Abstract
The host interferon (IFN) system plays an important role in protection against microbial infections. Salmonella enterica serovar Typhimurium is highly virulent in the mouse model, whereas mutants that lack DNA adenine methylase (Dam−) are highly attenuated and elicit fully protective immune responses against murine typhoid fever. We examined the expression of IFN-responsive genes in several mouse tissues following infection with Dam+ or Dam− Salmonella. Infection of mice with Dam+ Salmonella resulted in the induction of host genes known to be indicators of IFN bioactivity and regulated by either IFN-α/β (Mx1) or IFN-γ (class II transactivator protein [CIITA] and inducible nitric oxide synthase [iNOS]) or by both IFN-α/β and IFN-γ (RNA-specific adenosine deaminase [ADAR1] and RNA-dependent protein kinase [PKR]) in a tissue-specific manner compared to uninfected animals. Since the Mx1 promoter is IFN-α/β specific and the Mx1 gene is not inducible directly by IFN-γ, these data suggest a role of IFN-α/β in the host response to Salmonella infection. Mice infected with Dam− Salmonella showed reduced expression of the same set of IFN-stimulated genes (ISGs) as that observed after infection with wild-type Salmonella. The reduced capacity to induce ISGs persisted in Dam−-vaccinated mice after challenge with the virulent (Dam+) strain. Finally, although no Dam− organisms were recovered from the liver or spleen after oral infection of mice, ADAR, PKR, Mx, and CIITA expression levels were elevated in these tissues relative to those in uninfected mice, suggestive of the distant action of a signaling molecule(s) in the activation of ISG expression.
Much work regarding the construction of live bacterial vaccines has been performed in Salmonella spp. since they establish an infection by direct interaction with the gut-associated lymphoid tissue, resulting in strong mucosal responses. Additionally, Salmonella also invades and proliferates within host cells and thus is capable of eliciting strong cell-mediated immune responses (10, 18, 19, 40). Salmonella mutants lacking DNA adenine methylase (Dam) are highly attenuated for virulence (12, 16, 25) and are effective as live vaccines against Salmonella infection of mice (16) and chickens (8, 9). Further, Salmonella Dam− strains are sensitive to mediators of innate immunity and confer cross-protective immunity to heterologous Salmonella serotypes (15).
Although acute infections with Dam− Salmonella strains are most often asymptomatic, infection with wild-type Dam+ Salmonella is lethal in the mouse model (16). Little is known regarding the mechanistic basis of the difference in the responses. Among the potentially contributing parameters are altered cytokine responses, decreased ability to disseminate and colonize tissues, and impaired proliferation in target organs. To begin to understand the molecular basis of both the virulence attenuation and the immunity conferred by Dam-deficient strains, we investigated the host interferon (IFN) response to Salmonella infection in vaccinated versus nonvaccinated mice. The IFN response represents an early host defense that is engaged prior to the onset of the adaptive immune response (4). IFNs are commonly grouped into two types, whose functional relationships are distinguished by their receptors and intracellular signaling pathways, and by the physiologic conditions of their production and action. Members of the first type of IFNs, including IFN-α and IFN-β, play important roles in the host response to viral infection, e.g., through induction of cellular enzymes that act to directly interfere with viral replication cycles within infected cells (4, 34, 35, 41). These IFNs also activate natural killer cells and major histocompatibility complex I-restricted cytotoxic activities (4, 35, 39, 41). The second type of IFN, also termed IFN-γ, is involved in protection against both viral and bacterial infections (5, 39) via its role in both innate and adaptive immunity (4, 35). IFN-γ stimulates macrophage activation and specific cytotoxic immunity through induction of cellular proteins that affect CD4+ and CD8+ T-cell responses (4, 5, 41, 45).
Given the broadly based roles of IFN-regulated cellular gene products in the host response to infection, we sought to determine whether Salmonella Dam− and Dam+ strains differentially induced IFN-mediated responses and proliferated in target organs in mice. The principal objective of our study was to determine whether Salmonella infection alters the in vivo expression of IFN-stimulated genes (ISGs) whose protein products play central roles in promoting immune responses or otherwise affect macromolecular synthesis or localization within animal cells. Five ISGs were chosen for study. The IFN-α/β-inducible Mx1 gene encodes a GTPase activity that affects trafficking within animal cells and is a potent inhibitor of the replication of many RNA viruses (14, 35). Mx expression has been used extensively as a sensitive indicator of IFN-α/β bioactivity in a range of species (14, 21). The IFN-γ-inducible proteins, inducible nitric oxide synthase (iNOS) and the class II transactivator protein (CIITA), regulate, respectively, the production of the antimicrobial agent NO and the induction of major histocompatibility complex II molecules that facilitate the ability to present processed microbial antigens (35, 39). The RNA-dependent protein kinase (PKR) and RNA-specific adenosine deaminase (ADAR), inducible by both IFN-α/β and IFN-γ, affect macromolecular synthesis within animal cells through phosphorylation of translation factor eIF-2 and editing of RNA transcripts, respectively (34, 35). PKR and ADAR are critical in modulating many physiological responses associated with viral and possibly bacterial infections (35). In this study, we establish that Dam+ Salmonella induces ISGs in a tissue-specific fashion and that mice infected with Dam− Salmonella are diminished in this capacity.
MATERIALS AND METHODS
Bacterial strains.
The S. enterica serovar Typhimurium strains used in this study were derived from strain ATCC 14028 (CDC 6516-60). The construction of damΔ232 serovar Typhimurium (MT2188) was described previously (16).
Infection and challenge of mice and determination of bacterial load.
Oral infections were performed as described previously (16). Briefly, inoculum was prepared by growth of mutant and wild-type (WT) bacteria overnight in Luria-Bertani (LB) broth at 37°C with shaking. Female BALB/c mice (Charles River Laboratory), 6 to 8 weeks old, were perorally infected or perorally immunized by gastrointubation with 109 CFU of serovar Typhimurium in 0.2 ml of 0.2 M sodium phosphate buffer (pH 8.0). The mice were sacrificed after 6 days unless otherwise noted. Tissues were processed rapidly for RNA analyses and for bacterial-load determinations.
For challenge experiments, mice immunized with Dam− Salmonella were infected with 109 CFU of WT Salmonella 21 days postimmunization and sacrificed 6 days post-challenge. Colony counts per gram of tissue were determined by enumeration on LB agar plates: LB plates containing the base analog 2-aminopurine (2-AP) were used to distinguish the 2-AP-resistant WT (Dam+) challenge strain from the 2-AP-sensitive Dam− strain.
Cell culture and IFN treatment.
Mouse embryonic fibroblast (MEF) cells were generously provided by A. Koromilas, Lady Davis Institute, Montreal, Canada (1). MEF cells were maintained in Dulbecco modified Eagle medium supplemented with 10% (vol/vol) fetal bovine serum, 100 U of penicillin per ml and 100 μg of streptomycin per ml. IFN treatment was carried out with 1,000 U of human IFN-αA/D (PBL Biomedical Laboratories) per ml, which is fully active on mouse cells (36), for 6 h or with 250 U of mouse IFN-γ per ml for 24 h.
RNA isolation. (i) Cultured cells.
Total RNA was isolated from cultured MEF cells either untreated or IFN treated, as specified in the RNeasy Midi kit protocol (Qiagen).
(ii) Mouse tissue.
Mice were sacrificed, and the liver, spleen, Peyer's patches, cecum, heart, kidneys, lungs, and brain were rapidly excised and immediately frozen in liquid nitrogen. About 0.1 g of tissue was homogenized with Triazol reagent (Gibco-BRL) using 2-ml glass-to-glass homogenizers (Fisher). Total RNA was prepared as specified in the Triazol reagent protocol. RNA concentrations were determined spectrophotometrically, and RNA preparations were stored at −80°C.
Northern analysis.
Northern analysis of total RNA isolated from MEF cells or mouse tissues was performed essentially as described previously (13, 33). Briefly, total RNA (10 μg) from each sample was fractionated on denaturing formaldehyde-agarose gels. After electrophoresis, the gels were washed with diethyl pyrocarbonate-treated water and then with 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and transferred to nylon membranes (Hybond−N+; Amersham) using a capillary blotting apparatus, and the RNA was cross-linked to the membrane by exposure to UV light (Stratalinker 1800). The membranes were prehybridized at 48°C with ULTRAhyb solution (Ambion). The indicated 32P-labeled cDNA probes (50 to 100 ng) were prepared by the random-primer labeling procedure using [α-32P]TTP. The cDNA templates were prepared as restriction enzyme fragments, fractionated using agarose gel electrophoresis, and purified by the GENECLEAN II kit method (ISC BioExpress). The probes used were (i) PKR, prepared from the pGEX-KG-PKR plasmid containing the full-length mouse PKR protein kinase cDNA (2); (ii) ADAR1, prepared from the pBluescript-ADAR plasmid containing mouse ADAR, corresponding to exon 2 cDNA, generously provided by C. George of this laboratory; (iii) Mx1, derived from the pSP64-Mx1 plasmid containing the full-length mouse Mx1 GTPase cDNA, generously provided by J. Pavlovic, University of Zürich, Zürich, Switzerland; (iv) iNOS, prepared from the PUC19-iNOS plasmid, containing the full-length iNOS (Nos2) cDNA, generously provided by C. Nathan, Cornell Medical School, New York; (v) L3-2 ribosomal protein, derived from pGEM-4Z plasmid, generously provided by I. Campbell, Scripps Research Institute, La Jolla, Calif.; and, (vi) β-actin probe, as previously described (33). Approximately 106 cpm of probe was used per ml of the ULTRAhyb solution. Hybridization was carried out overnight at 48°C. The membranes were washed twice for 5 min, each time in 2 × SSC containing 0.1% sodium dodecyl sulfate, and then twice for 15 min in 0.1 × SSC containing 0.1% sodium dodecyl sulfate, at 48°C, prior to autoradiography or quantitation using a Bio-Rad GS-525 molecular imager system.
RT-PCR analysis.
Reverse transcription (RT) of 2.5 μg of mouse total RNA was carried out at 42°C, using random hexamer oligonucleotides (Amersham) as primers and Superscript RNase H− reverse transcriptase (Gibco-BRL). A 1-μl sample from the RT reaction was then subjected to PCR analysis, using Taq DNA polymerase (Roche). For detection of the ADAR1 a and b splice variants, the primer pair (+)1900 and (−)2577 was used in the amplification at an annealing temperature of 58°C. For CIITA amplification, the primers (+)525 (5′-GAAGAGACGCTTCCCGGAAG-3′) and (−)1079 (5′-CTTTCCTGGCTCTTGTTGC-3′) were used at an annealing temperature of 60°C. For glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplification, the primer pair (+)144 (5′-ACCCCTTCATTGACCTCAACT-3′) and (−)727 (5′-ATGCCAGTGAGCTTCCCGTT-3′) was used at an annealing temperature of 54°C. The PCR products were subjected to agarose gel electrophoresis and visualized by ethidium bromide under UV light. Quantitation was performed using an Alpha Innotech 4400 imager.
RESULTS
Salmonella Dam+ infection of mice leads to increased expression of IFN-stimulated genes compared to Dam− infection.
The actions of IFNs are mediated through the modulation of expression of target genes (ISGs). Little is known about the possible differential expression of ISGs in mice infected with S. enterica serovar Typhimurium. Here we examined the expression levels of host ISGs known to be regulated by either IFN-α/β, IFN-γ, or both, in serovar Typhimurium-infected mice. Four target genes were examined by Northern hybridization: PKR and ADAR1, which are inducible by both type IFN-α/β and IFN-γ in mouse and human cell lines (14); iNOS, which is selectively induced by IFN-γ; and the Mx1 GTPase, which is selectively induced by IFNα/β in mice and in cell culture (4, 34, 35).
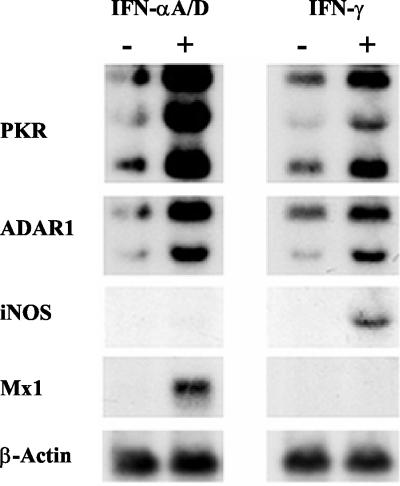
Because the BALB/c mouse model had been utilized to study the role of Dam in bacterial virulence (16) and because the mouse genotype can affect expression of cytokine genes (31), the IFN inducibility of the target genes was first verified by using cultured MEF cells derived from BALB/c mice. As anticipated, the transcript levels of ADAR1, PKR, and Mx1, but not of iNOS, were increased in MEF cells treated with recombinant IFN-αA/D relative to levels observed in untreated MEF cells (Fig. 1). Treatment of MEF cells with recombinant IFN-γ resulted in the accumulation of iNOS, PKR, and ADAR1, but not Mx1, transcripts (Fig. 1). Consistent with earlier observations, IFN induced three PKR transcripts, of ≈2.5, ≈4, and ≈6 kb (1), and single transcripts of ≈3.0 kb for Mx1 (14) and ≈4.0 kb for iNOS (26). Two IFN-inducible ADAR1 transcripts were observed, with the ≈4-kb transcript being somewhat more inducible than the ≈6.5-kb transcript. β-Actin transcript levels were comparable in untreated and IFN-treated MEF cells in culture (Fig. 1).
FIG. 1.
Northern analysis of IFN-α- and IFN-γ-responsive gene expression in cultured MEF cells. Total RNA was isolated from 80% confluent MEF cells, either left untreated (−) or treated (+) with recombinant IFN-αA/D or IFN-γ, as indicated. RNA samples (10 μg) were fractionated by formaldehyde-agarose gel electrophoresis, transferred to a nylon membrane, and then hybridized with 32P-labeled cDNA probes for mouse PKR, ADAR1, iNOS, and Mx1. The mouse β-actin probe was included as a control.
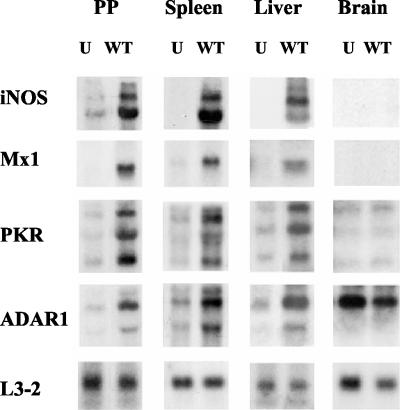
Next, the expression levels of IFN-regulated genes were examined in tissues recovered from BALB/c mice that were infected orally with wild-type (WT) S. enterica serovar Typhimurium (Fig. 2). RNA was isolated from eight tissues (liver, spleen, Peyer's patches, cecum, lung, heart, kidney, and brain) after different times of infection (1, 3, and 6 days) with Dam+ Salmonella, as well as from tissues of uninfected mice. Transcript levels of iNOS, Mx1, PKR, ADAR1, and the product of a housekeeping gene, ribosomal protein L3-2, were measured by Northern analysis. ISG induction was observed in most tissues examined on days 1 and 3 postinfection, but the induction at these early time points was subject to mouse-to-mouse variability and the level of ISG induction was lower and much less consistent than the level seen on day 6 (data not shown). Thus, day 6 was the time point analyzed in subsequent experiments. Salmonella infection resulted in the induction of ISG expression (Table 1), most significantly in the Peyer's patches, spleen and liver of WT-infected mice relative to uninfected mice (Fig. 2). Brains showed high levels of ADAR1 but low levels of iNOS, Mx1, and PKR transcripts, none of which were increased following WT infection (Fig. 2). Thus, the expression levels of IFN-responsive transcripts in tissues from WT-Salmonella-infected mice were increased in a manner comparable to that seen for cultured MEF cells treated with IFNs (Fig. 1).
FIG. 2.
Northern analysis of IFN-responsive gene expression in tissues recovered from uninfected and Salmonella-infected mice. Total RNA was isolated from Peyer's patches (PP), spleen, liver, and brain tissue (0.1 g) from BALB/c mice, either uninfected (U) or 6 days after oral infection with WT Salmonella. RNA samples (10 μg) were fractionated by formaldehyde-agarose gel electrophoresis, transferred to a nylon membrane, and then hybridized with 32P-labeled cDNA probes for mouse iNOS, Mx1, PKR, and ADAR1. The mouse ribosomal L3-2 protein probe was included as a control.
TABLE 1.
IFN-stimulated gene expression levels in tissues from mice infected with Dam+ or Dam− Salmonellaa
| Tissue | Strain | Level of expression ofb:
|
|||
|---|---|---|---|---|---|
| iNOS | Mx1 | ADAR1 | PKR | ||
| PP | WT | +++ | +++ | +++ | ++ |
| Dam− | + | UD | + | NI | |
| Liver | WT | +++ | ++ | +++ | ++ |
| Dam− | UD | + | ++ | + | |
| Spleen | WT | +++ | + | + | ++ |
| Dam− | UD | + | + | + | |
| Cecum | WT | + | UD | NI | ++ |
| Dam− | NI | UD | NI | + | |
| Lungs | WT | ++ | + | NI | + |
| Dam− | NI | NI | NI | + | |
| Heart | WT | UD | + | NI | ++ |
| Dam− | UD | + | NI | ++ | |
| Kidneys | WT | UD | UD | + | + |
| Dam− | UD | UD | + | + | |
| Brain | WT | UD | UD | NI | NI |
| Dam− | UD | UD | NI | NI | |
The induction levels of iNOS, Mx1, ADAR1, and PKR transcripts were determined from Northern analysis of RNA samples isolated from tissues of uninfected mice and from mice 6 days after infection with either Dam+ or Dam− Salmonella. Northern analysis of RNA samples were quantified using a phospho-imager system.
NI, no increase in mRNA transcript amount from the level seen in uninfected tissue; +, mRNA amount increased less than two-fold in infected compared to uninfected tissue; ++, mRNA amount increased two- to fourfold in infected compared to uninfected tissue; +++, mRNA amount increased by more than fourfold in infected compared to uninfected tissue; UD, transcript level undetectable.
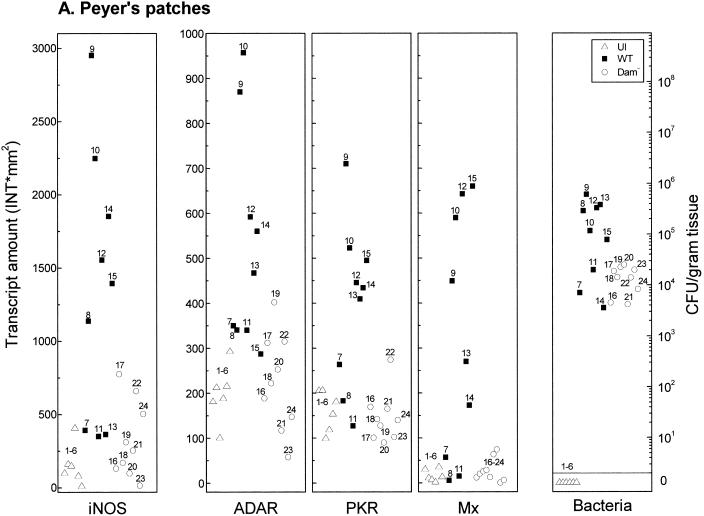
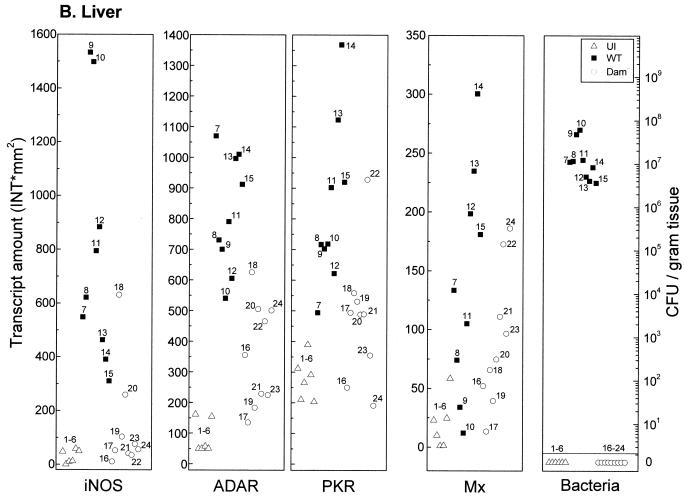
Salmonella Dam− strains are attenuated for virulence and elicit fully protective immune responses in vaccinated mice (15, 16). To begin to understand the molecular basis of both the virulence attenuation and the elicitation of immunity, the expression levels of ISGs were determined in vaccinated (Dam−) and nonvaccinated mice. Transcript levels of iNOS, PKR, ADAR1, and Mx1 were determined in tissues recovered from uninfected mice or from mice infected for 6 days with either the Dam− mutant or WT (Dam+) S. enterica serovar Typhimurium. Ribosomal protein L3-2 was used as an internal control to normalize target transcript amounts between different tissues of individual animals. As evidenced by measurement of transcript levels, mice infected with Dam− Salmonella exhibited a diminished capacity to induce all four ISGs in Peyer's patches (Fig. 3A), liver (Fig. 3B), and spleen (Fig. 3C) compared to wild-type (Dam+) infected mice.
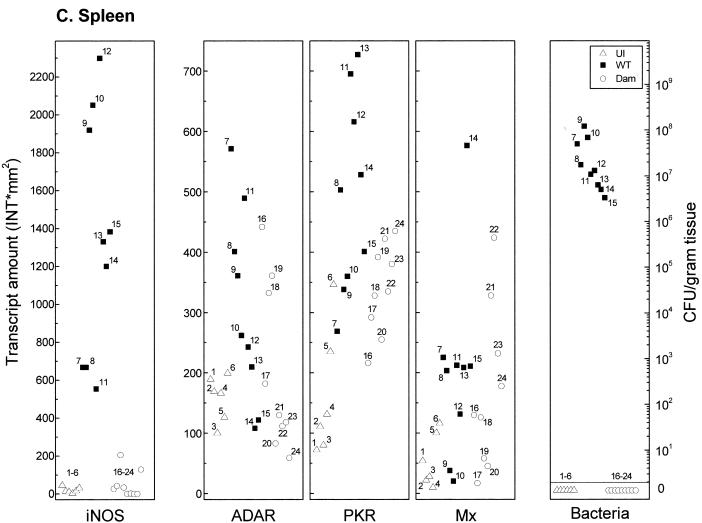
FIG. 3.
Comparison of IFN-responsive transcript levels and bacterial counts in tissues recovered from mice infected with Dam+ or Dam− Salmonella. Total RNA was isolated, and bacteria were enumerated from individual tissue samples taken from uninfected (U) BALB/c mice (open triangles, numbered 1 to 6), from mice 6 days after infection with WT Salmonella (solid squares, numbered 7 to 15), and from mice infected with Dam− Salmonella (open circles, numbered 16 to 24). (A) Peyer's patches; (B) liver; (C) spleen. For measurement of transcript amounts (INT∗mm2), RNA samples (10 μg) were analyzed by Northern analysis with 32P-labeled cDNA probes for mouse iNOS, ADAR1, PKR, Mx1, and L3-2, as shown for Fig. 1. Hybridization signals were quantitated using a GS525 phosphorimaging system. Transcript levels of the four IFN-responsive mRNAs were normalized between samples by using the constitutively expressed L3-2 ribosomal protein transcript as the standard.
Quantitation of mRNA levels using tissues from WT-infected mice, Dam−-infected mice, and uninfected mice showed that while differences between individual animals can occur, the iNOS, ADAR1, PKR, and Mx1 transcript levels were typically elevated in tissues from WT-infected mice compared to uninfected or Dam−-infected animals. It is noteworthy that although iNOS levels were increased in Peyer's patches from some of the Dam−-infected mice, they remained quite low relative to the increases seen in the individual WT-infected animals (Fig. 3A). iNOS levels in the liver (Fig. 3B) and spleen (Fig. 3C), with minor exceptions, were comparable between uninfected and Dam−-infected mice. Basal iNOS transcript levels in the eight tissues examined from uninfected mice were generally very low and bordered on the limit of detection under our standard conditions of Northern analysis, which readily detected iNOS transcripts in all tissues from WT-infected mice except the heart, kidneys, and brain (Table 1).
Bacterial load represents one parameter that may affect the magnitude of induction of ISGs at a given tissue site. Expression levels of iNOS, PKR, ADAR1, and Mx1 and bacterial counts were determined in tissues recovered from uninfected mice or from mice on day 6 after infection with either Dam− or Dam+ serovar Typhimurium (Expression levels and bacterial counts corresponding to individual tissues are labeled with unique identifiers in Fig. 3.) Notably, tissues associated with the highest level of ISG expression did not always show the highest bacterial count in infections with Dam+ or Dam− organisms (Fig. 3). For example, in both the liver (Fig. 3B) and the spleen (Fig. 3C), bacterial loads were strikingly different between Dam+ and Dam− infections, with significant numbers of Dam+ organisms (≈5 × 106 to 1 × 108 CFU/g) but below threshold levels for Dam− mutant bacteria. However, ADAR1, PKR, and Mx1 expression levels generally were elevated in the liver and spleen of Dam−-infected mice relative to uninfected mice (Fig. 3B and C), suggestive of the distant action of a signaling molecule. By contrast, Peyer's patches (Fig. 3A) showed more comparable levels of Dam+ and Dam− organisms, yet ADAR1, PKR, and Mx1 transcript levels were much lower than those of Dam+-infected animals, similar to levels observed in uninfected mice.
Expression of CIITA transcripts in tissue sites of mice infected with Dam+ and Dam− Salmonella.
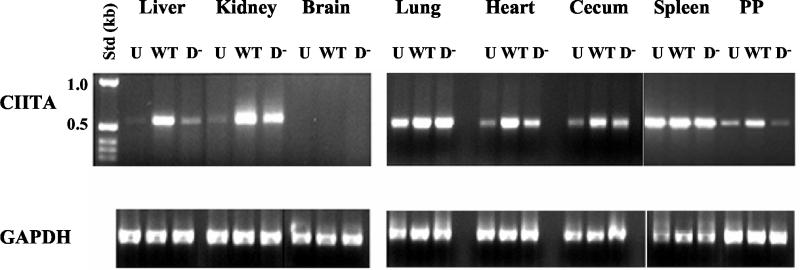
The transcription factor CIITA activates the transcription of major histocompatibility complex class II antigen genes and is encoded by an IFN-γ-inducible gene (27, 35). CIITA RNA transcript levels can be low in some tissues even following induction by IFN-γ (32, 35). Since induction of CIITA expression was not readily detected by Northern analysis of RNA isolated from tissues of Dam+ Salmonella-infected mice, RT-PCR analysis was used to assess CIITA transcript levels in eight mouse tissues (liver, kidney, brain, lungs, heart, cecum, spleen, and Peyer's patches).
CIITA RNA amounts were increased in most tissues following infection with WT Salmonella, with some notable exceptions (Fig. 4). The spleen, for example, showed high basal CIITA RNA expression levels in the absence of Salmonella infection, consistent with other reports (32); by contrast, no CIITA RNA was detected in the brain of either uninfected or infected mice. Note that although no Dam− Salmonella organisms were recovered from kidneys, lungs, heart, or cecum at 6 days postinfection, increased CIITA expression was observed in these tissues compared to those of uninfected mice. These data indicate that factors that induce CIITA expression can act distal to the site of Salmonella infection.
FIG. 4.
Expression of transcription factor CIITA in mouse tissues. RNA was isolated from the indicated tissues of BALB/c mice that were uninfected (U) or from mice 6 days after infection with WT or Dam− (D−) Salmonella. RNA was reverse transcribed using random primers, and then PCR was performed using primer pairs specific for either CIITA or GAPDH as a control. Products obtained for liver, kidney, brain, lung, heart, cecum, spleen, and Peyer's patch (PP) samples are shown and correspond to the predicted 554-bp CIITA and 583-bp GAPDH fragments. Std (kb): numbers correspond to standard DNA fragments in kilobases.
Differential accumulation of ADAR1 alternative splice variants in cultured MEF cells and infected mouse tissues.
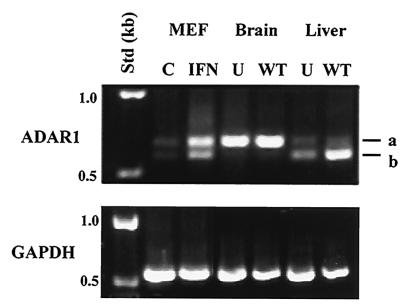
The RNA editing enzyme ADAR1 is expressed from two distinct splice variants, designated a and b, in J774.1 cultured macrophage-like cells. The two splice variants can be distinguished by PCR analysis: the b form is a 5′ splice variant with a 78-nucleotide deletion within exon 7 (23). The ADAR1 splice variant proteins display differential A-I editing activities for some natural RNA substrates, for example, the serotonin-2C and glutamate receptor pre-mRNA sites (24, 35). Figure 5 shows that both the a and b splice variants of ADAR1 were present in cultured MEF cells. Additionally, the a and b splice variants were increased following IFN treatment, with the a variant being more abundant than the b variant.
FIG. 5.
Differential expression of ADAR1 splice variants in mouse tissues. PCR products were generated using cDNA templates prepared with RNA isolated from cultured MEF cells that were untreated (C) or treated with IFN-αA/D (IFN) and from the brain and liver of uninfected (U) BALB/c mice or from mice 6 days after infection with WT Salmonella. a and b designate the PCR fragments corresponding to the ADAR1-a and b splice variants. Expression of GAPDH in mouse tissues and MEF cells was analyzed by PCR using the same cDNA templates as were used for ADAR1 expression. Std (kb): numbers correspond to standard DNA fragments in kilobases.
Next, we examined the ADAR1 splice variants in the brain and liver of uninfected and infected mice (Fig. 5). The a splice variant of ADAR1 was constitutively expressed at high levels relative to the b variant in brain tissue derived from uninfected and WT-Salmonella-infected mice. In contrast, while both the a and b variants were present in liver tissue from BALB/c mice, the b variant was most abundant and its expression was significantly increased following infection with WT Salmonella. These data are consistent with the conclusion that ADAR1 splice variants are expressed in a tissue-specific fashion (23). Moreover, expression levels of an ADAR1 splice variant changed during Dam+ infection, suggesting that differential A-I RNA editing activity for RNA substrates may occur in some infected tissue sites.
Mice immunized with Dam− Salmonella show reduced IFN-stimulated gene expression after challenge with WT bacteria.
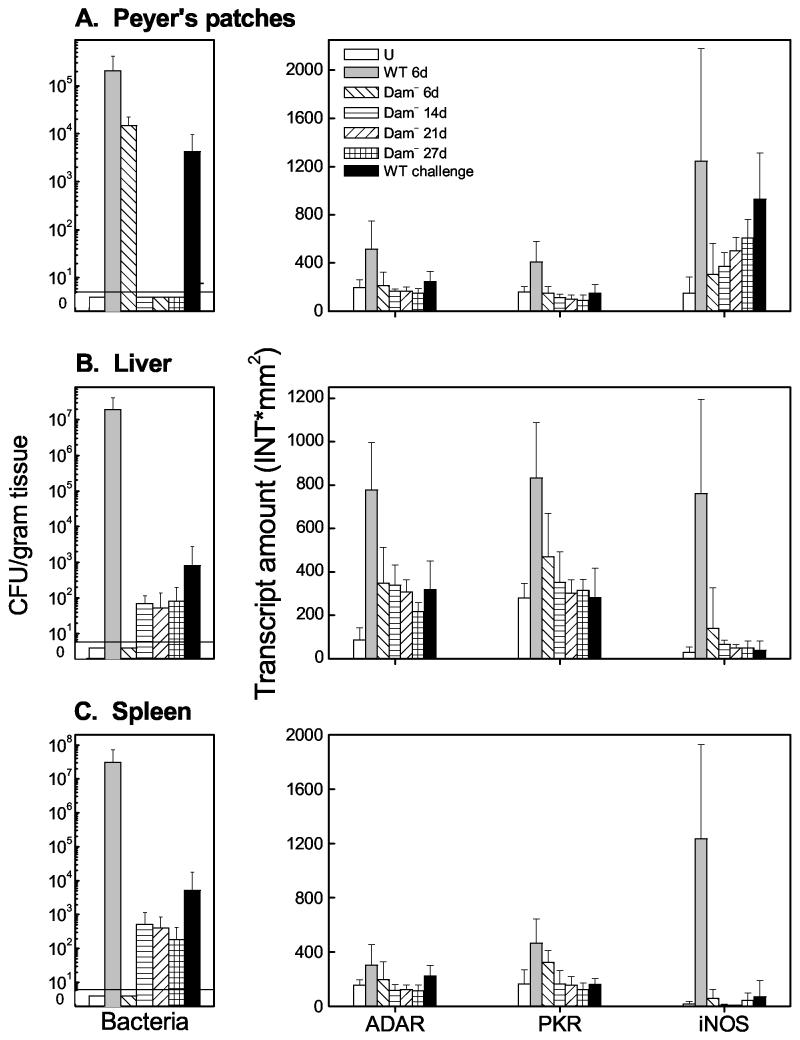
Mice infected with Dam− Salmonella failed to express ISGs at the levels observed after infection with WT Salmonella (Fig. 3). Here, ISG expression levels were measured in mice vaccinated with Dam− Salmonella and subsequently challenged with WT bacterial 21 days postimmunization. WT Salmonella organisms were either not detected or detected at significantly smaller numbers 6 days post-challenge (Fig. 6). The transcript levels of ADAR1 and PKR remained relatively low in the Peyer's patches, liver, and spleen (Fig. 6) compared to the levels observed in nonvaccinated mice 6 days after infection with WT Salmonella. Similarly, the level of iNOS transcript in Dam−-immunized mice subsequently challenged with WT bacteria was also low in the liver and spleen 6 days post-challenge (Fig. 6B and C). These data indicate that mice vaccinated with Dam− Salmonella show a diminished capacity to induce both IFN-α/β- and IFN-γ-regulated genes after WT challenge.
FIG.6.
Comparison of IFN-responsive transcript levels and bacterial counts in mice immunized with Dam− Salmonella and challenged with WT (Dam+) Salmonella. Total RNA was isolated, and bacteria were enumerated from tissue samples (Peyer's patches [A], liver [B], and spleen [C]) taken from groups of 6 to 10 uninfected (U) BALB/c mice or from mice infected with WT or Dam− Salmonella. Mice were orally infected with either Dam+ or Dam− Salmonella, and tissues were recovered at 6, 14, 21, or 27 days postinfection as indicated. WT challenge refers to mice that were infected with Dam− Salmonella, challenged with WT Salmonella on day 21, and had their tissues harvested 6 days after the WT challenge. Bacterial colony counts (CFU per gram) were obtained from harvested tissues. At 6 days post-challenge, no Dam− organisms (from the immunization) were recovered from the Peyer's patches or spleen; Dam− organisms from the immunization were recovered from the livers of two of six mice (125 and 100 CFU/g, respectively). For measurement of transcript amounts (INT∗mm2), RNA samples (10 μg) were analyzed by Northern hybridization with 32P-labeled cDNA probes for mouse ADAR1, PKR, iNOS, and L3-2, as shown for Fig. 3. Hybridization signals were quantitated using a GS525 phosphorimager. Transcript amounts of the three IFN-responsive mRNAs, ADAR, PKR, and iNOS, were normalized using the constitutively expressed L3-2 ribosomal protein transcript as the standard. Standard error measurements were determined from independent analyses of six to nine mice and are indicated by error bars.
DISCUSSION
Very little is known about the expression of IFN-stimulated enzymes following bacterial infection of mice or the relative capacity of an attenuated bacterial vaccine strain to affect the expression of IFN-responsive target genes in infected animals. Because of the potential for synergistic activation of target ISG transcription by different cytokine activators including IFN-α/β and IFN-γ (7, 37, 39) and because IFN signaling can be blocked or otherwise modulated following pathogen infection (20, 35), we determined directly the expression levels of ISG transcripts in vivo. Examination of mouse tissues following Salmonella infection by the oral route showed a tissue-specific elevated expression of host genes known to be regulated by either IFN-α/β (Mx1) or IFN-γ (CIITA and iNOS) or both (ADAR1 and PKR). Mice infected with the attenuated Salmonella Dam− vaccine strain showed a considerably reduced expression of the same set of ISGs even under conditions of comparable bacterial load at a given tissue site (e.g., Peyer's patches). Such vaccinated animals also exhibited reduced expression of ISGs after infection with the WT (Dam+) strain (Fig. 6). The differential expression of ISGs in infected hosts is consistent with a possible role in the host response to bacterial infection (6, 39).
IFN-γ can play an important role in host protection against microbial infections, as evidenced from studies with knockout animals that functionally lack either IFN-γ or a component of the IFN-γ signal transduction pathway (4, 35, 39). The role of IFN-α/β in the defense against bacteria has been considerably less well studied than that of IFN-γ, although limited interactions and functional relationships have been established. For example, treatment of macrophages with IFN-α or IFN-β enhanced their antimicrobial activity against Mycobacterium avium and other pathogens (43). Additionally, IFN-α and IFN-β are produced on bacterial or protozoal infection of macrophages and fibroblasts in vitro and during the immune response to these pathogens in vivo (43). To our knowledge, this is the first report that the expression of ISGs, Mx1, Adar1, and Pkr, is significantly elevated following bacterial infection. This finding indicates an involvement of IFN-α/β in the host response to Salmonella infection, since the Mx1 promoter is IFN-α/β specific and the Mx1 gene is not inducible directly by IFN-γ (4, 14, 35). It is possible that IFN-α/β induction during Salmonella infection, implied by the increased expression of IFN-α/β response genes (Mx1, Adar1, and Pkr), is involved in regulation of IFN-γ expression (28).
The ability of lipopolysaccharide (LPS) to stimulate IFN-γ production is established (38, 45). Both WT (Dam+) and Dam− Salmonella carry LPS, yet their ability to modulate the expression of IFN-α/β- and IFN-γ-selective ISG gene targets differed significantly, as shown here. An interesting possibility is that envelope differences exist between the mutant and WT Salmonella strains. That is, Dam− strains may alter the form or presentation of the LPS in a manner that gives rise to a differential ability of Dam+ and Dam− bacteria to induce IFNs. Indeed, consistent with the possibility, it was recently reported that the Dam− mutation disrupts Salmonella cell envelope integrity (30). The ability of virulence genes such as Dam to affect the host IFN response to infection is further illustrated by studies with Salmonella PhoP− and Aro− mutant strains (3, 42). PhoP− Salmonella shows significant attenuation in IFN-γ−/− mutant mice relative to normal mice. By contrast to the PhoP− mutant, the Aro− mutant of Salmonella is highly virulent in mice lacking IFN-γ but not in normal mice, suggesting that the role of the IFN response in protection versus pathogenesis is dependent, in part, on the genetic background of the bacterial pathogen (3, 42).
Considerable progress has been made in the characterization of ISG transcripts in cell culture systems, but much less is known from studies in animals (35). For example, expression and regulation of the A-I editing enzyme ADAR1 has been extensively studied in human cell lines, where alternative promoters have been shown to drive the expression of the Adar1 gene (13). We established here that the single Adar1 mouse gene, which maps to chromosome 3F2 (44), encodes two different sized mRNA transcripts (≈4 and ≈6.5 kb) both in mouse MEF cells in culture and in mouse tissues including Peyer's patches, liver, and spleen. Interestingly, both ADAR1 transcripts were detected in all tissues examined, except for the brain, in which only the≈6.5-kb transcript was detectable, with comparable amounts seen in brains from uninfected and infected animals (Fig. 2 and 5). These results suggest that expression of the murine ADAR1 gene in mice and in cell culture is driven by a combination of constitutively active and inducible promoters similar to that established for human ADAR1 in cell culture (13). Notably, these results also imply that the integrity of the blood-brain barrier in WT-Salmonella-infected mice was not compromised, even at 6 days postinfection when death was imminent.
Similar to ADAR1 (13), transcription of the CIITA gene is driven by differential useage of multiple promoters that mediate constitutive and IFN-γ-inducible expression of the CIITA factor in different cells and tissues (27, 35). Most cell types do not express significant basal CIITA in the absence of IFN-γ (27, 29, 35). Consistent with this observation, we found that CIITA transcript levels were low in infected mice but were increased in most tissues, for example the liver, kidneys, heart, and cecum, following infection with WT Salmonella. However, consistent with earlier reports (31), CIITA basal expression already was high in the spleens of uninfected mice and was not further increased following infection.
By contrast to our results seen for induced CIITA expression with whole-tissue-derived RNA from Dam+-infected animals, it has been reported that CIITA expression is reduced during dendritic cell maturation in culture by a variety of antigen-mediated stimuli including Salmonella infection (22). The basis of this difference may reside in the cells examined and the conditions of infection. CIITA expression is not limited to dendritic cells since other professional antigen-presenting cells such as B cells and macrophages also express CIITA; moreover, nonprofessional antigen-presenting cells such as T cells, endothelial cells, and fibroblasts express CIITA under certain conditions (17). Further, CIITA expression in dendritic cells is largely driven by promoters I and III, not by the inducible promoter IV that is responsive to IFN-γ (27).
iNOS is associated with both antimicrobial and immunosuppressive activity (26). The specific induction of iNOS in the Peyer's patches (but not the deep tissues) of Dam−-Salmonella-infected mice may contribute to the reduced capacity of a subsequent WT challenge to either induce IFN-responsive genes or establish a successful infection (Fig. 6). The robust induction of iNOS transcript levels in Dam+-Salmonella-infected mice 6 days postinfection might well represent the synergistic effect of IFN-γ induced by the bacterial infection as well as bacterial components such as LPS, acting at the gamma-activated sequence element of the iNOS promoter (11). The differences that we observed in iNOS transcript levels in mice infected with Dam+ versus Dam− Salmonella agree well with the recent finding for NO gas production by infected murine splenocytes, wherein NO levels following immunization with the Dam− Salmonella strain were well below those observed after infection with WT or AroA− strains (15).
This report describes the enhanced expression of host target genes regulated by IFN-α/β, in addition to those regulated by IFN-γ, in response to Salmonella infection. The differential expression of ISGs at tissue sites of mice infected with WT Salmonella compared to avirulent Dam− mutant Salmonella suggests a possible role for ISGs in the host response to bacterial infection. Further investigation of the role of differential expression of ISGs will provide insight into the molecular basis of bacterial pathogenesis and the design of therapeutics to control either the infecting bacteria or the host immune responses that may contribute to bacterial pathogenicity.
Acknowledgments
We thank Robert Sinsheimer and Rafael Curiel for critically reading the manuscript. We thank Itay Shtrichman and Carol Inatsuka for technical assistance.
This work was supported by National Institutes of Health research grants AI-12520 and AI-20611 (to C.E.S.); the Santa Barbara Cottage Hospital Research Program, USDA research grant 2000-02539, and University-Industry Cooperative Research BioStar grant (2001-1287) (to M.J.M); and a postdoctoral fellowship grant from the Cancer Center of Santa Barbara (to D.M.H.).
Editor: D. L. Burns
REFERENCES
- 1.Abraham, N., D. F. Stojdi, P. I. Ducan, N. Methot, T. Ishii, M. Dube, B. C. Vanderhyden, H. L. Atkins, D. A. Gray, M. W. McBurney, A. E. Koromilas, E. G. Brown, N. Sonenberg, and J. B. Bell. 1999. Characterization of transgenic mice with targeted disruption of the catalytic of the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem. 274:5953-5962. [DOI] [PubMed] [Google Scholar]
- 2.Aebi, M., J. Fah, N. Hurt, C. E. Samuel, D. Thomis, L. Bazzigher, J. Pavlovic, O. Haller, and P. Staeheli. 1989. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol. Cell. Biol. 9:5062-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao, S., K. W. Beagley, M. P. France, J. Shen, and A. J. Husband. 2000. Interferon-γ plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology 99:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biron, C. A., and G. C. Sen. 2001. Interferons and other cytokines, p. 321-351. In D. M. Knipe, P. M. Howley, D. E. Griffin, M. Martine, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 5.Bochm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 6.Bogdan, C. 2000. The function of type I interferons in antimicrobial immunity. Curr. Opin. Immunol. 12:419-424. [DOI] [PubMed] [Google Scholar]
- 7.Czarniecki, C. W., C. W. Fennie, D. B Powers, and D. A. Estell. 1984. Synergistic antiviral and antiproliferative activities of Escherichia coli-derived human alpha, beta, and gamma interferons. J. Virol. 49:490-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. 2001. A Salmonella DNA adenine methylase mutant elicits protective immune responses to homologous and heterologous serovars in chickens. Infect. Immun. 69:7950-7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. Salmonella DNA adenine methylase mutants prevent colonization of newly hatched chickens by homologous and heterologous serotypes. Int. J. Food Microbiol., in press. [DOI] [PubMed]
- 10.Eisenstein, T. K. 1999. Mucosal immune defense: the Salmonella typhimurium model, p. 51-109. In Y. Paterson, (ed.), Intracellular bacterial vaccine vectors. Wiley-Liss, Inc., New York, N.Y.
- 11.Gao, J., D. C. Morrison, T. J. Parmely, S. W. Russell, and W. J. Murphy. 1997. An interferon-γ-activated site (GAS) is necessary for the full expression of the mouse iNOS gene in response to interferon-γ and lipopolysaccharide. J. Biol. Chem. 272:1226-1230. [DOI] [PubMed] [Google Scholar]
- 12.Garcia del Portillo, F., M. G. Pucciarelli, and J. Casadesus. 1999. DNA adenine methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc. Natl. Acad. Sci. USA 96:11578-11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George, C. X., and C. E. Samuel. 1999. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structure that initiate from different promoters, one constitutively active and the other interferon inducible. Proc. Natl. Acad. Sci. USA 96:4621-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haller, O., M. Frese, and G. Kochs. 1998. Mx proteins: mediators of innate resistance to RNA viruses. Rev. Sci. Technol. Off. Int. Epizootol. 17:220-230. [DOI] [PubMed] [Google Scholar]
- 15.Heithoff, D. M., E. Y. Enioutina, R. A. Daynes, R. L. Sinsheimer, D A. Low, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect. Immun. 69:6725-6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heithoff, D. M., R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284:967-970. [DOI] [PubMed] [Google Scholar]
- 17.Holling, T. M., N. van der Stoep, E. Quinten, and P. J. van den Elsen. 2002. Activated human T cells accomplish MHC class II expression through T cell-specific occupation of class II transactivator promoter III. J. Immunol. 168:763-770. [DOI] [PubMed] [Google Scholar]
- 18.Hone, D. M., M. T. Shata, D. W. Pascual, and G. K. Lewis. 1999. Mucosal vaccination with Salmonella vaccine vectors, p. 171-221. In Y. Paterson (ed.), Intracellular bacterial vaccine vectors. Wiley-Liss, Inc., New York, N.Y.
- 19.Jones, B. D., and S. Falkow. 1996. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Genet. 14:533-561. [DOI] [PubMed] [Google Scholar]
- 20.Katze, M. G. 1995. Regulation of the interferon-induced PKR: can viruses cope? Trends Microbiol. 3:75-78. [DOI] [PubMed] [Google Scholar]
- 21.Kim, C. H., M. C. Johnson, J. D. Drennan, B. E. Simon, E. Thomann, and J. A. Leong. 2000. DNA vaccines encoding viral glycoproteins induce nonspecific immunity and Mx protein synthesis in fish. J. Virol. 74:7048-7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landmann, S., A. Muhlethaler-Mottet, L. Bernasconi, T. Suter, J. M. Waldburger, K. Masternak, J. F. Arrighi, C. Hauser, A. Fontana, and W. Reith. 2001. Maturation of dendritic cells is accompanied by rapid transcriptional silencing of class II transactivator (CIITA) expression. J. Exp. Med. 194:379-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, Y., C. X. George, J. B. Patterson, and C. E. Samuel. 1997. Functionally distinct double-stranded RNA binding domains associated with alternative splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase. J. Biol. Chem. 272:4419-4428. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Y., R. B. Emson, and C. E. Samuel. 1999. Serotonin-2C receptor pre-mRNA editing in rat brain and in vitro by splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase ADAR1. J. Biol. Chem. 274:18351-18358. [DOI] [PubMed] [Google Scholar]
- 25.Low, D. A., N. J. Weyand, and M. J. Mahan. 2001. The roles of DNA methylation in the control of bacterial gene expression and virulence. Infect. Immun. 69:7197-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacMicking, J., Q. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 27.Muhlethaler-Mottet, A., L. A. Otten, V. Steimle, and B. Mach. 1997. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 16:2851-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen, K. B., L. P. Cousens, L. A. Doughty, G. C. Pien, J. E. Durbin, and C. A. Biron. 2000. Interferon α/β-mediated inhibition and promotion of interferon γ: STAT1 resolves a paradox. Nat. Immunol. 1:70-76. [DOI] [PubMed] [Google Scholar]
- 29.Piskurich, J. F., Y. Wang, M. W. Linhoff, L. C. White, and J. P. Ting. 1998. Identification of distinct regions of 5′ flanking DNA that mediate constitutive, IFN-gamma, STAT1, and TGF-beta-regulated expression of the class II transactivator gene. J. Immunol. 160:233-240. [PubMed] [Google Scholar]
- 30.Pucciarelli, M. G., A. I. Prieto, J. Casadesus, and F. Garcia-Del Portillo. 2002. Envelope instability in DNA adenine methylase mutants of Salmonella enterica. Microbiology 148:1171-1182. [DOI] [PubMed] [Google Scholar]
- 31.Raj, N. B., S. C. Cheung, I., Rosztoczy, and P. M. Pitha. 1992. Mouse genotype affects inducible expression of cytokine genes. J. Immunol. 148:1934-1940. [PubMed] [Google Scholar]
- 32.Ramassar, V., N. Goes, M. Hobart, and P. F. Halloran. 1996. Evidence for the in vivo role of class II transactivator in basal and IFNγ induced class II expression in mouse tissues. Transplantation 62:1901-1907. [DOI] [PubMed] [Google Scholar]
- 33.Rende-Fournier, R., L. G. Ortega, C. X. George, and C. E. Samuel. 1997. Interaction of the human protein kinase PKR with the mouse homolog occurs via the N-terminal region of PKR and does not inactivate autophosphorylation activity of mouse PKR. Virology 238:410-423. [DOI] [PubMed] [Google Scholar]
- 34.Samuel, C. E. 1991. Antiviral action of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activity. Virology 183:1-11. [DOI] [PubMed] [Google Scholar]
- 35.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuel, C. E., and G. S. Knutson. 1982. Mechanism of interferon action. Kinetics of induction of the antiviral state and protein phosphorylation in mouse fibroblasts treated with natural and cloned interferons. J. Biol. Chem. 257:11791-11795. [PubMed] [Google Scholar]
- 37.Samuel, C. E., and G. S. Knutson. 1983. Mechanism of interferon action: human leukocyte and immune interferons regulate the expression of different genes and induce different antiviral states in human amnion U cells. Virology 130:474-484. [DOI] [PubMed] [Google Scholar]
- 38.Salkowski, C. A., G. R. Detore, and S. N. Vogel. 1997. Lipopolysaccharide and monophosphoryl lipid A differentially regulate interleukin-12, gamma interferon, and interleukin-10 mRNA production in murine macrophages. Infect. Immun. 65:3239-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shtrichman, R., and C. E. Samuel. 2001. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 4:251-259. [DOI] [PubMed] [Google Scholar]
- 40.Sirard, J. C., F. Niedergang, and J. P. Kraehenbuhl. 1999. Live attenuated Salmonella: a paradigm of mucosal vaccines. Immunol. Rev. 171:5-26. [DOI] [PubMed] [Google Scholar]
- 41.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 42.VanCott, J. L., S. N. Chatfield, M. Roberts, D. M. Hone, E. L. Hohmann, D. W. Pascual, M. Yamamoto, H. Kiyono, and J. R. McGhee. 1998. Regulation of host immune response by modification of Salmonella virulence genes. Nat. Med. 4:1247-1252. [DOI] [PubMed] [Google Scholar]
- 43.Waldburger, J. M., K. Masternak, A. Muhlethaler-Moffet, J. Vilard, M. Peretti, S. Landman, and W. Reith. 2000. Lessons from the bare lymphocyte syndrome: molecular mechanisms regulating MHC class II expression. Immunol. Rev. 178:148-165. [DOI] [PubMed] [Google Scholar]
- 44.Weier, H. U. G., C. X. George, R. A. Lersch, S. Breitweser, J. F. Cheng, and C. E. Samuel. 2000. Assignment of the RNA-specific adenosine deaminase gene (Adar) to mouse chromosome 3F2 by in situ hybridization. Cytogenet. Cell Genet. 89:214-215. [DOI] [PubMed] [Google Scholar]
- 45.Young, H. A. 1996. Regulation of interferon-γ gene expression. J. Interferon Cytokine Res. 16:563-568. [DOI] [PubMed] [Google Scholar]