Abstract
Entamoeba histolytica trophozoites are covered by lipophosphoglycan-peptidoglycan molecules which may be key virulence factors. We found that pretreatment of severe combined immunodeficient mice bearing human intestinal xenografts with a monoclonal antibody to the amebic lipophosphoglycan-peptidoglycan molecules can prevent or significantly reduce the human intestinal inflammation and tissue damage that are normally seen with E. histolytica colonic infection.
The protozoan parasite Entamoeba histolytica invades the mucosal tissue of the human colon, causing colonic inflammation and tissue damage. E. histolytica trophozoites are covered by complex lipophosphoglycans-proteophosphoglycans (LPGs-PPGs), glycosylphosphatidylinositol-anchored molecules which appear to contain a peptide moiety (2, 4, 6, 9). The amebic LPGs-PPGs have been linked to amebic virulence, as they are a rare example of molecules that differ significantly in structure between the pathogenic E. histolytica and the morphologically identical, but genetically distinct, nonpathogenic commensal Entamoeba dispar (1, 5, 6). In addition, passive immunization with a monoclonal antibody (EH5) to the amebic LPGs-PPGs inhibited amebic liver abscess formation in severe combined immunodeficient (SCID) mice (4). A critical unanswered question is whether antibodies to the amebic LPGs-PPGs, or to any other amebic antigen, can prevent or reduce disease in the human colon. Here, we show that a combination of passive immunization and direct intraluminal injection of the monoclonal antibody EH5 provides protection against amebic colitis in SCID mice with human colonic xenografts (SCID-HU-INT mice).
SCID-HU-INT mice were generated by engrafting human colonic sections into the rear flanks and suprascapular regions of 6- to 8-week-old SCID mice as previously described (7). After a 12-week engraftment period, groups of eight SCID-HU-INT mice received intraperitoneal (i.p.) and intraluminal (human colonic xenograft) injections of 200 μg of purified monoclonal antibody EH5 or of the isotype-matched control monoclonal antibody 1B10 (antiphosphorylcholine). A third group of eight SCID-HU-INT mice received similar inoculations with phosphate-buffered saline (PBS). Eight SCID-HU-INT mice were not challenged with amebas and served as uninfected controls. Twenty-four hours after antibody injections, the human colonic xenografts were challenged with 106 E. histolytica HM-1:IMSS trophozoites. After 24 h of infection, all mice received an intraluminal (human colonic xenograft) injection of 50 μl of fluorescein isothiocyanate-labeled dextran (FITC-dextran), and 4 h later, the animals were sacrificed and sera were obtained for fluorescence measurement as previously described (8). The human colonic xenografts were sectioned, and segments were homogenized for interleukin 8 (IL-8), IL-1β, and myeloperoxidase (MPO) assays, as previously described (8). Colonic segments were also fixed for histology and stained with hematoxylin and eosin as previously described (7).
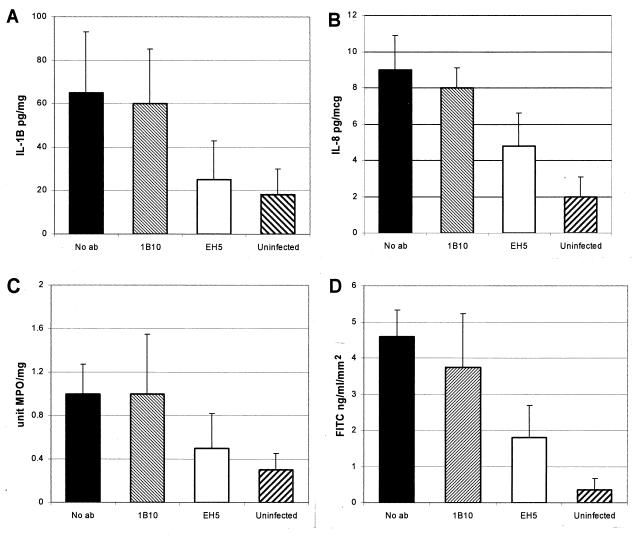
Infection of human colonic xenografts with E. histolytica trophozoites results in marked tissue damage and inflammation, with invasion of amebas into mucosal and submucosal layers (7, 8, 10). Elevations in levels of the proinflammatory cytokine IL-1β and the chemokine IL-8, as well as marked neutrophil influx into the infected human colonic xenograft, are consistent components of infection (8). These findings were reproduced in SCID-HU-INT mice receiving either PBS or the control monoclonal antibody 1B10. As shown in Fig. 1A, levels of IL-1β were significantly higher in E. histolytica-infected human colonic xenografts obtained from SCID-HU-INT mice receiving 1B10 (P ≤ 0.05) or no antibody (P ≤ 0.05) than in those obtained from uninfected controls. In contrast, levels of IL-1β were significantly lower in E. histolytica-infected human colonic xenografts from SCID-HU-INT mice passively immunized with EH5 than in animals treated with 1B10 (P ≤ 0.05) or PBS (P ≤ 0.05) (Fig. 1A) and were not significantly different from those seen in uninfected colonic xenografts (P = 0.4). IL-8 levels (Fig. 1B) were also significantly lower in E. histolytica-infected human colonic xenografts obtained from SCID-HU-INT mice passively immunized with EH5 than in those obtained from SCID-HU-INT mice treated with 1B10 (P ≤ 0.05) or PBS (P ≤ 0.05). However, levels of IL-8 in E. histolytica-infected human colonic xenografts from SCID-HU-INT mice passively immunized with EH5 were higher than those seen in uninfected human intestinal xenografts (P ≤ 0.05). MPO levels, a measure of neutrophil influx into the intestinal xenografts, were significantly lower in human colonic xenografts from SCID-HU-INT mice pretreated with EH5 than in E. histolytica-infected human colonic xenografts from SCID-HU-INT mice pretreated with PBS (P ≤ 0.05) or the control monoclonal antibody 1B10 (Fig. 1C). Levels of MPO in human colonic xenografts from EH5-pretreated SCID-HU-INT mice were not significantly different from those seen in uninfected human colonic xenografts (P = 0.3). Tissue damage caused by E. histolytica infection of human colonic xenografts results in a loss of the intestinal permeability barrier and can be quantified by measuring the flux of FITC-dextran from the lumen of the xenograft to the serum of the SCID-HU-INT mouse (8). We found significant differences in damage to E. histolytica-infected human colonic xenografts between SCID-HU-INT mice treated with EH5 and those receiving PBS or 1B10, with higher levels of FITC-dextran seen in the sera of the PBS (P ≤ 0.01)- or 1B10 (P ≤ 0.01)-treated SCID-HU-INT mice (Fig. 1D). Pretreatment with EH5 did not provide complete protection against damage to the intestinal permeability barrier, as levels of FITC-dextran were significantly higher in E. histolytica-infected, EH5-treated SCID-HU-INT mice than in uninfected SCID-HU-INT mice (P ≤ 0.05).
FIG. 1.
Effect of passive immunization of SCID-HU-INT mice with monoclonal antibody EH5 on inflammation and tissue damage in human colonic xenografts infected with E. histolytica. Values are the means of results for eight animals ± the standard deviation for E. histolytica-infected human colonic xenografts from SCID-HU-INT mice passively immunized with monoclonal antibody EH5, 1B10, PBS (No ab), or uninfected human colonic xenografts. (A) Levels of IL-1β, expressed as picograms of IL-1β per milligram of total protein; (B) levels of IL-8, expressed as picograms of IL-8 per microgram of total protein; (C) levels of MPO, expressed as units of MPO per milligram of total protein; and (D) levels of FITC in serum, expressed as nanograms of FITC per milliliter of serum per millimeter squared of tissue. All levels measured (A to D) were significantly lower in E. histolytica-infected human colonic xenografts from SCID-HU-INT mice treated with EH5 than in E. histolytica-infected colonic xenografts from SCID-HU-INT mice treated with the control monoclonal antibody 1B10 or with no antibody.
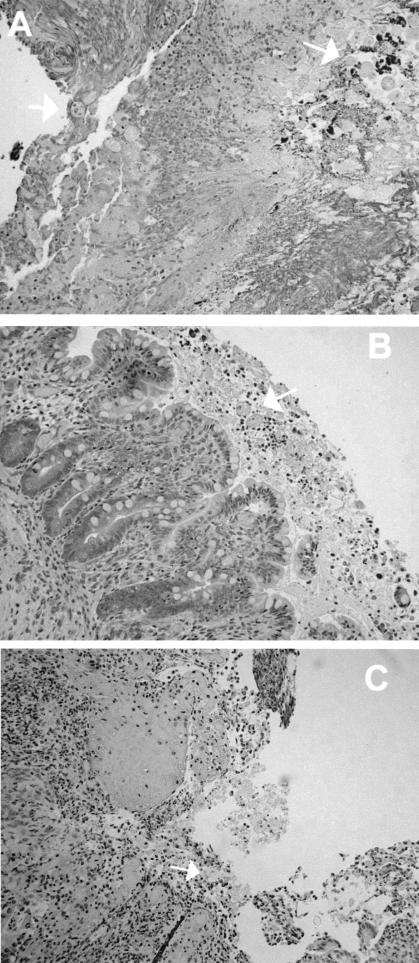
Examination of histological sections obtained from human colonic xenografts from SCID-HU-INT mice treated with 1B10 consistently revealed areas of mucosal and submucosal invasion by E. histolytica trophozoites, with mucosal damage and inflammation (Fig. 2A). These findings did not differ from those seen in E. histolytica-infected human colonic xenografts from untreated SCID-HU-INT mice (data not shown) (7, 8). Among the eight human colonic xenografts obtained from EH5-treated SCID-HU-INT mice, sections from five showed no evidence of invasive infection and, when ameba were seen, they were clumped within the lumen (Fig. 2B). In three other human colonic xenografts obtained from EH5-treated mice, areas of amebic invasion into the mucosa and submucosa, along with marked inflammation, were seen in some sections and were indistinguishable from those seen in E. histolytica-infected human colonic xenografts from 1B10- or PBS-treated SCID-HU-INT mice (Fig. 2C).
FIG. 2.
Morphologic findings in E. histolytica-infected human colonic xenografts from SCID-HU-INT mice. Photomicrographs of hematoxylin-and-eosin-stained sections of E. histolytica-infected human colonic xenografts from SCID-HU-INT mice treated with 1B10 (A) or EH5 (B and C) are shown. (A) Extensive mucosal destruction and inflammation and multiple E. histolytica trophozoites (white arrows) that have invaded the mucosa and submucosal tissues are visible in a section from a 1B10-treated SCID-HU-INT mouse. (B) In an E. histolytica-infected human colonic xenograft from an EH5-treated SCID-HU-INT mouse, amebic trophozoites are clumped together in the lumen but the mucosa remains intact and no signs of invasion arevisible. (C) In a section from a different EH-5-treated SCID-HU-INT mouse, mucosal destruction and inflammation are present and amebic trophozoites can be seen invading the mucosa and submucosal regions. Magnification, ×176 (all panels).
Our data indicate that pretreatment of SCID-HU-INT mice with a monoclonal antibody to the E. histolytica LPG-PPG molecule can significantly reduce disease in E. histolytica-infected human colonic xenografts. Treatment with EH5 prior to the initiation of amebic infection reduced the resultant parasite-induced gut inflammation, as measured by IL-1β, IL-8, and MPO levels, and the E. histolytica-induced tissue damage, as measured histologically and by the integrity of the mucosal permeability barrier. These data provide direct evidence that preexisting antibodies to a key amebic surface antigen can protect against amebic colitis in the human intestine in vivo. Our findings are consistent with those of recent clinical studies in which the presence of mucosal immunoglobulin A antibodies to the surface lectin of E. histolytica was associated with resistance to reinfection with E. histolytica in Bangladeshi children (3).
In our study, antibodies were administered i.p., as well as directly into the lumen of the human colonic xenograft, in order to maximize gut antibody levels. It is possible that i.p. administration alone, or direct intraluminal administration of EH5 alone, would have been protective, and work is in progress to address this possibility. Antibodies to EH5 did not completely protect all passively immunized SCID-HU-INT mice in this study, and this was apparent from both the analyses of IL-8 and FITC levels and the histological analysis. Where disease was detected, pathologic findings were identical to those seen in E. histolytica-infected human colonic xenografts from control SCID-HU-INT mice. There was a correlation between histological findings of tissue damage and higher FITC levels in the three EH5-treated mice with invasive disease (mean, 2.6 ng of FITC/ml of serum/mm2 of tissue [±7]) and those in the five EH5-treated SCID-HU-INT mice without signs of disease (mean, 1.32 ng/ml/mm2 ± 0.6; P ≤ 0.05), but differences in cytokine or MPO levels between these groups did not reach statistical significance (data not shown). The differences in efficacy among EH5-treated SCID-HU-INT mice were not secondary to differences in antibody levels, as murine immunoglobulin G levels (representing EH5 in these SCID mice) in the human intestinal xenografts were similar in all EH5-treated mice (data not shown).
The mechanisms by which EH5 provided protection in this setting are not clearly established, but previous studies have shown that antibodies to the amebic LPGs-PPGs can block amebic adherence to target cells (9). Consistent with the idea that effector functions of antibodies may not be necessary for protection in this setting, we have found that Fab fragments of EH5 appear to be as effective as the intact antibody in preventing disease (Z. Zhang and S.L. Stanley, Jr., unpublished data). Because these studies used SCID-HU-INT mice to investigate human colonic disease in vivo, it remains to be established whether active immunization with the amebic LPGs-PPGs can engender protective immunity against amebic colitis.
Acknowledgments
We thank Lynne Foster for technical assistance.
This work was supported by NIH grant AI30084 (to S.L.S.), NIH grant DK52574 for the Washington University Digestive Diseases Research Core Center, and NIH grant HD00836 to the Birth Defects Research Laboratory at the University of Washington, Seattle. S.L. Stanley, Jr., is a Burroughs Wellcome Scholar in Molecular Parasitology.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bhattacharya, A., R. Arya, C. G. Clark, and J. P. Ackers. 2000. Absence of lipophosphoglycan-like glycoconjugates in Entamoeba dispar. Parasitology 120:31-35. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya, A., R. Prasad, and D. L. Sacks. 1992. Identification and partial characterization of a lipophosphoglycan from a pathogenic strain of Entamoeba histolytica. Mol. Biochem. Parasitol. 56:161-168. [DOI] [PubMed] [Google Scholar]
- 3.Haque, R., I. M. Ali, R. B. Sack, B. M. Farr, G. Ramakrishnan, and W. A. Petri, Jr. 2001. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J. Infect. Dis. 183:1787-1793. [DOI] [PubMed] [Google Scholar]
- 4.Marinets, A., T. Zhang, N. Guillen, P. Gounon, B. Bohle, U. Vollmann, O. Scheiner, G. Wiedermann, S. L. Stanley, Jr., and M. Duchene. 1997. Protection against invasive amebiasis by a single monoclonal antibody directed against a lipophosphoglycan antigen localized on the surface of Entamoeba histolytica. J. Exp. Med. 186:1557-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moody, S., S. Becker, Y. Nuchamowitz, and D. Mirelman. 1997. Virulent and avirulent Entamoeba histolytica and Entamoeba dispar differ in their cell surface phosphorylated glycolipids. Parasitology 114:95-104. [DOI] [PubMed] [Google Scholar]
- 6.Moody-Haupt, S., J. H. Patterson, D. Mirelman, and M. J. McConville. 2000. The major surface antigens of Entamoeba histolytica trophozoites are GPI-anchored proteophosphoglycans. J. Mol. Biol. 297:409-420. [DOI] [PubMed] [Google Scholar]
- 7.Seydel, K. B., E. Li, P. E. Swanson, and S. L. Stanley, Jr. 1997. Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect. Immun. 65:1631-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seydel, K. B., E. Li, Z. Zhang, and S. L. Stanley, Jr. 1998. Epithelial cell-initiated inflammation plays a crucial role in early tissue damage in amebic infection of human intestine. Gastroenterology 115:1446-1453. [DOI] [PubMed] [Google Scholar]
- 9.Stanley, S. L., Jr., H. Huizenga, and E. Li. 1992. Isolation and partial characterization of a surface glycoconjugate of Entamoeba histolytica. Mol. Biochem. Parasitol. 50:127-138. [DOI] [PubMed] [Google Scholar]
- 10.Stenson, W. F., Z. Zhang, T. Riehl, and S. L. Stanley, Jr. 2001. Amebic infection in the human colon induces cyclooxygenase-2. Infect. Immun. 69:3382-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]