Abstract
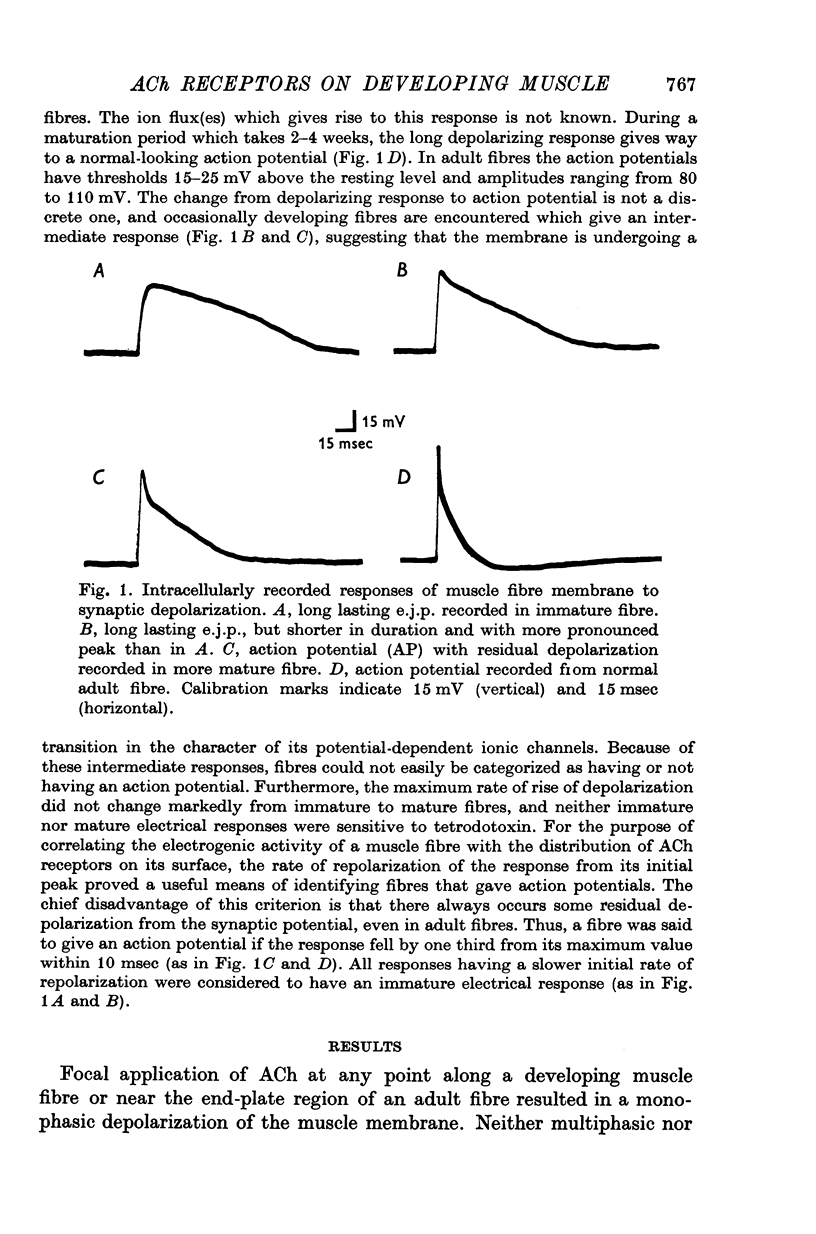
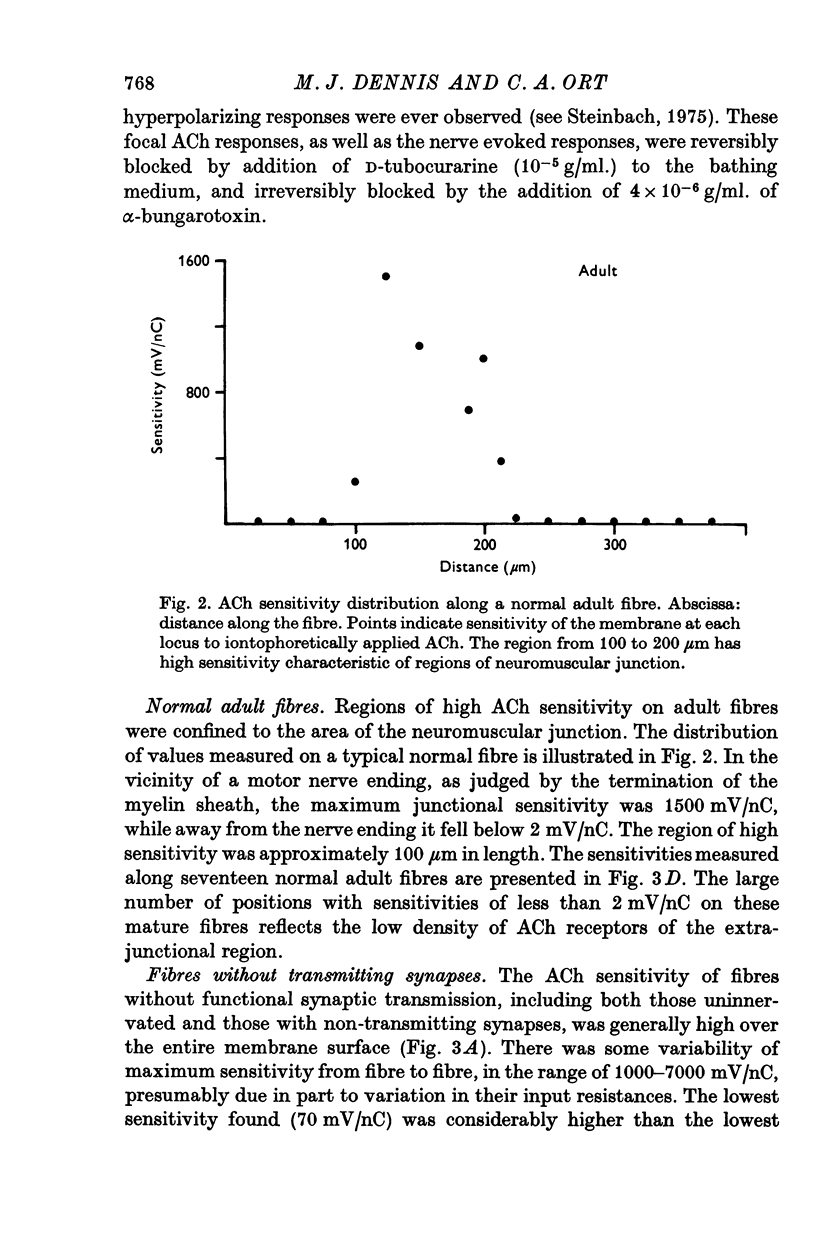
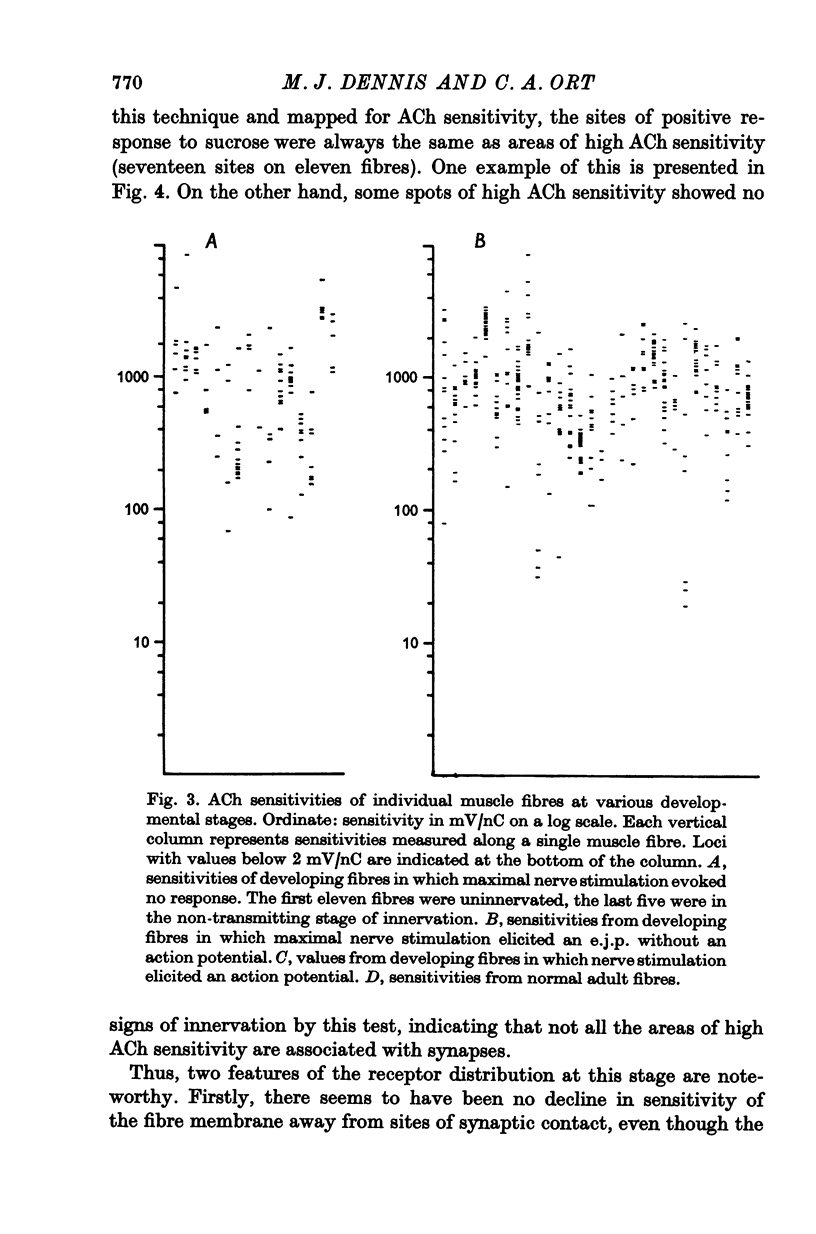
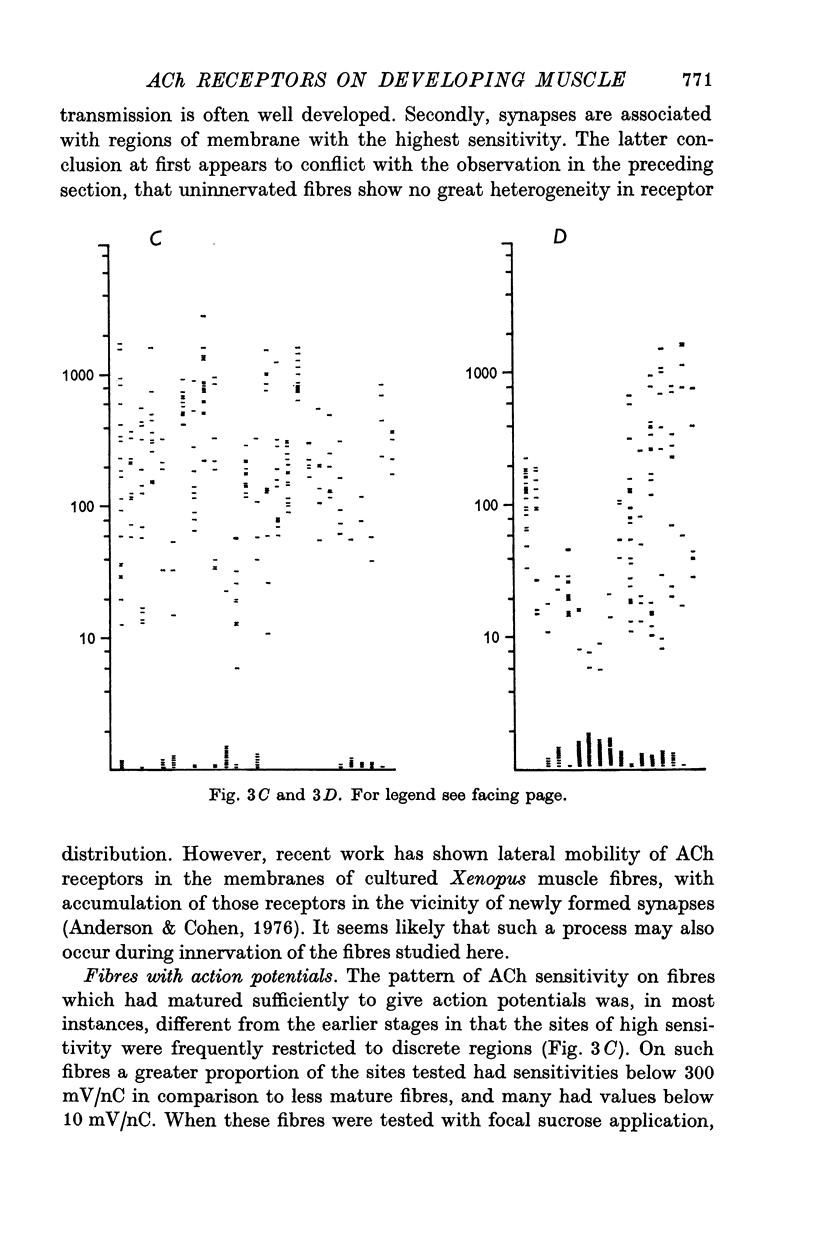
1. Muscle fibres developing during limb regeneration were examined for responsiveness to acetylcholine (ACh) applied iontophoretically along the membrane. 2. Fibres which were uninnervated as well as those with non-transmitting synapses had high over-all sensitivities, with only minor variations along their length. 3. Functionally innervated fibres in which depolarization did not yet elicit action potentials had high over-all sensitivities, even when the synaptic potentials ahd amplitudes of 40-50 mV. In these the membrane in the vicinity of synapses tended to have sensitivities above the background level. 4. Upon the appearance of action potentials, several weeks after fibre innervation, the responsiveness to ACh began to decline in synapse free regions of the membrane. In mature muscle the sensitivity to ACh is restricted to sites of synaptic contact.
Full text
PDF


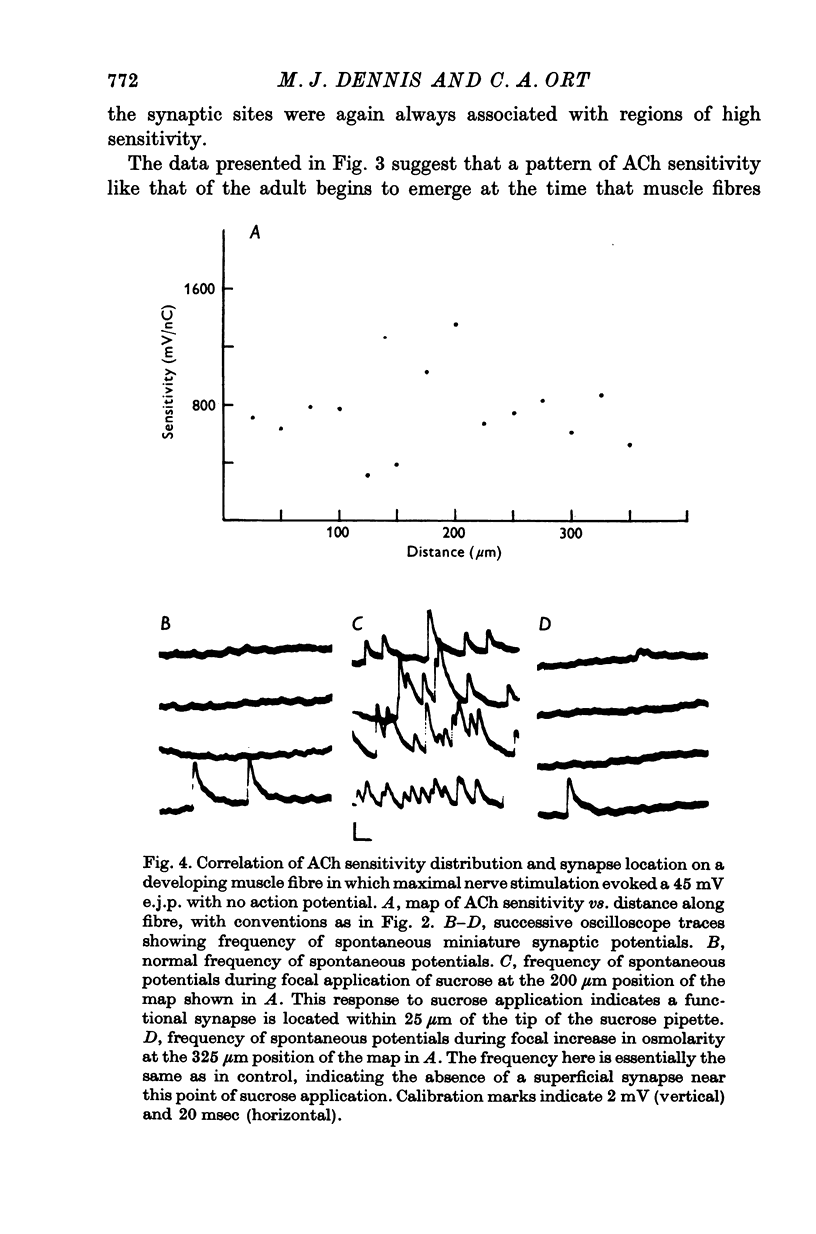
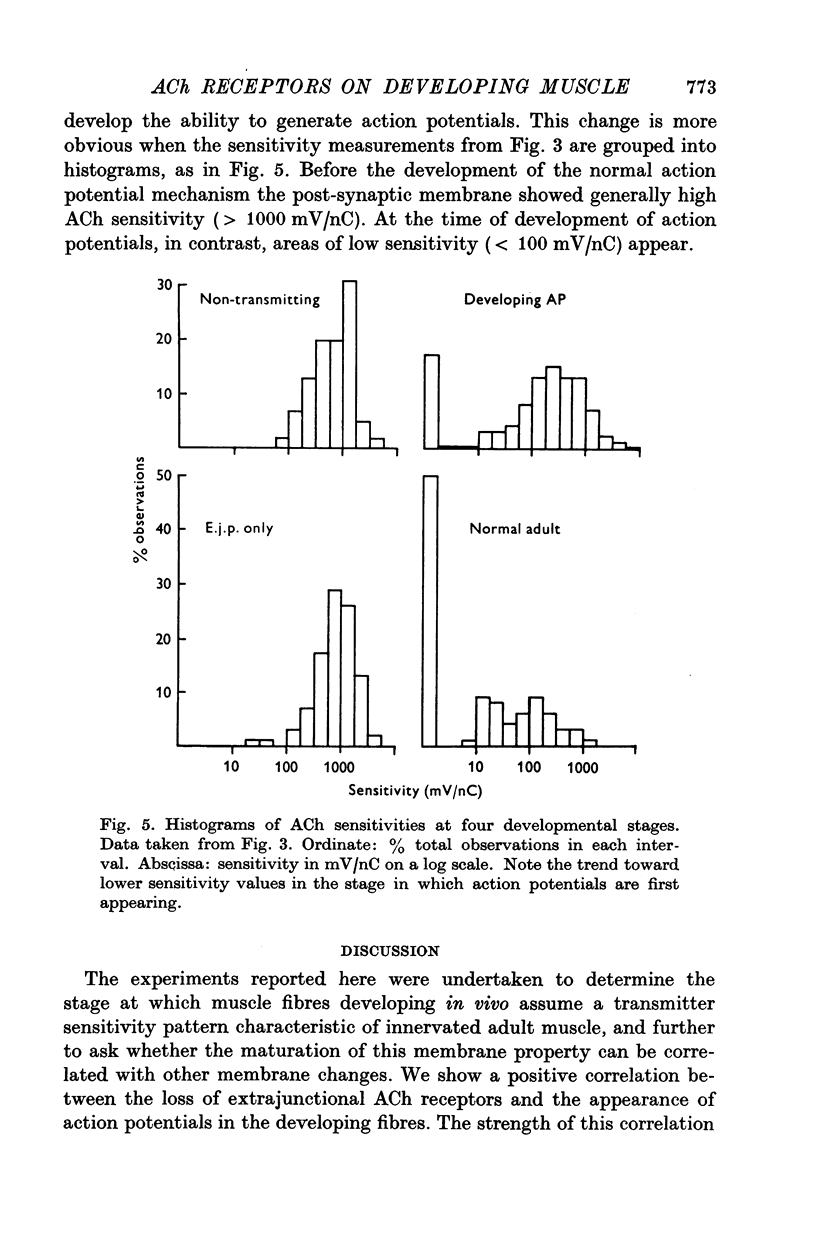



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. K., Hall Z. W. Increased extrajunctional acetylcholine sensitivity produced by chronic acetylcholine sensitivity produced by chronic post-synaptic neuromuscular blockade. J Physiol. 1975 Jan;244(3):659–676. doi: 10.1113/jphysiol.1975.sp010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. A., Fischbach G. D. Regulation of muscle acetylcholine sensitivity by muscle activity in cell culture. Science. 1973 Jul 6;181(4094):76–78. doi: 10.1126/science.181.4094.76. [DOI] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Ort C. A. Physiological properties of nerve-muscle junctions developing in vivo. Cold Spring Harb Symp Quant Biol. 1976;40:435–442. doi: 10.1101/sqb.1976.040.01.041. [DOI] [PubMed] [Google Scholar]
- Dennis M. J. Physiological properties of junctions between nerve and muscle developing during salamander limb regeneration. J Physiol. 1975 Jan;244(3):683–702. doi: 10.1113/jphysiol.1975.sp010820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B., Witzke F. Trophic regulation of acetylcholine sensitivity of muscle: effect of electrical stimulation. Science. 1972 May 5;176(4034):514–516. doi: 10.1126/science.176.4034.514. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Berg D. K., Cohen S. A., Frank E. Enrichment of nerve--muscle synapses in spinal cord--muscle cultures and identification of relative peaks of ACh sensitivity at sites of transmitter release. Cold Spring Harb Symp Quant Biol. 1976;40:347–357. doi: 10.1101/sqb.1976.040.01.034. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Cohen S. A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev Biol. 1973 Mar;31(1):147–162. doi: 10.1016/0012-1606(73)90326-6. [DOI] [PubMed] [Google Scholar]
- Gutmann E. Neurotrophic relations. Annu Rev Physiol. 1976;38:177–216. doi: 10.1146/annurev.ph.38.030176.001141. [DOI] [PubMed] [Google Scholar]
- Letinsky M. S. Acetylcholine sensitivity changes in tadpole tail muscle fibers innervated by developing motor neurons. J Neurobiol. 1975 Nov;6(6):609–617. doi: 10.1002/neu.480060607. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Control of ACh sensitivity in rat muscle fibers. Cold Spring Harb Symp Quant Biol. 1976;40:263–274. doi: 10.1101/sqb.1976.040.01.027. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol. 1975 Nov;252(3):603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives J., Silman I., Amsterdam A. Appearance and disappearance of acetycholine receptor during differentiation of chick skeletal muscle in vitro. Cell. 1976 Apr;7(4):543–550. doi: 10.1016/0092-8674(76)90204-x. [DOI] [PubMed] [Google Scholar]
- Purves D., Sakmann B. The effect of contractile activity on fibrillation and extrajunctional acetylcholine-sensitivity in rat muscle maintained in organ culture. J Physiol. 1974 Feb;237(1):157–182. doi: 10.1113/jphysiol.1974.sp010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y., Fischman D. A. Morphological and physiological evidence for the development of functional neuromuscular junctions in vitro. Dev Biol. 1973 Mar;31(1):200–225. doi: 10.1016/0012-1606(73)90332-1. [DOI] [PubMed] [Google Scholar]
- Steinbach J. H. Acetylcholine responses on clonal myogenic cells in vitro. J Physiol. 1975 May;247(2):393–405. doi: 10.1113/jphysiol.1975.sp010937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge D. A. Physiological characteristics of re-innervation of skeletal muscle in the mouse. J Physiol. 1974 Aug;241(1):141–153. doi: 10.1113/jphysiol.1974.sp010645. [DOI] [PMC free article] [PubMed] [Google Scholar]


