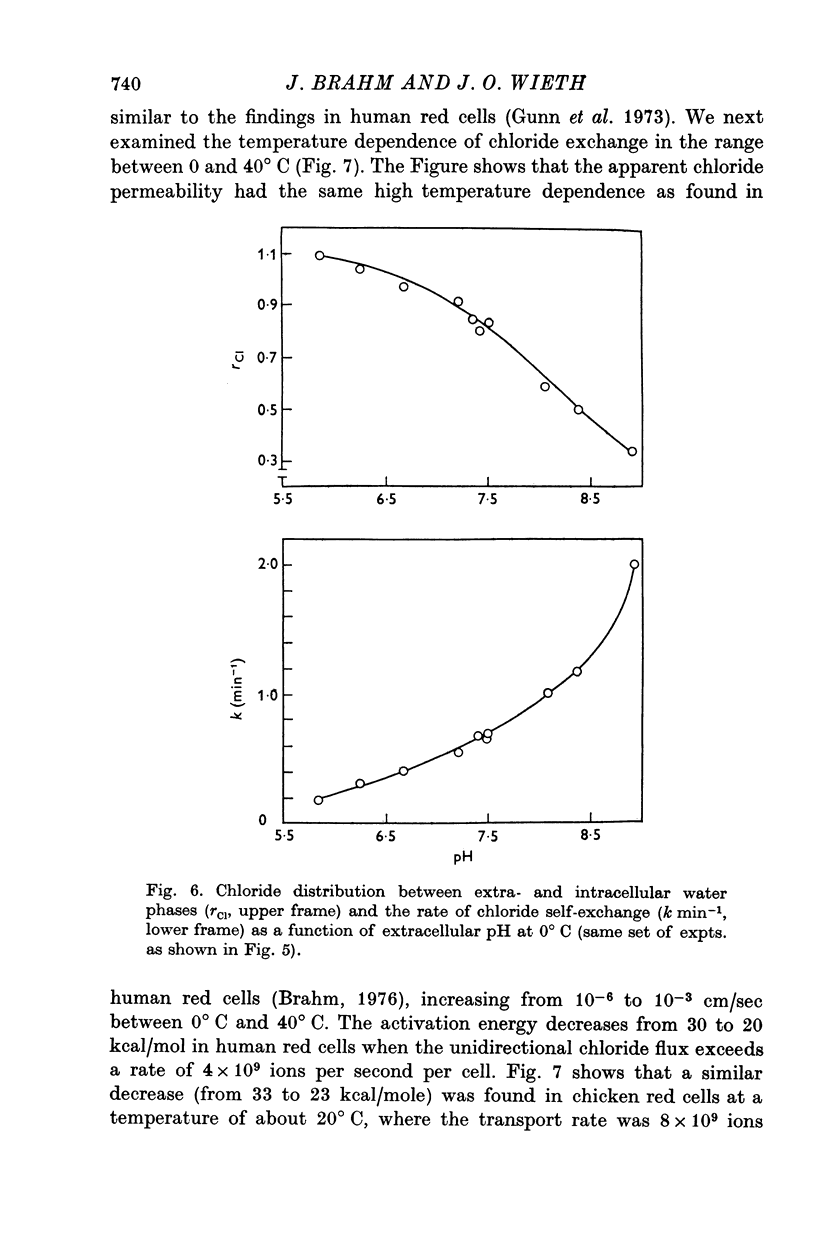
1. Urea and water permeabilities of chicken erythrocytes are considerably lower than those of mammalian red cells. 2. The permeabilities to urea, thiourea and to N-methylurea (about 10(-6) cm/sec at 25 degrees C) were independent of concentration within a very broad range, and we found no evidence of interaction between transport of analogue molecules. The activation energies were between 17 and 19 kcal/mole, and urea transport was not inhibited by phloretin, which inhibits urea transport in mammalian red cells. 3. The water permeability of chicken red cells (as measured by the diffusion of tritiated water) was 1-35 X 10(-3) cm/sec at 25 degrees C. The activation energy was 10 kcal/mole, and the water permeability was not affected by phloretin or parachloromercuribenzoate. 4. It is concluded that the urea and water permeabilities of the chicken erythrocyte membrane are similar to those of a non-porous bimolecular phospholipid membrane. 5. Like the red cells of other animal species the chicken red cell membrane contains an anion transport system, mediating a rapid exchange of chloride across the cell membranes. The pH dependence, temperature dependence, and sensitivity to inhibitors were similar to the properties of the anion transport system found in mammalian red cells. Our study shows, therefore, that the transport system offers a highly specific pathway to the exchange of anions, without presenting an inspecific leak to the permeation of water and urea.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S., Finkelstein A., Katz I., Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol. 1976 Jun;67(6):749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson E. S., Rossi Fanelli M. R., Giacometti G. M., Rosenberg A., Antonini E. Effects of ligand binding on the rates of hydrogen exchange in myoglobin and hemoglobin. Biochemistry. 1973 Jul 3;12(14):2699–2706. doi: 10.1021/bi00738a024. [DOI] [PubMed] [Google Scholar]
- Blum R. M., Forster R. E. The water permeability of erythrocytes. Biochim Biophys Acta. 1970 Jun 2;203(3):410–423. doi: 10.1016/0005-2736(70)90181-1. [DOI] [PubMed] [Google Scholar]
- Brown P. A., Feinstein M. B., Sha'afi R. I. Membrane proteins related to water transport in human erythrocytes. Nature. 1975 Apr 10;254(5500):523–525. doi: 10.1038/254523a0. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Carlsen A., Wieth J. O. Glycerol transport in human red cells. Acta Physiol Scand. 1976 Aug;97(4):501–513. doi: 10.1111/j.1748-1716.1976.tb10290.x. [DOI] [PubMed] [Google Scholar]
- Cass A., Finkelstein A. Water permeability of thin lipid membranes. J Gen Physiol. 1967 Jul;50(6):1765–1784. doi: 10.1085/jgp.50.6.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmark M. Chloride transport in human red cells. J Physiol. 1975 Aug;250(1):39–64. doi: 10.1113/jphysiol.1975.sp011042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmark M., Wieth J. O. Temperature dependence of chloride, bromide, iodide, thiocyanate and salicylate transport in human red cells. J Physiol. 1972 Aug;224(3):583–610. doi: 10.1113/jphysiol.1972.sp009914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer R. E., Macey R. I. Perturbation of red cell volume: rectification of osmotic flow. Biochim Biophys Acta. 1970 Jan 6;196(1):53–65. doi: 10.1016/0005-2736(70)90165-3. [DOI] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Chloride transport in human erythrocytes and ghosts: a quantitative comparison. J Physiol. 1976 Nov;262(3):679–698. doi: 10.1113/jphysiol.1976.sp011615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galey W. R., Owen J. D., Solomon A. K. Temperature dependence of nonelectrolyte permeation across red cell membranes. J Gen Physiol. 1973 Jun;61(6):727–746. doi: 10.1085/jgp.61.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazitt Y., Ohad I., Loyter A. Phosphorylation and dephosphorylation of membrane proteins as a possible mechanism for structural rearrangement of membrane components. Biochim Biophys Acta. 1976 Jun 4;436(1):1–14. doi: 10.1016/0005-2736(76)90214-5. [DOI] [PubMed] [Google Scholar]
- Gunn R. B., Dalmark M., Tosteson D. C., Wieth J. O. Characteristics of chloride transport in human red blood cells. J Gen Physiol. 1973 Feb;61(2):185–206. doi: 10.1085/jgp.61.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter F. R., George J., Ospina B. Possible carriers in erythrocytes. J Cell Physiol. 1965 Jun;65(3):299–311. doi: 10.1002/jcp.1030650303. [DOI] [PubMed] [Google Scholar]
- Jenkins R. E., Tanner J. A. The major human erythrocyte membrane protein. Evidence for an S-shaped structure which traverses the membrane twice and contains a duplicated set of sites. Biochem J. 1975 Jun;147(3):393–399. doi: 10.1042/bj1470393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. L., Solomon A. K. Interaction between phloretin and the red blood cell membrane. J Gen Physiol. 1976 Apr;67(4):381–397. doi: 10.1085/jgp.67.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. A., Hays L., Hays R. M. Evolution of a facilitated diffusion pathway for amides in the erythrocyte. Am J Physiol. 1974 Jun;226(6):1327–1332. doi: 10.1152/ajplegacy.1974.226.6.1327. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G., MARSHALL J. K. The atachment of phloretin and analogues to human erythrocytes in connection with inhibition of sugar transport. J Biol Chem. 1959 Nov;234:3022–3026. [PubMed] [Google Scholar]
- Lepke S., Fasold H., Pring M., Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4'-diisothiocyano stilbene-2,2'-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol. 1976 Oct 20;29(1-2):147–177. doi: 10.1007/BF01868957. [DOI] [PubMed] [Google Scholar]
- MURDAUGH H. V., Jr, DOYLE E. M. Effect of hemoglobin on erythrocyte urea concentration. J Lab Clin Med. 1961 May;57:759–762. [PubMed] [Google Scholar]
- Macey R. I., Farmer R. E. Inhibition of water and solute permeability in human red cells. Biochim Biophys Acta. 1970 Jul 7;211(1):104–106. doi: 10.1016/0005-2736(70)90130-6. [DOI] [PubMed] [Google Scholar]
- Macey R. I., Karan D. M., Farmer R. E. Properties of water channels in human red cells. Biomembranes. 1972;3:331–340. doi: 10.1007/978-1-4684-0961-1_22. [DOI] [PubMed] [Google Scholar]
- Owen J. D., Solomon A. K. Control of nonelectrolyte permeability in red cells. Biochim Biophys Acta. 1972 Dec 1;290(1):414–418. doi: 10.1016/0005-2736(72)90087-9. [DOI] [PubMed] [Google Scholar]
- Poznansky M., Tong S., White P. C., Milgram J. M., Solomon A. K. Nonelectrolyte diffusion across lipid bilayer systems. J Gen Physiol. 1976 Jan;67(1):45–66. doi: 10.1085/jgp.67.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price H. D., Thompson T. E. Properties of liquid bilayer membranes separating two aqueous phases: temperature dependence of water permeability. J Mol Biol. 1969 May 14;41(3):443–457. doi: 10.1016/0022-2836(69)90287-3. [DOI] [PubMed] [Google Scholar]
- Rabinowitz L., Gunther R. A. Urea transport in elasmobranch erythrocytes. Am J Physiol. 1973 May;224(5):1109–1115. doi: 10.1152/ajplegacy.1973.224.5.1109. [DOI] [PubMed] [Google Scholar]
- Redwood W. R., Haydon D. A. Influence of temperature and membrane composition on the water permeability of lipid bilayers. J Theor Biol. 1969 Jan;22(1):1–8. doi: 10.1016/0022-5193(69)90075-7. [DOI] [PubMed] [Google Scholar]
- Rothstein A., Cabantchik Z. I., Knauf P. Mechanism of anion transport in red blood cells: role of membrane proteins. Fed Proc. 1976 Jan;35(1):3–10. [PubMed] [Google Scholar]
- Toyoshima Y., Thompson T. E. Chloride flux in bilayer membranes: chloride permeability in aqueous dispersions of single-walled, bilayer vesicles. Biochemistry. 1975 Apr 8;14(7):1525–1531. doi: 10.1021/bi00678a028. [DOI] [PubMed] [Google Scholar]
- Wieth J. O. Effects of hexoses and anions on the erythritol permeability of human red cells. J Physiol. 1971 Mar;213(2):435–453. doi: 10.1113/jphysiol.1971.sp009392. [DOI] [PMC free article] [PubMed] [Google Scholar]


