Abstract
1. The contribution of Na, K and Ca ions to the generation of slow waves in the circular muscle of the guinea-pig stomach was studied.
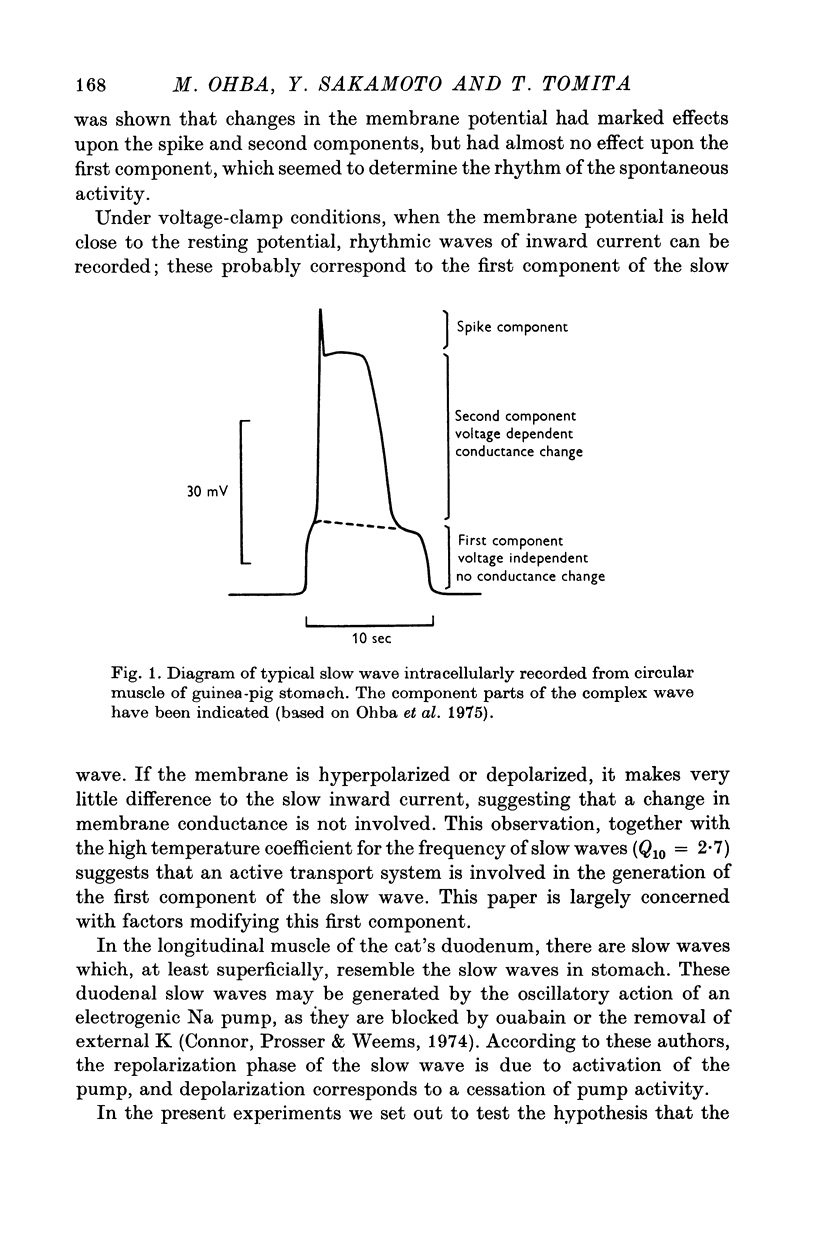
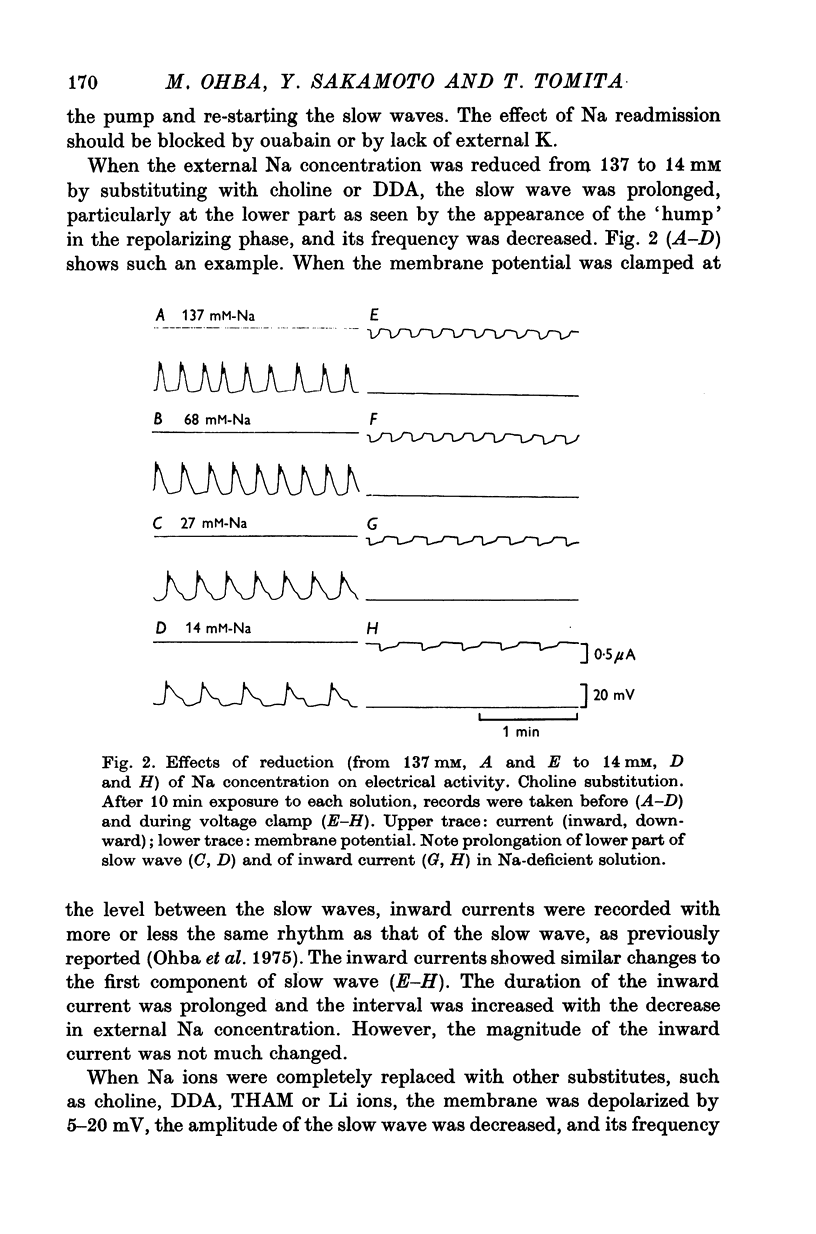
2. The slow waves had a lower, potential-independent (first) and an upper, potential-dependent (second) component. Reduction of the external Na prolonged the first component, but complete removal of Na depolarized the membrane and caused deterioration of the slow wave.
3. Readmission of Na (5-10 mM) restored the slow wave; this action was not abolished by ouabain.
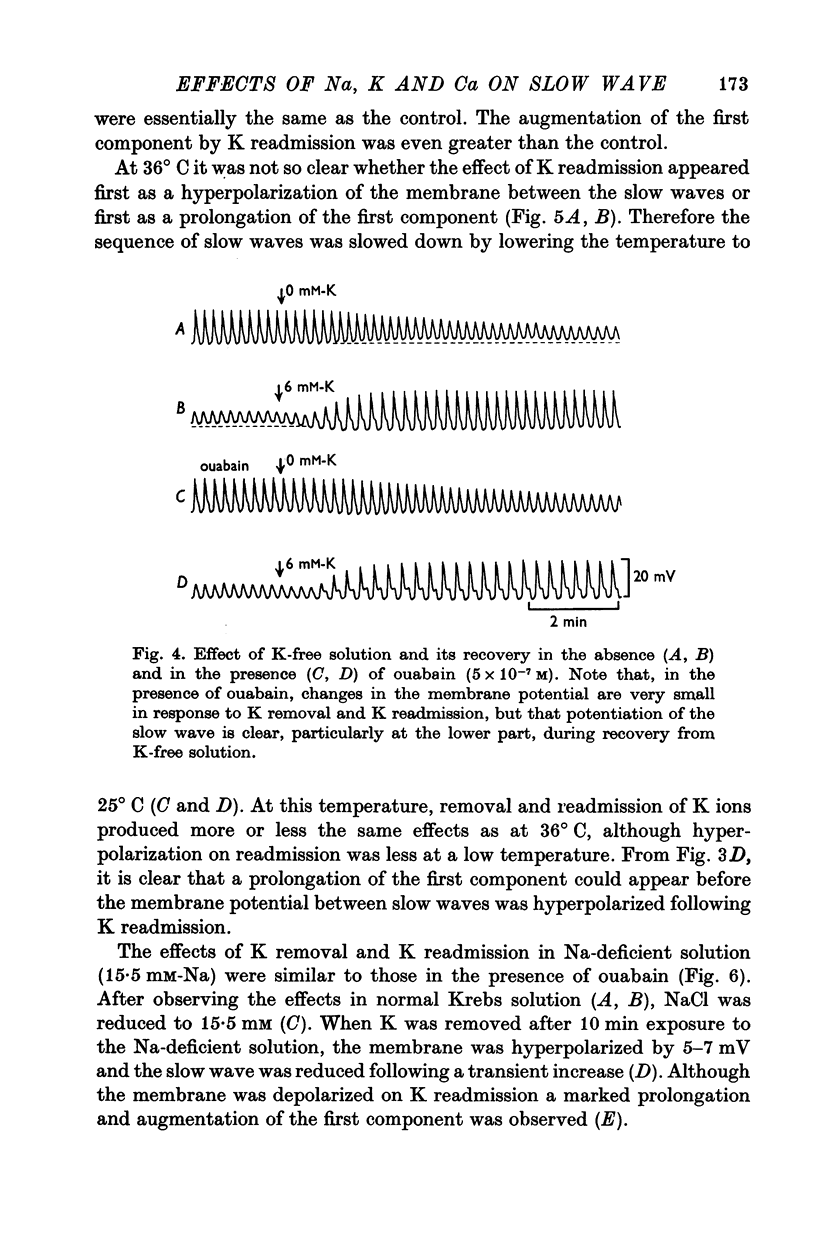
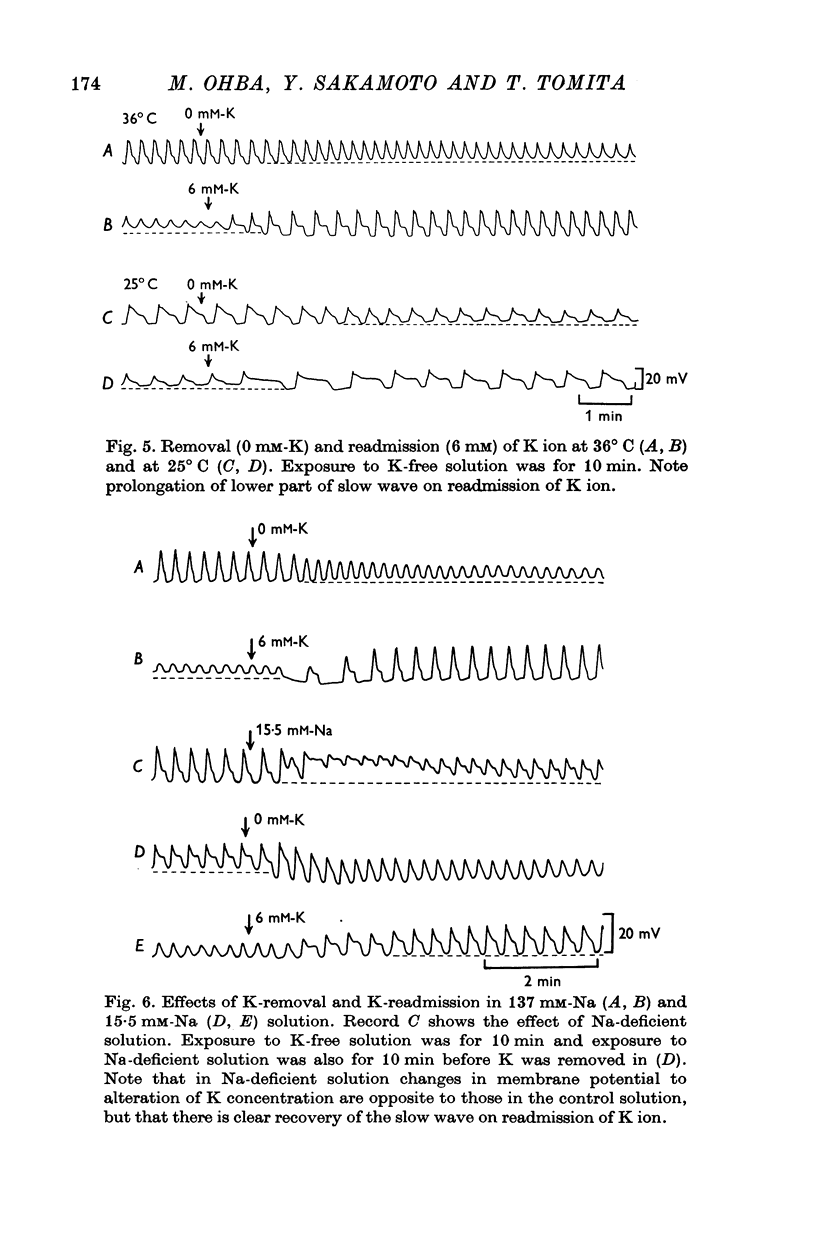
4. Removal of K depolarized the membrane and slightly reduced the amplitude and duration of the slow waves. Readmission of K hyperpolarized the membrane and increased the amplitude and duration of the slow waves, particularly of the first component. Ouabain blocked the effects on the membrane potential, but not the effects on the slow waves.
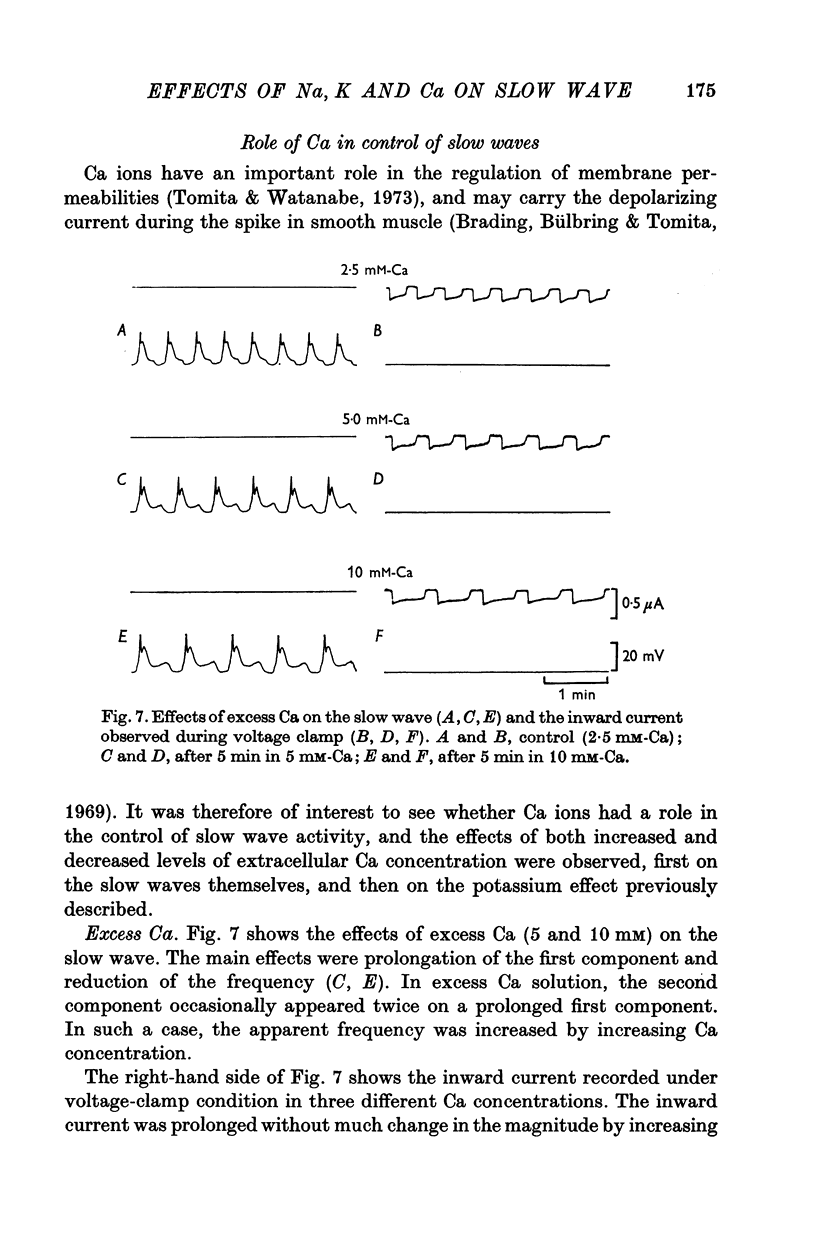
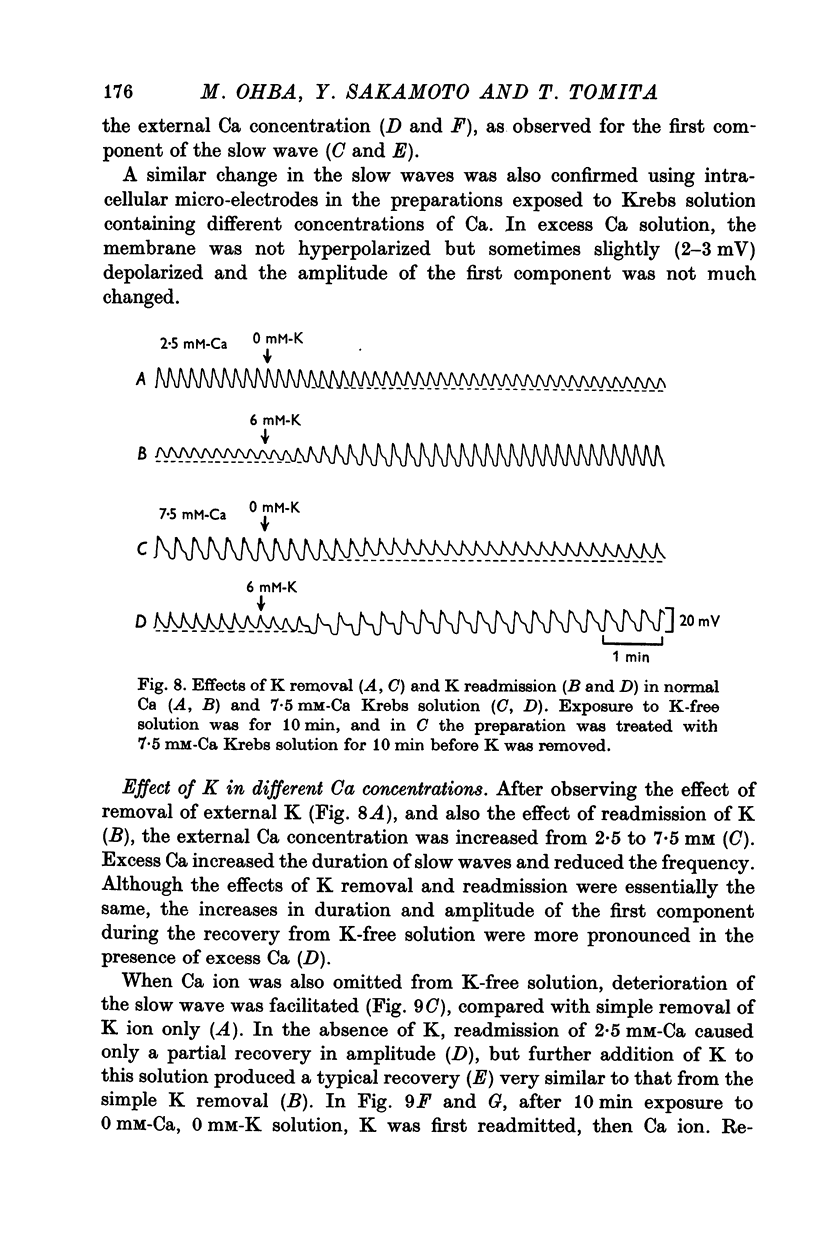
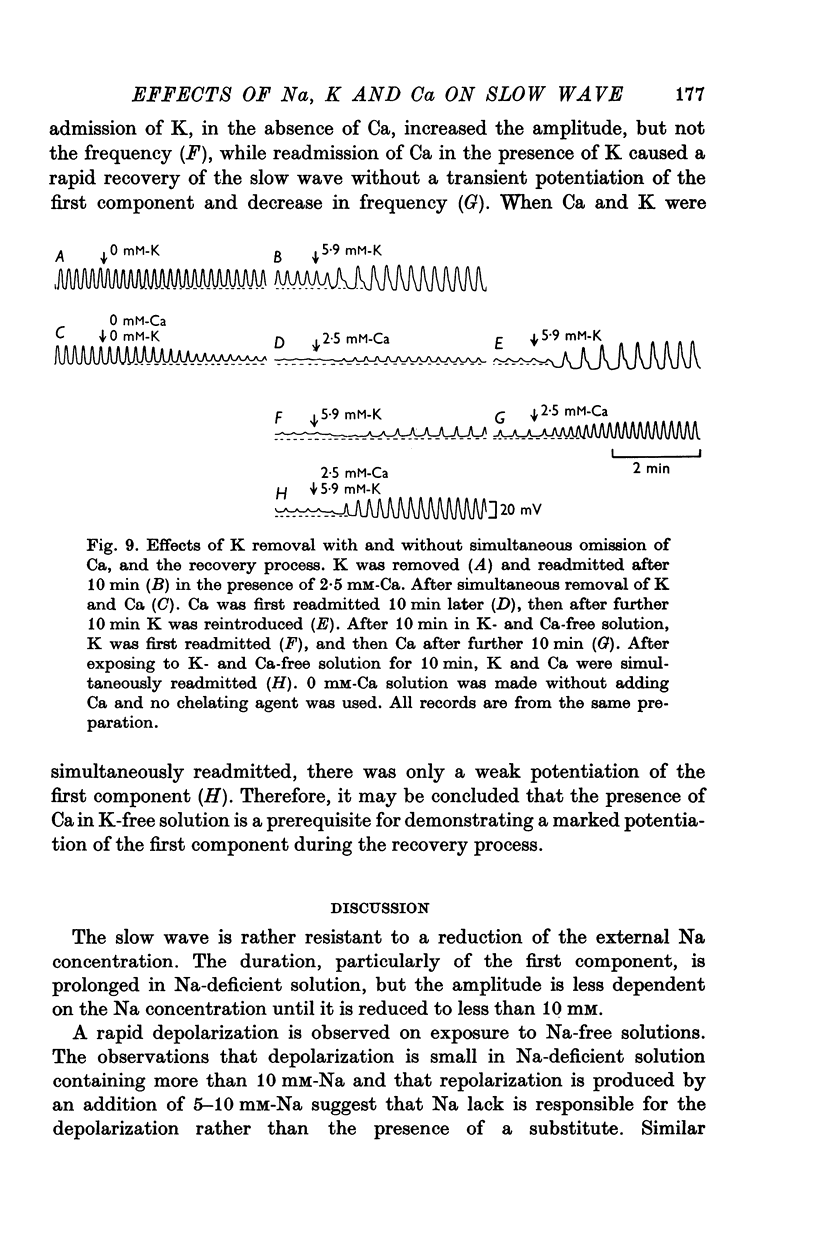
5. An increase in extracellular Ca prolonged the first component and reduced the frequency. Removal of extracellular Ca abolished the slow wave activity. Excess Ca enhanced, and low Ca reduced the effects of altering the concentrations of external K.
6. It is concluded that the ouabain-sensitive Na-K pump may not be directly involved in generating slow waves, but that some other metabolic process is involved, which is regulated to a large extent by Ca, and possibly also by Na and K.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Electrogenic sodium pump in smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Sep;217(2):297–313. doi: 10.1113/jphysiol.1971.sp009572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Prosser C. L., Weems W. A. A study of pace-maker activity in intestinal smooth muscle. J Physiol. 1974 Aug;240(3):671–701. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIEL E. E. EFFECTS OF INTRA-ARTERIAL PERFUSIONS ON ELECTRICAL ACTIVITY AND ELECTROLYTE CONTENTS OF DOG SMALL INTESTINE. Can J Physiol Pharmacol. 1965 Jul;43:551–577. doi: 10.1139/y65-056. [DOI] [PubMed] [Google Scholar]
- Job D. D. Ionic basis of intestinal electrical activity. Am J Physiol. 1969 Nov;217(5):1534–1541. doi: 10.1152/ajplegacy.1969.217.5.1534. [DOI] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Prosser C. L., Job D. D. Ionic dependence of slow waves and spikes in intestinal muscle. Am J Physiol. 1969 Nov;217(5):1542–1547. doi: 10.1152/ajplegacy.1969.217.5.1542. [DOI] [PubMed] [Google Scholar]
- Ohba M., Sakamoto Y., Tomita T. The slow wave in the circular muscle of the guinea-pig stomach. J Physiol. 1975 Dec;253(2):505–516. doi: 10.1113/jphysiol.1975.sp011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osa T. Effect of removing the external sodium on the electrical and mechanical activities of the pregnant mouse myometrium. Jpn J Physiol. 1971 Dec;21(6):607–625. doi: 10.2170/jjphysiol.21.607. [DOI] [PubMed] [Google Scholar]
- Osa T. The effects of sodium, calcium and manganese on the electrical and mechanical activities of the myometrial smooth muscle of pregnant mice. Jpn J Physiol. 1973 Apr;23(2):113–133. doi: 10.2170/jjphysiol.23.113. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. Factors controlling myogenic activity in smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):73–85. doi: 10.1098/rstb.1973.0010. [DOI] [PubMed] [Google Scholar]
- Tomita T., Yamamoto T. Effects of removing the external potassium on the smooth muscle of guinea-pig taenia coli. J Physiol. 1971 Feb;212(3):851–868. doi: 10.1113/jphysiol.1971.sp009360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins J. R., Cranefield P. F. Effect on membrane potential and electrical activity of adding sodium to sodium-depleted cardiac purkinje fibers. J Gen Physiol. 1974 Oct;64(4):473–493. doi: 10.1085/jgp.64.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]


