Abstract
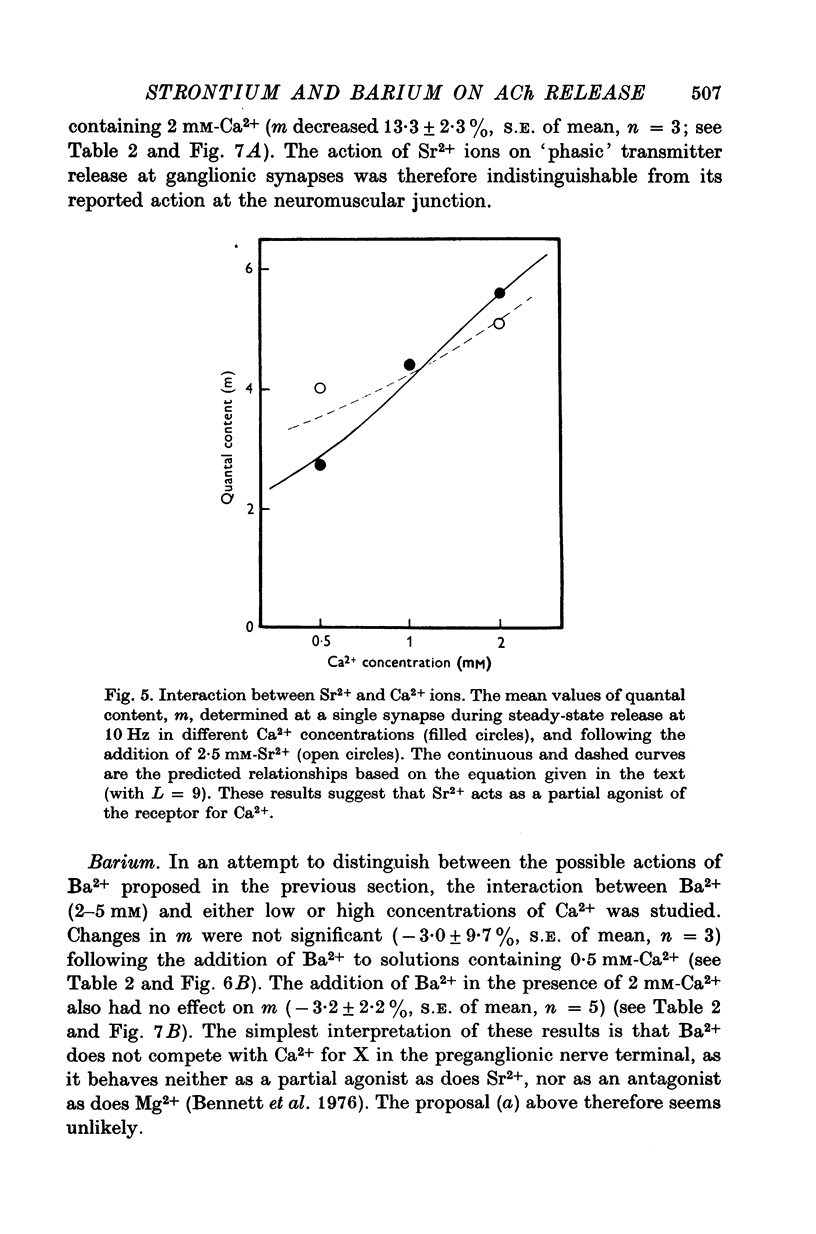
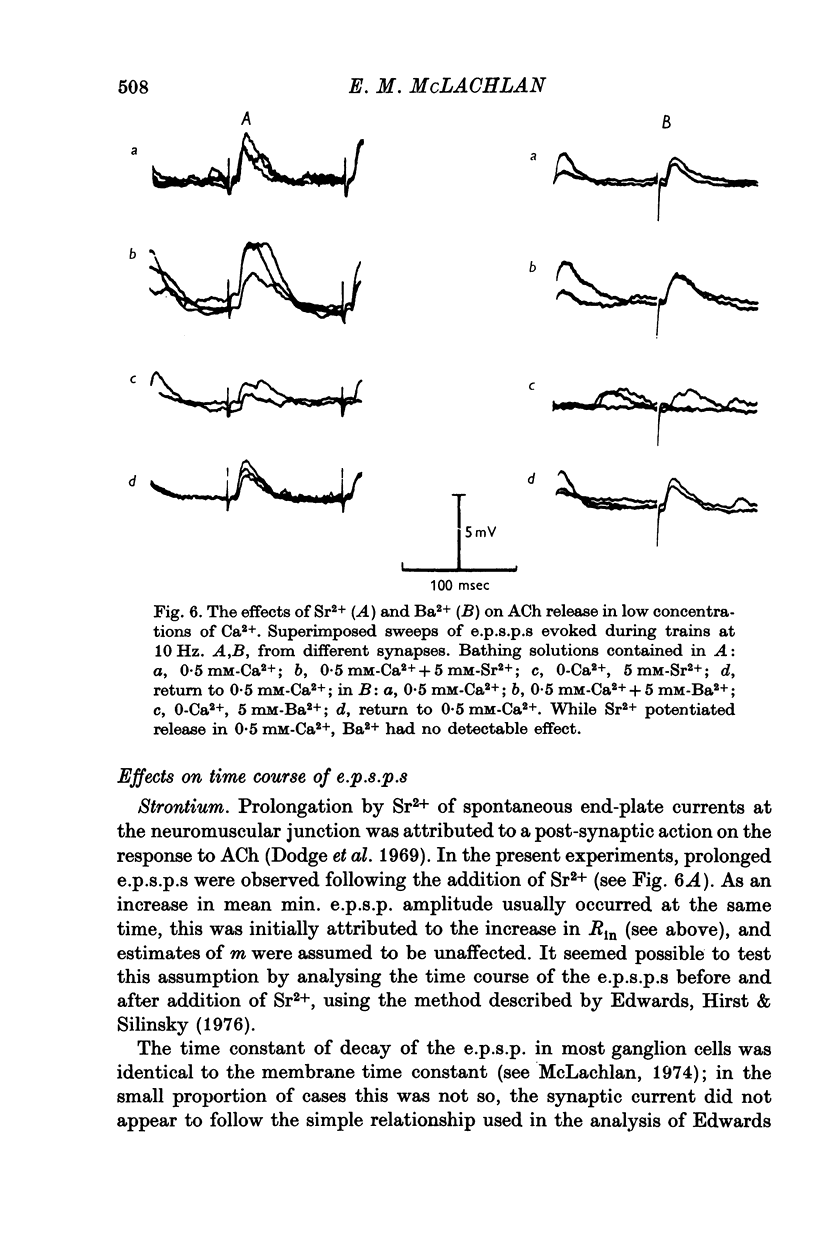
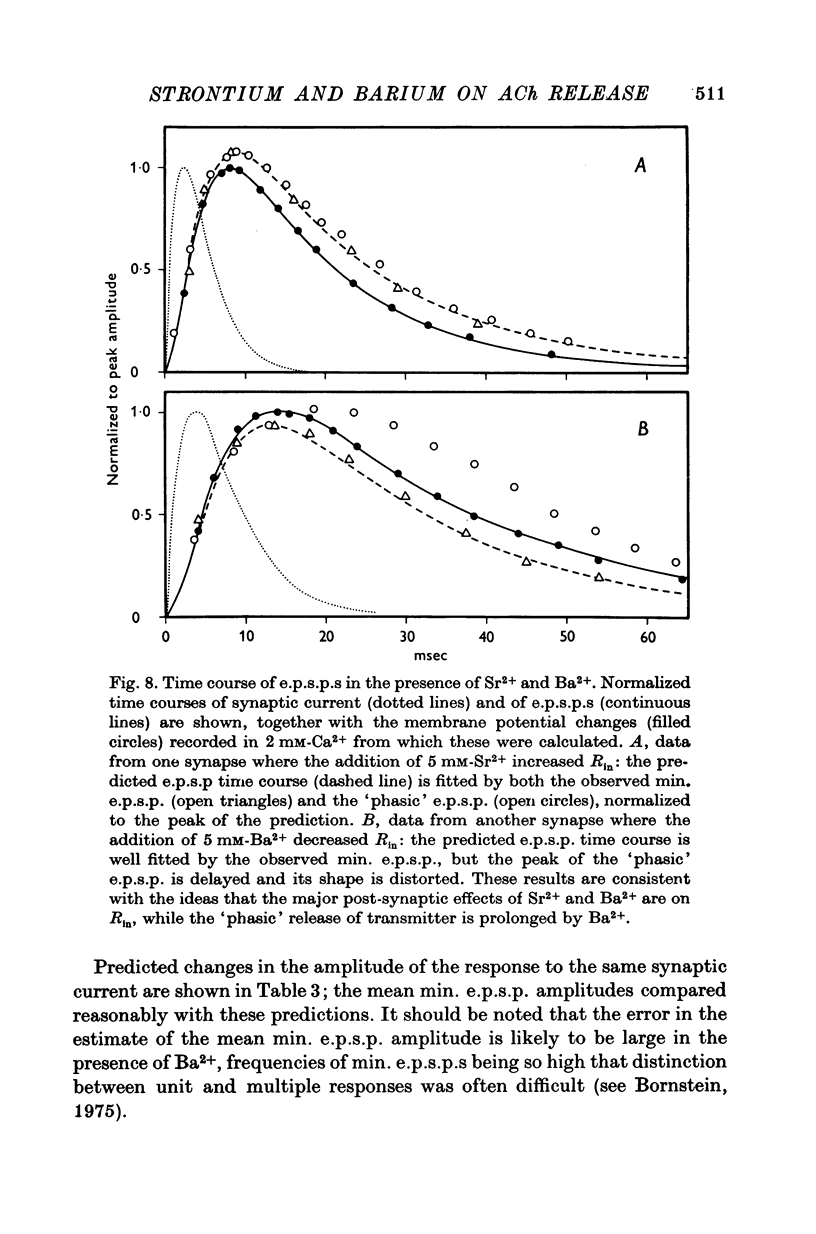
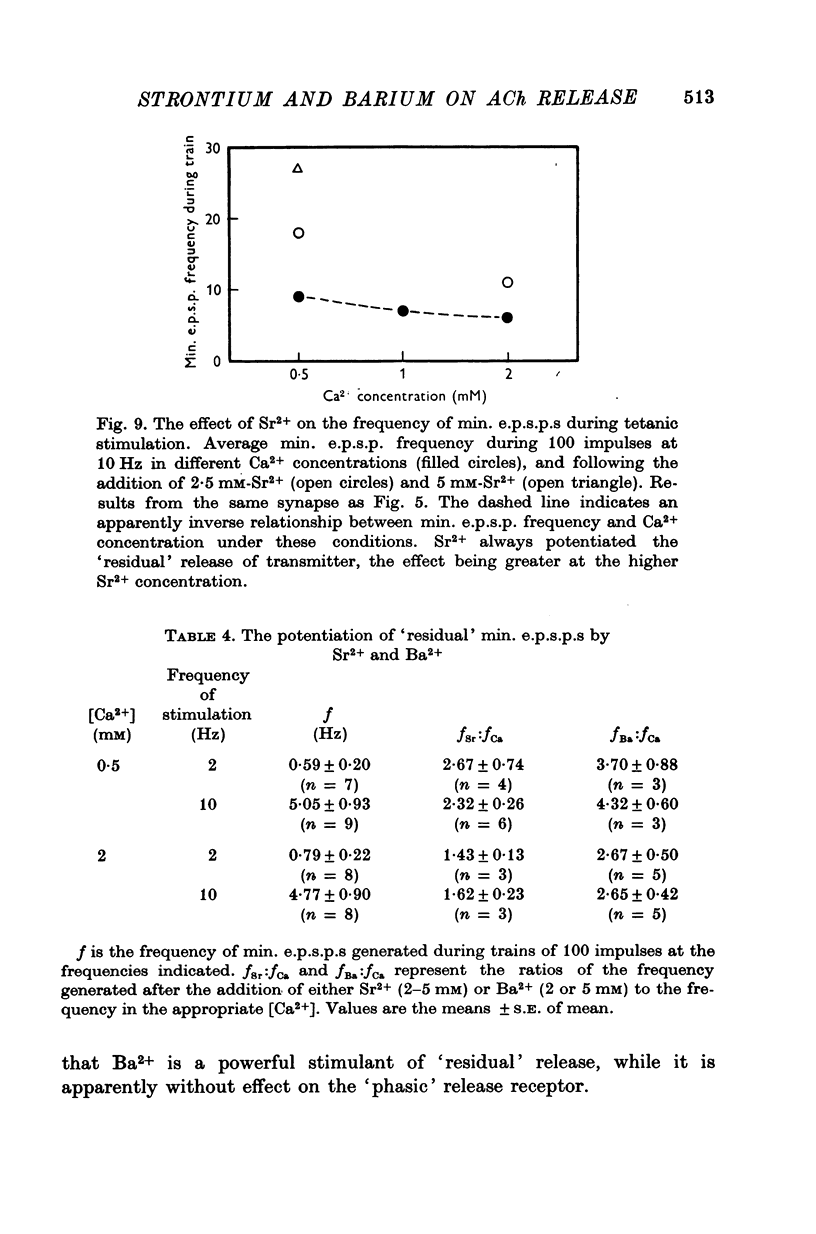
1. A study has been made of the effects of Sr2+ and Ba2+ ions at synapses in isolated superior cervical ganglia of guinea-pigs. Intracellular recordings of membrane potential were made from ganglion cells in the presence of different concentrations of Ca2+, Sr2+ and Ba2+ ions. 2. The addition of Sr2+ (2-5 mM) caused little change in resting membrane potential; in contrast, Ba2+ (1-6 mM) always depolarized the cells and prolonged the duration of action potentials. 3. The resting frequency of spontaneous miniature excitatory post-synaptic potentials (min. e.p.s.p.s) was briefly accelerated by the addition of either Sr2+ or Ba2+, but subsequently returned to about control levels. 4. Following replacement of Ca2+ by Sr2+, e.p.s.p.s could always be evoked during repetitive stimulation of preganglionic axons at a fixed latency after the nerve impulses ('phasic' transmitter release). Replacement of Ca2+ by Ba2+ produced many asynchronous e.p.s.p.s during trains of impulses ('residual' transmitter release). 5. By analysis of the interaction between Sr2+ and Ca2+, Sr2+ was shown to have a partial agonist action on 'phasic' transmitter release. The same analysis applied to Ba2+ failed to demonstrate either a partial agonist or antagonist action. 6. Both Sr2+ and Ba2+ prolonged e.p.s.p.s. Changes in Sr2+ could mainly be attributed to its effect on cell input resistance; Ba2+ may also prolong the time course of transmitter release. 7. The increased frequency of min. e.p.s.p.s which occurs during repetitive stimulation was potentiated by both Sr2+ and Ba2+, Ba2+ being about twice as potent as Sr2+. This activation of 'residual' transmitter release is independent of the action of these ions on 'phasic' release. 8. It is concluded that the reported maintenance by Ba2+ of acetyl-choline output from perfused ganglia results from the asynchronous release of large numbers of quanta during trains of impulses.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaes E., Meiri U., Rahamimoff H., Rahamimoff R. Proceedings: Possible role of mitochondria in transmitter release. J Physiol. 1974 Aug;241(1):30P–31P. [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Balnave R. J., Gage P. W. The inhibitory effect of manganese on transmitter release at the neuromuscular junction of the toad. Br J Pharmacol. 1973 Feb;47(2):339–352. doi: 10.1111/j.1476-5381.1973.tb08332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Hall R. The effect of calcium ions on the binomial statistic parameters which control acetylcholine release at synapses in striated muscle. J Physiol. 1975 May;247(2):429–446. doi: 10.1113/jphysiol.1975.sp010939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Pettigrew A. G. The effect of calcium ions on the binomial statistic parameters that control acetylcholine release at preganglionic nerve terminals. J Physiol. 1976 Jun;257(3):597–620. doi: 10.1113/jphysiol.1976.sp011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P. R., Mambrini J. Modification of transmitter release by ions which prolong the presynaptic action potential. J Physiol. 1970 Oct;210(3):681–695. doi: 10.1113/jphysiol.1970.sp009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Crawford A. C. The dependence of evoked transmitter release on external calcium ions at very low mean quantal contents. J Physiol. 1974 Jul;240(2):255–278. doi: 10.1113/jphysiol.1974.sp010609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., LYWOOD D. W., STRAUB R. W. The stimulant effect of barium on the release of acetylcholine from the superior cervical ganglion. J Physiol. 1961 May;156:515–522. doi: 10.1113/jphysiol.1961.sp006690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. STIMULANT ACTION OF BARIUM ON THE ADRENAL MEDULLA. Nature. 1964 Jul 18;203:305–307. doi: 10.1038/203305a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Miledi R., Rahamimoff R. Strontium and quantal release of transmitter at the neuromuscular junction. J Physiol. 1969 Jan;200(1):267–283. doi: 10.1113/jphysiol.1969.sp008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Hirst G. D., Silinsky E. M. Interaction between inhibitory and excitatory synaptic potentials at a peripheral neurone. J Physiol. 1976 Aug;259(3):647–663. doi: 10.1113/jphysiol.1976.sp011487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. The effect of calcium on the myelinated nerve fibre. J Physiol. 1957 Jul 11;137(2):245–260. doi: 10.1113/jphysiol.1957.sp005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. The effects of osmotic pressure changes on the spontaneous activity at motor nerve endings. J Physiol. 1956 Dec 28;134(3):689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN B. F., SUCKLING E. E. Effect of several cations on transmembrane potentials of cardiac muscle. Am J Physiol. 1956 Aug;186(2):317–324. doi: 10.1152/ajplegacy.1956.186.2.317. [DOI] [PubMed] [Google Scholar]
- Hales C. N., Milner R. D. Cations and the secretion of insulin from rabbit pancreas in vitro. J Physiol. 1968 Nov;199(1):177–187. doi: 10.1113/jphysiol.1968.sp008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I. Microphysiology of vertebrate neuromuscular transmission. Physiol Rev. 1973 Jul;53(3):674–723. doi: 10.1152/physrev.1973.53.3.674. [DOI] [PubMed] [Google Scholar]
- Hurlbut W. P., Longenecker H. B., Jr, Mauro A. Effects of calcium and magnesium on the frequency of miniature end-plate potentials during prolonged tetanization. J Physiol. 1971 Dec;219(1):17–38. doi: 10.1113/jphysiol.1971.sp009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A. Stimulus-secretion coupling on the oxytocin release from the isolated posterior pituitary lobe. Jpn J Physiol. 1968 Aug 15;18(4):471–480. doi: 10.2170/jjphysiol.18.471. [DOI] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. W., Wernig A. The binomial nature of transmitter release at the crayfish neuromuscular junction. J Physiol. 1971 Nov;218(3):757–767. doi: 10.1113/jphysiol.1971.sp009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Van Der Kloot W. Effects of the ionophore X-537A on acetylcholine release at the frog neuromuscular junction. J Physiol. 1976 Jul;259(1):177–198. doi: 10.1113/jphysiol.1976.sp011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. Action of Co and Ni at the frog neuromuscular junction. Nat New Biol. 1973 Sep 12;245(141):52–53. doi: 10.1038/newbio245052a0. [DOI] [PubMed] [Google Scholar]
- Laskowski M. B., Thies R. Interactions between calcium and barium on the spontaneous release of transmitter from mammalian motor nerve terminals. Int J Neurosci. 1972 Jul;4(1):11–16. doi: 10.3109/00207457209147159. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. An analysis of the release of acetylcholine from preganglionic nerve terminals. J Physiol. 1975 Feb;245(2):447–466. doi: 10.1113/jphysiol.1975.sp010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. Changes in statistical release parameters during prolonged stimulation of preganglionic nerve terminals. J Physiol. 1975 Dec;253(2):477–491. doi: 10.1113/jphysiol.1975.sp011201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. Electrophysiological evidence for the second store of ACh in preganglionic nerve terminals. Brain Res. 1975 Nov 14;98(2):373–376. doi: 10.1016/0006-8993(75)90017-7. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M. The formation of synapses in mammalian sympathetic ganglia reinnervated with preganglionic or somatic nerves. J Physiol. 1974 Feb;237(1):217–242. doi: 10.1113/jphysiol.1974.sp010479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Activation of transmitter release by strontium and calcium ions at the neuromuscular junction. J Physiol. 1971 Jul;215(3):709–726. doi: 10.1113/jphysiol.1971.sp009493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Yaari Y. Delayed release of transmitter at the frog neuromuscular junction. J Physiol. 1973 Jan;228(1):241–257. doi: 10.1113/jphysiol.1973.sp010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. Estimation of parameters for a model of transmitter release at synapses. Biometrics. 1976 Mar;32(1):61–68. [PubMed] [Google Scholar]
- Rotshenker S., Erulkar S. D., Rahamimoff R. Reduction in the frequency of miniature end-plate potentials by nerve stimulation in low calcium solutions. Brain Res. 1976 Jan 16;101(2):362–365. doi: 10.1016/0006-8993(76)90277-8. [DOI] [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. I. The resting cell and its alteration by extrinsic factors. Pharmacol Rev. 1958 Mar;10(1):59–164. [PubMed] [Google Scholar]
- WERMAN R., GRUNDFEST H. Graded and all-or-none electrogenesis in arthropod muscle. II. The effects of alkali-earth and onium ions on lobster muscle fibers. J Gen Physiol. 1961 May;44:997–1027. doi: 10.1085/jgp.44.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]


