Abstract
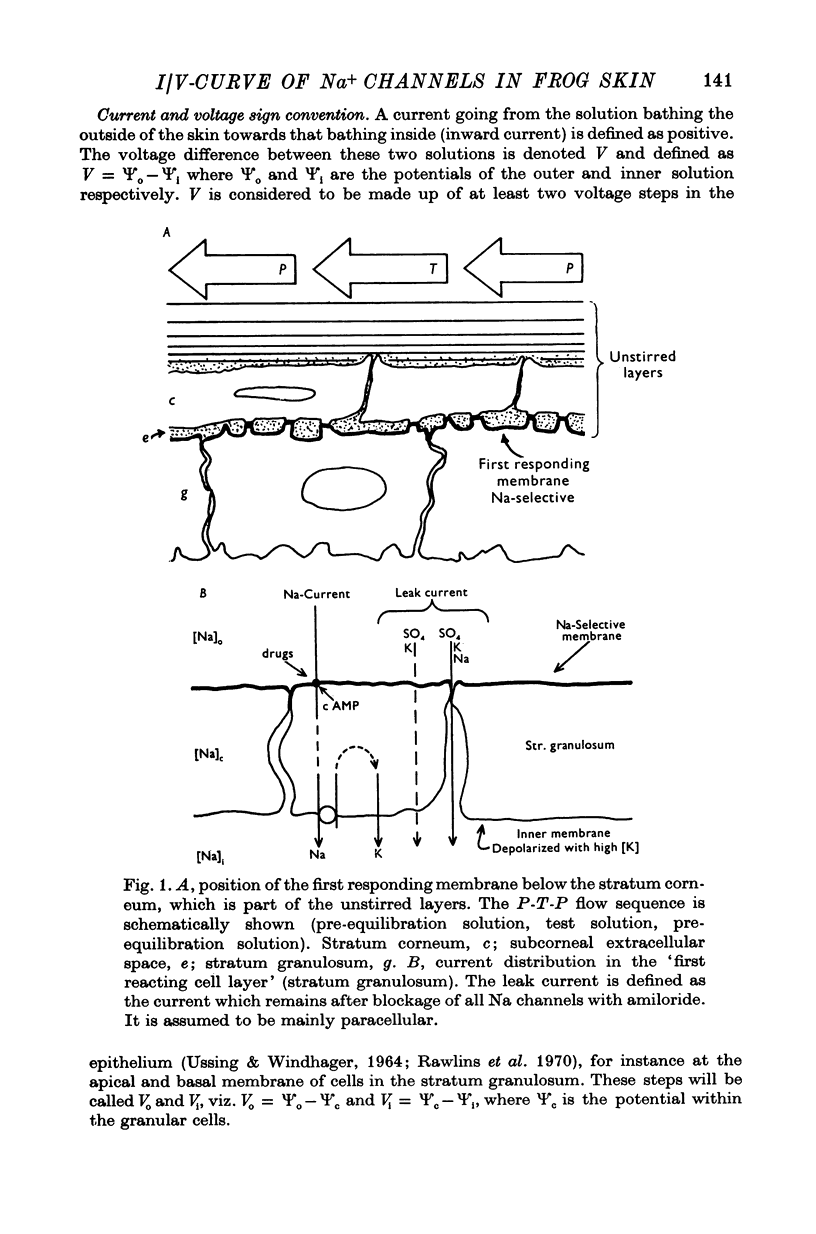
1. The inward facing membranes of in vitro frog skin epithelium were depolarized with solutions of high K concentration. The electrical properties of the epithelium are then expected to be governed by the outward facing, Na-selective membrane.
2. In this state, the transepithelial voltage (V) was clamped to zero and step-changes of Na activity in the outer solution ((Na)o) were performed with a fast-flow chamber at constant ionic strength, while the short-circuit current was recorded.
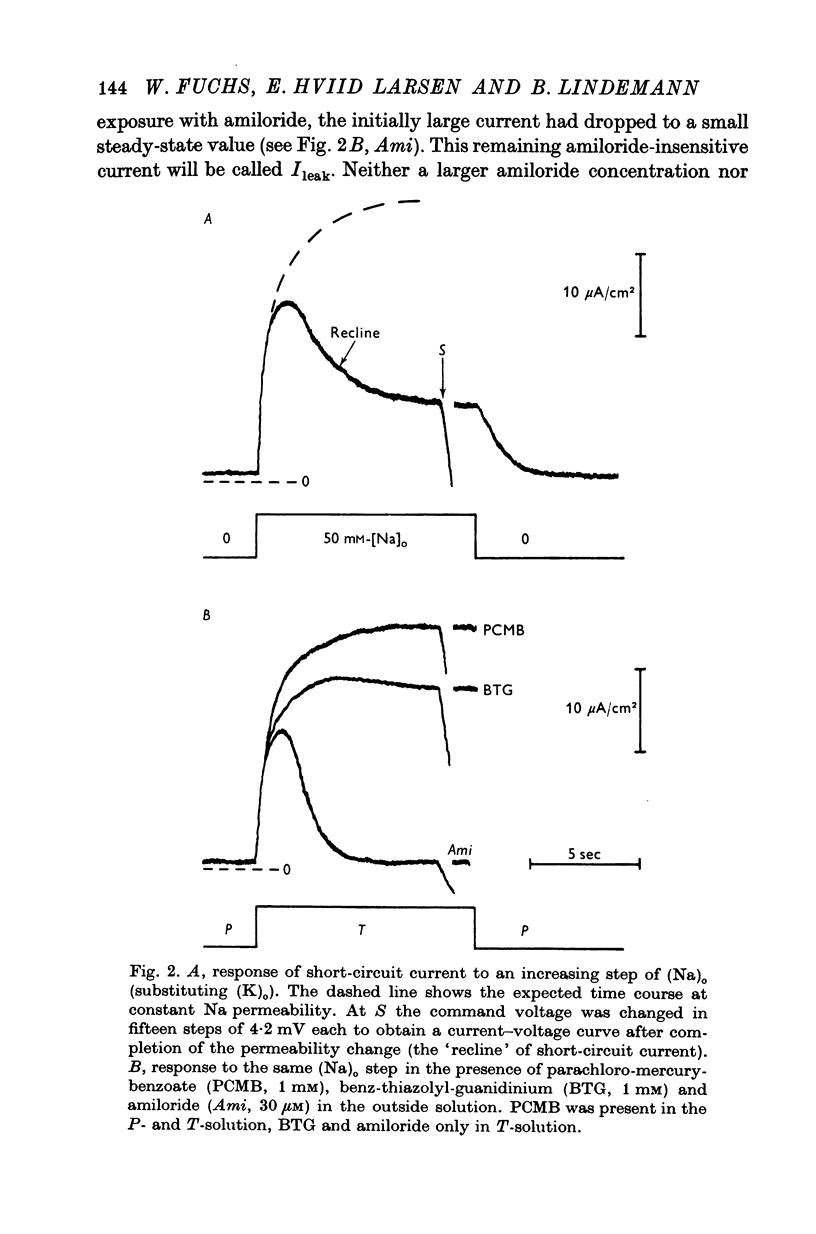
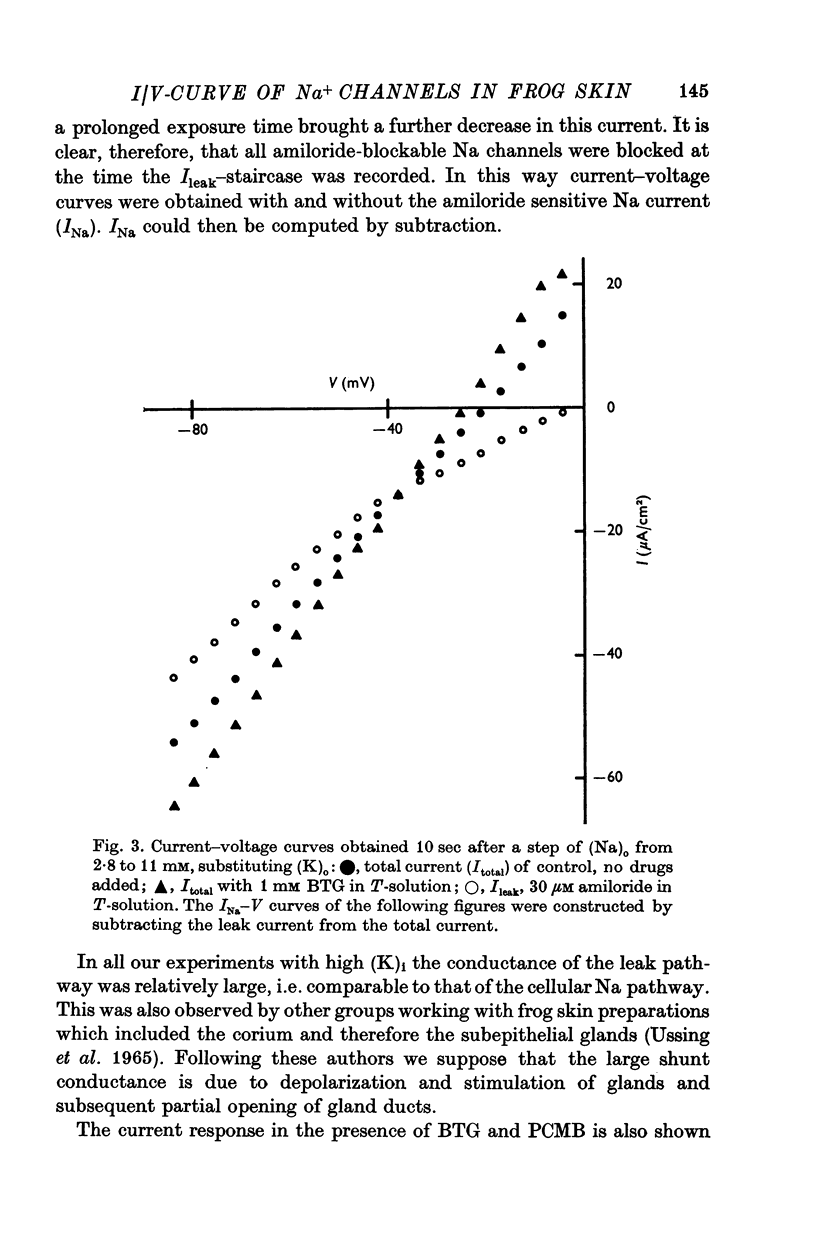
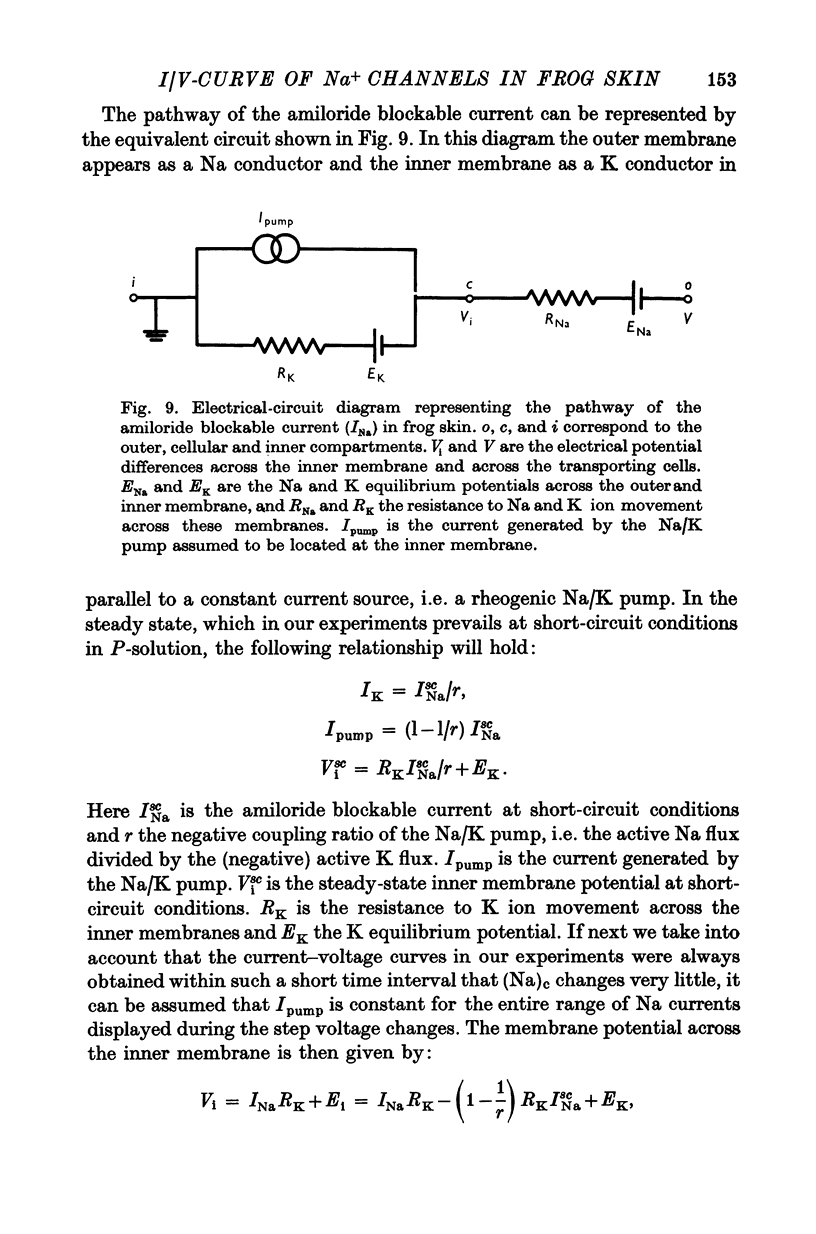
3. At pre-selected times after a step-change of (Na)o the current response (I) to a fast voltage staircase was recorded. This procedure was repeated after blocking the Na channels with amiloride to obtain the current—voltage curve of transmembrane and paracellular shunt pathways. The current—voltage curve of the Na channels was computed by subtracting the shunt current from the total current.
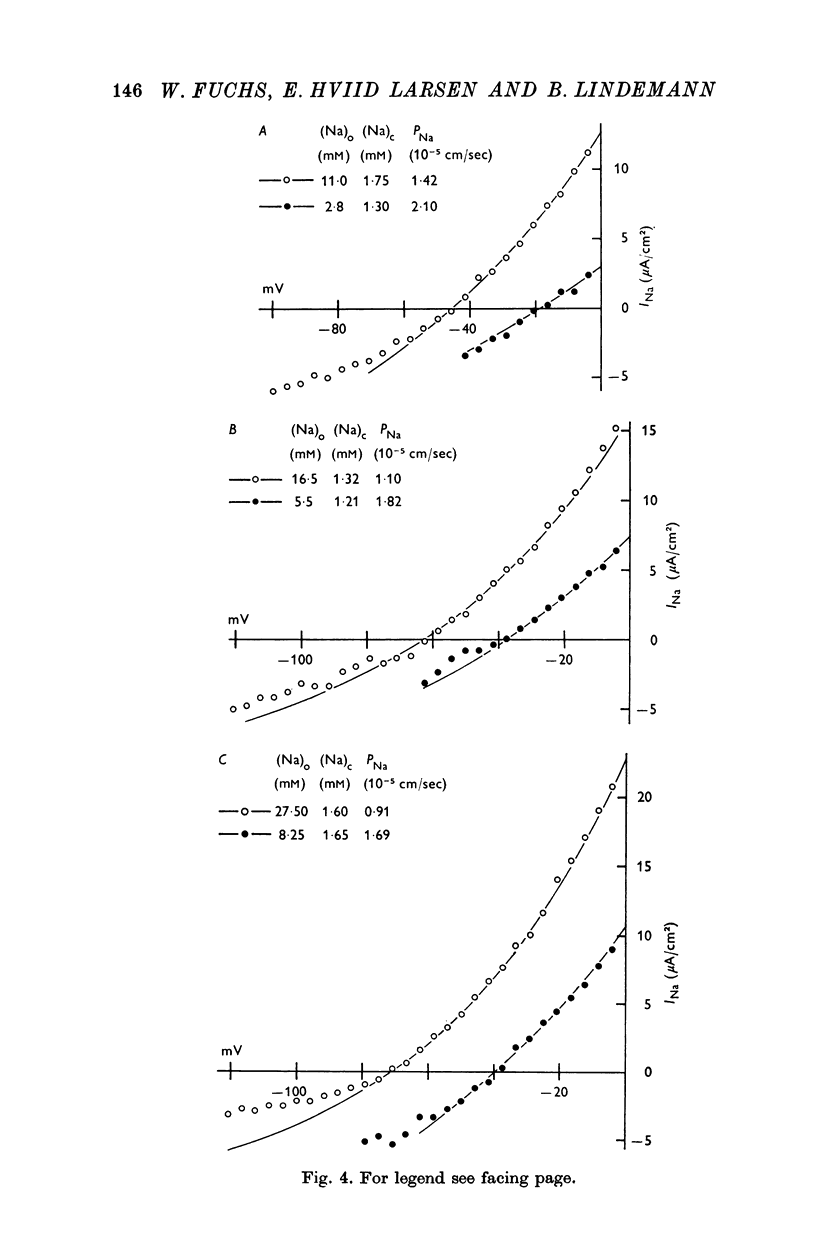
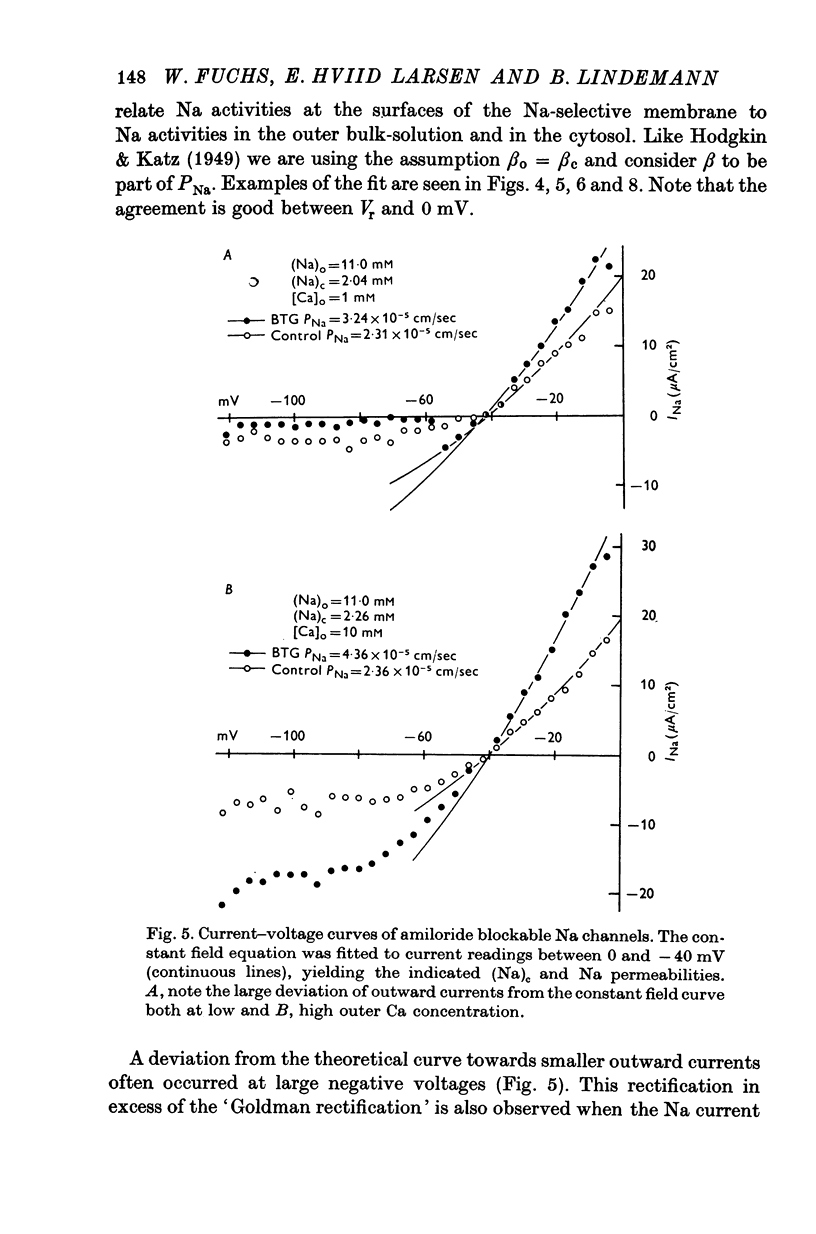
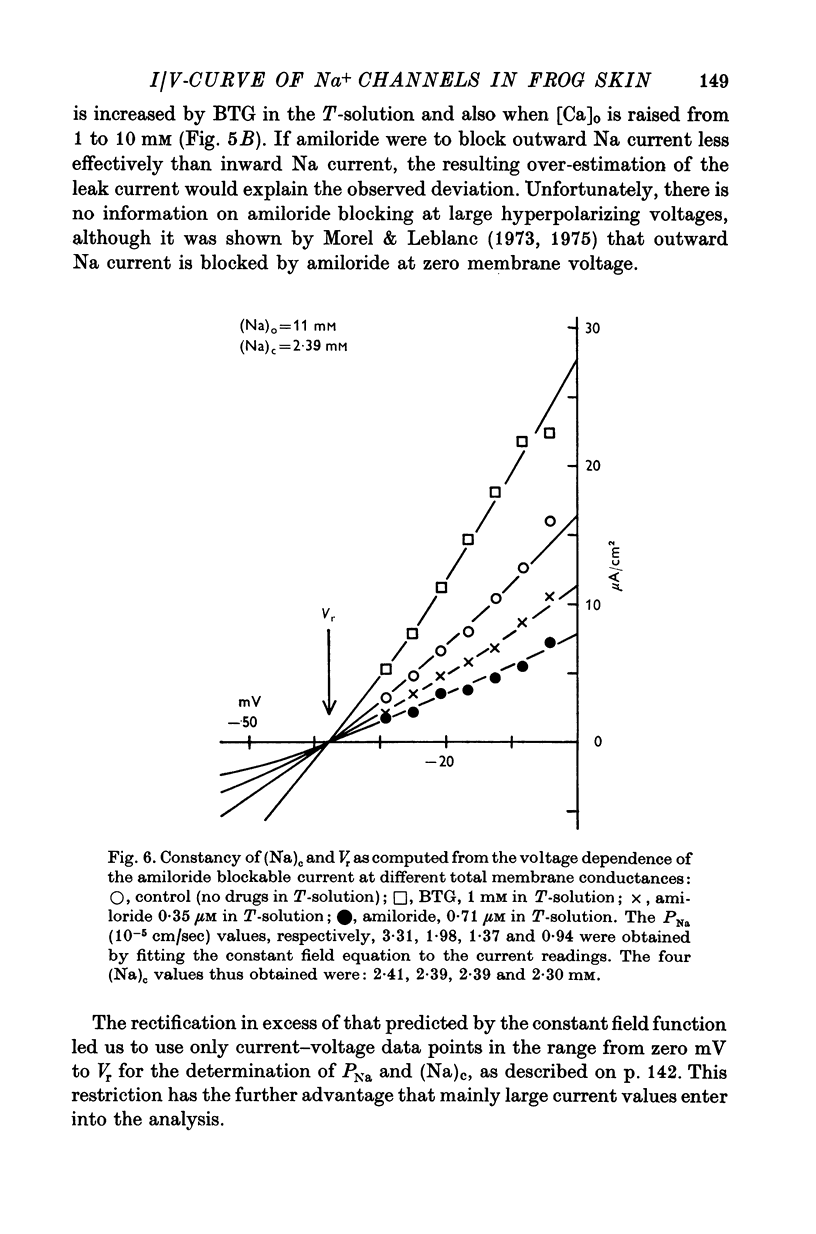
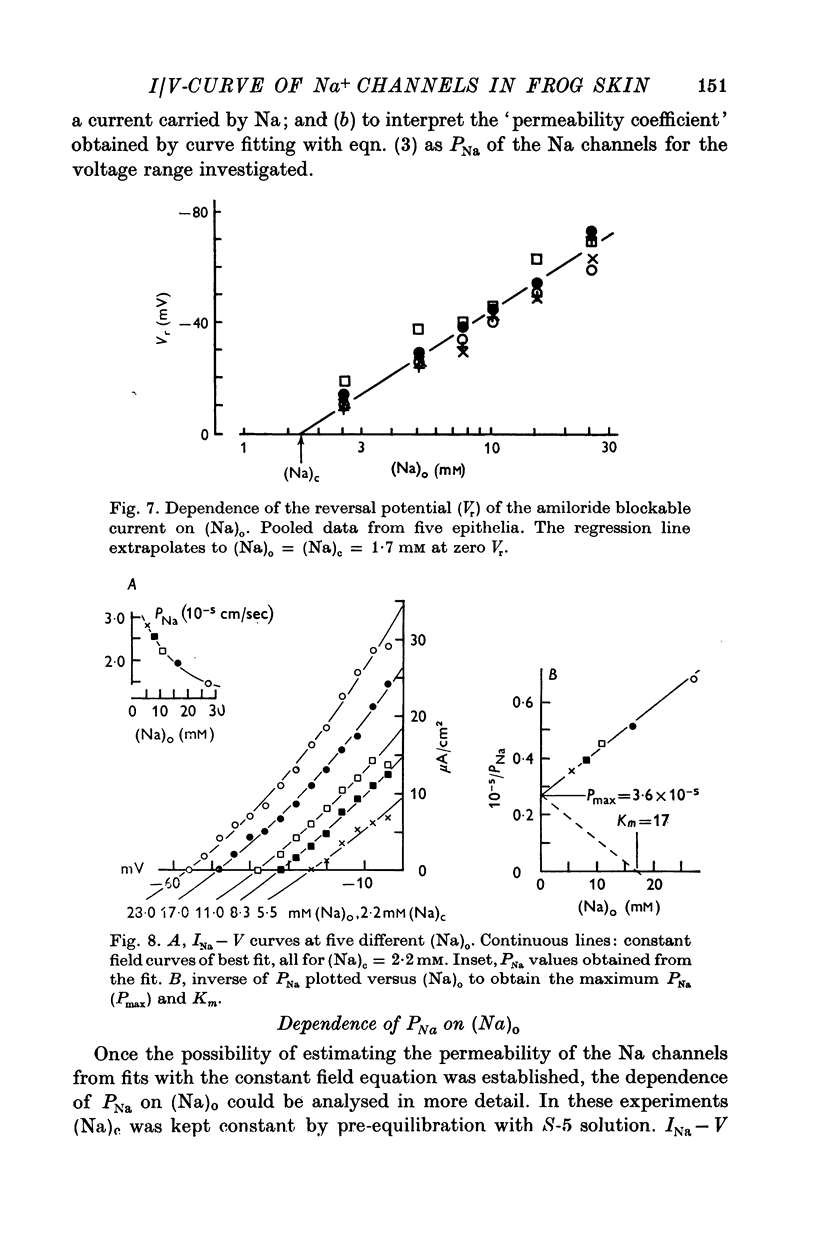
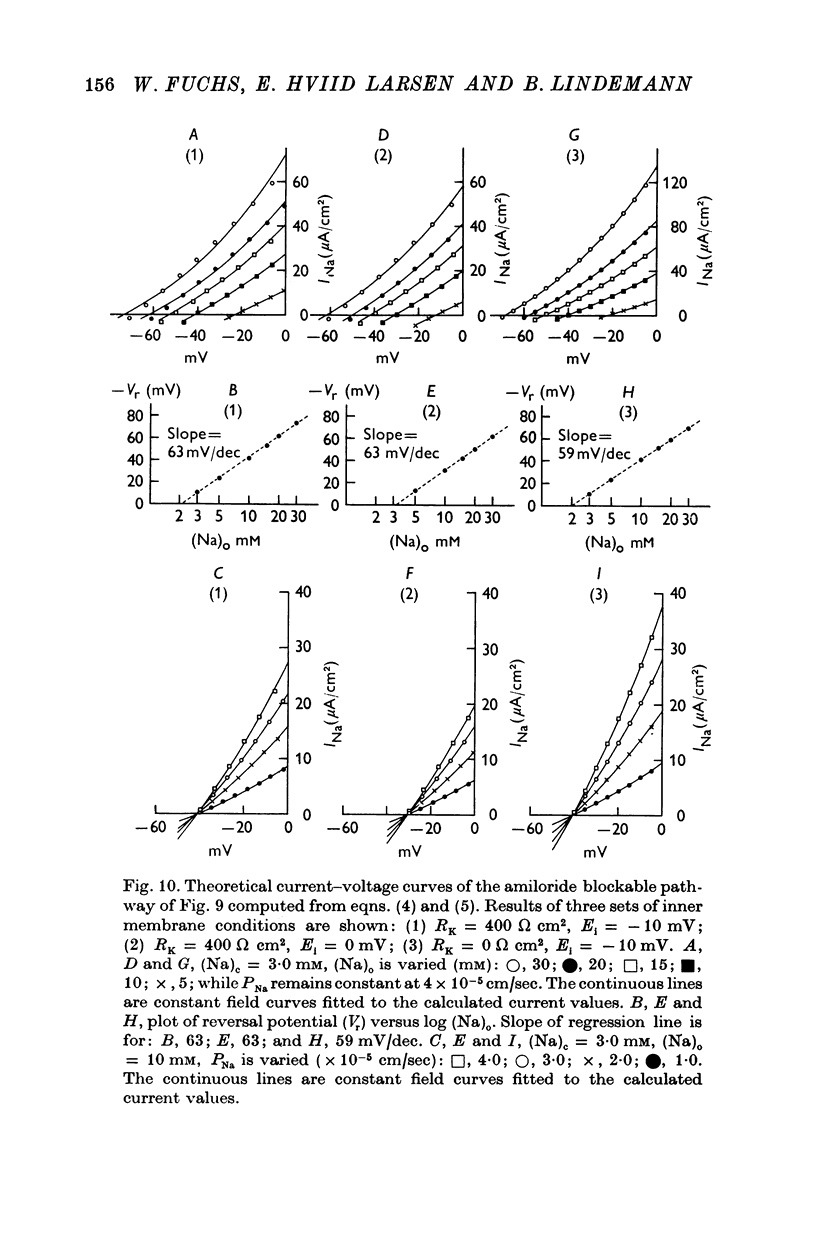
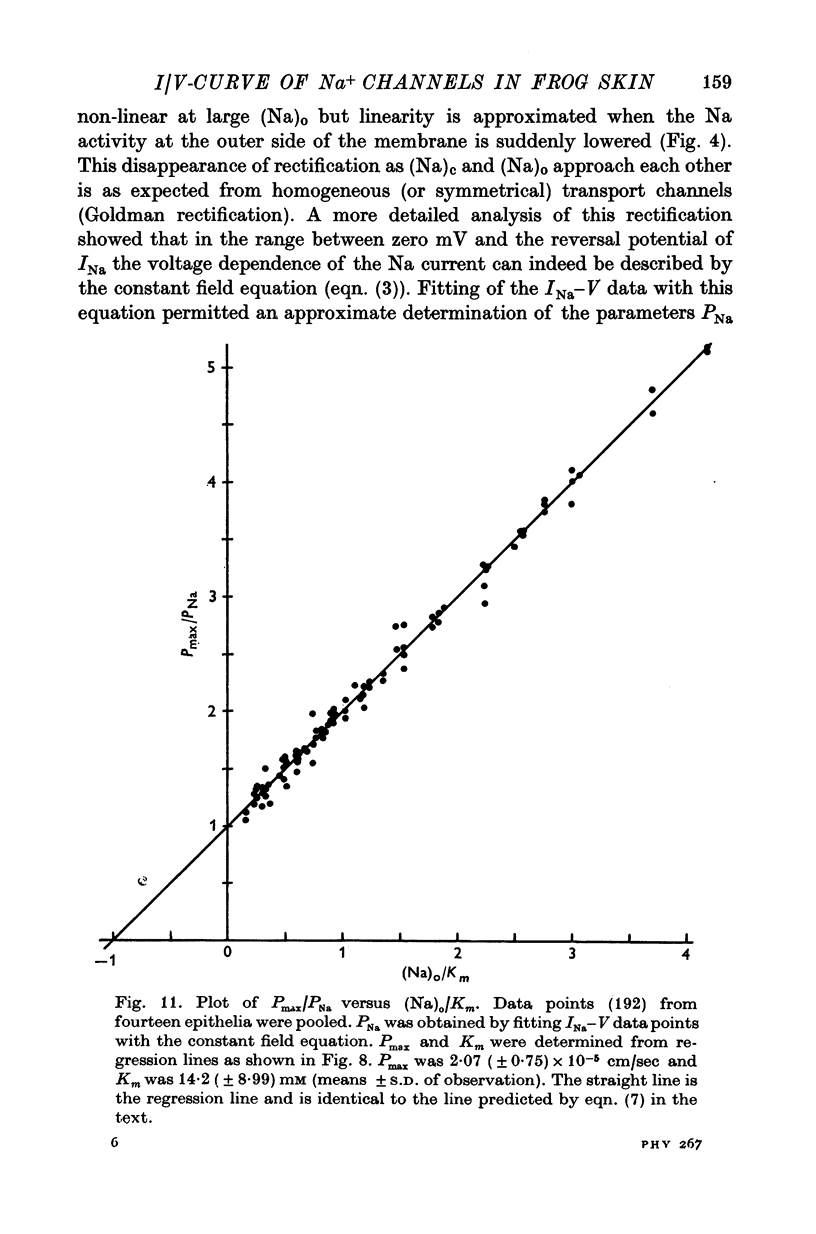
4. The instantaneous INa—V curve thus obtained at a given (Na)o could easily be fitted with the constant field equation in the range between -50 and zero mV. This fit yielded approximate estimates of PNa, the Na— permeability of the Na-selective membrane (at this (Na)o) and the cellular Na activity, (Na)c. As residual properties of the serosal membrane were ignored the computed values are expected to underestimate the true ones.
5. At constant (Na)c, the steady-state value of 1/PNa increases linearly with (Na)o. Error analysis and the effect of drugs show that the dependence is not due to the residual properties of the inward facing membranes but reflects the true behaviour of PNa.
6. The steady-state PNa at a given (Na)o is smaller than the transient PNa observed right after a stepwise increase of (Na)o to this value. The time constant of PNa-relaxation is in the order of seconds.
7. In conclusion, Na transport through open Na-selective channels of the outward facing membrane of the stratum granulosum cells can be described as an electrodiffusion process which as such does not saturate with increasing (Na)o. However, when added to the outer border of the membrane Na causes a decrease of PNa within several seconds. It is considered that binding of Na results in closure of Na channels.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biber T. U., Curran P. F. Direct measurement of uptake of sodium at the outer surface of the frog skin. J Gen Physiol. 1970 Jul;56(1):83–99. doi: 10.1085/jgp.56.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber T. U., Sanders M. L. Influence of transepithelial potential difference on the sodium uptake at the outer surface of the isolated frog skin. J Gen Physiol. 1973 May;61(5):529–551. doi: 10.1085/jgp.61.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke R., Lindemann B. Design of a fast voltage clamp for biological membranes, using discontinuous feedback. Rev Sci Instrum. 1974 May;45(5):656–661. doi: 10.1063/1.1686708. [DOI] [PubMed] [Google Scholar]
- CEREIJIDO M., CURRAN P. F. INTRACELLULAR ELECTRICAL POTENTIALS IN FROG SKIN. J Gen Physiol. 1965 Mar;48:543–557. doi: 10.1085/jgp.48.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Amiloride and the sodium channel. Naunyn Schmiedebergs Arch Pharmacol. 1974;281(3):261–269. doi: 10.1007/BF00500595. [DOI] [PubMed] [Google Scholar]
- Dörge A., Gehring K., Nagel W., Thurau K. Localization of sodium in frog skin by electron microprobe analysis. Naunyn Schmiedebergs Arch Pharmacol. 1974;281(3):271–280. doi: 10.1007/BF00500596. [DOI] [PubMed] [Google Scholar]
- Dörge A., Nagel W. Effect of amiloride on sodium transport in frog skin. II. Sodium transport pool and unidirectional fluxes. Pflugers Arch. 1970;321(2):91–101. doi: 10.1007/BF00586365. [DOI] [PubMed] [Google Scholar]
- Ehrlich E. N., Crabbé J. The mechanism of action of amipramizide. Pflugers Arch. 1968;302(1):79–96. doi: 10.1007/BF00586783. [DOI] [PubMed] [Google Scholar]
- Erlij D., Smith M. W. Sodium uptake by frog skin and its modification by inhibitors of transepithelial sodium transport. J Physiol. 1973 Jan;228(1):221–239. doi: 10.1113/jphysiol.1973.sp010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlij D., Smith M. W. Sodium uptake by the outside surface of frog skin. J Physiol. 1971 Oct;218 (Suppl):33P–34P. [PubMed] [Google Scholar]
- Finkelstein A., Mauro A. Equivalent Circuits as Related to Ionic Systems. Biophys J. 1963 May;3(3):215–237. doi: 10.1016/s0006-3495(63)86817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs W., Gebhardt U., Lindemann B. Delayed voltage responses to fast changes of (Na) 0 at the outer surface of frog skin epithelium. Biomembranes. 1972;3:483–498. doi: 10.1007/978-1-4684-0961-1_33. [DOI] [PubMed] [Google Scholar]
- Gebhardt U. A fast voltage clamp with automatic compensation for changes of extracellular resistivity. Pflugers Arch. 1974 Feb 18;347(1):1–7. [PubMed] [Google Scholar]
- Gebhardt U., Lindemann B. Speed of voltage threshold shift after step-changes of (Na)o and (Ca)o at the outer surface of frog skin. Pflugers Arch. 1974 Feb 18;347(1):9–18. doi: 10.1007/BF00587050. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann K., Lindemann B., Schnakenberg J. Current-voltage curves of porous membranes in the presence of pore-blocking ions. I. Narrow pores containing no more than one moving ion. Biophys J. 1972 Jun;12(6):683–702. doi: 10.1016/S0006-3495(72)86112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman S. I., Miller D. A. In vitro techniques for avoiding edge damage in studies of frog skin. Science. 1971 Jul 9;173(3992):146–148. doi: 10.1126/science.173.3992.146. [DOI] [PubMed] [Google Scholar]
- Leblanc G. The mechanism of lithium accumulation in the isolated frog skin epithelium. Pflugers Arch. 1972;337(1):1–18. doi: 10.1007/BF00587867. [DOI] [PubMed] [Google Scholar]
- Lindemann B., Gebhardt U., Fuchs W. A flow-chamber for concentration-step experiments with epithelial membranes. TIT J Life Sci. 1972;2(1):15–26. [PubMed] [Google Scholar]
- Lindemann B. Letter: Impalement artifacts in microelectrode recordings of epithelial membrane potentials. Biophys J. 1975 Nov;15(11):1161–1164. doi: 10.1016/S0006-3495(75)85892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B., Van Driessche W. Sodium-specific membrane channels of frog skin are pores: current fluctuations reveal high turnover. Science. 1977 Jan 21;195(4275):292–294. doi: 10.1126/science.299785. [DOI] [PubMed] [Google Scholar]
- MACROBBIE E. A., USSING H. H. Osmotic behaviour of the epithelial cells of frog skin. Acta Physiol Scand. 1961 Nov-Dec;53:348–365. doi: 10.1111/j.1748-1716.1961.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Morel F., Leblanc G. Transient current changes and Na compartimentalization in frog skin epithelium. Pflugers Arch. 1975 Jul 21;358(2):135–157. doi: 10.1007/BF00583924. [DOI] [PubMed] [Google Scholar]
- Nagel W., Dörge A. Effect of Amiloride on sodium transport of frog skin. I. Action on intracellular sodium content. Pflugers Arch. 1970;317(1):84–92. doi: 10.1007/BF00586701. [DOI] [PubMed] [Google Scholar]
- Nagel W. The intracellular electrical potential profile of the frog skin epithelium. Pflugers Arch. 1976 Sep 30;365(2-3):135–143. doi: 10.1007/BF01067010. [DOI] [PubMed] [Google Scholar]
- Rawlins F., Mateu L., Fragachan F., Whittembury G. Isolated toad skin epithelium: transport characteristics. Pflugers Arch. 1970;316(1):64–80. doi: 10.1007/BF00587897. [DOI] [PubMed] [Google Scholar]
- Rotunno C. A., Vilallonga F. A., Fernández M., Cereijido M. The penetration of sodium into the epithelium of the frog skin. J Gen Physiol. 1970 Jun;55(6):716–735. doi: 10.1085/jgp.55.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARE L., USSING H. H. EFFECT OF POTASSIUM ON THE MOVEMENT OF WATER ACROSS THE ISOLATED AMPHIBIAN SKIN. Acta Physiol Scand. 1965 May-Jun;64:109–118. doi: 10.1111/j.1748-1716.1965.tb04159.x. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- USSING H. H., BIBER T. U., BRICKER N. S. EXPOSURE OF THE ISOLATED FROG SKIN TO HIGH POTASSIUM CONCENTRATIONS AT THE INTERNAL SURFACE. II. CHANGES IN EPITHELIAL CELL VOLUME, RESISTANCE, AND RESPONSE TO ANTIDIURETIC HORMONE. J Gen Physiol. 1965 Jan;48:425–433. doi: 10.1085/jgp.48.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H., WINDHAGER E. E. NATURE OF SHUNT PATH AND ACTIVE SODIUM TRANSPORT PATH THROUGH FROG SKIN EPITHELIUM. Acta Physiol Scand. 1964 Aug;61:484–504. [PubMed] [Google Scholar]
- Zeiske W., Lindemann B. Chemical stimulation of Na + current through the outer surface of frog skin epithelium. Biochim Biophys Acta. 1974 Jun 13;352(2):323–326. doi: 10.1016/0005-2736(74)90223-5. [DOI] [PubMed] [Google Scholar]


