Abstract
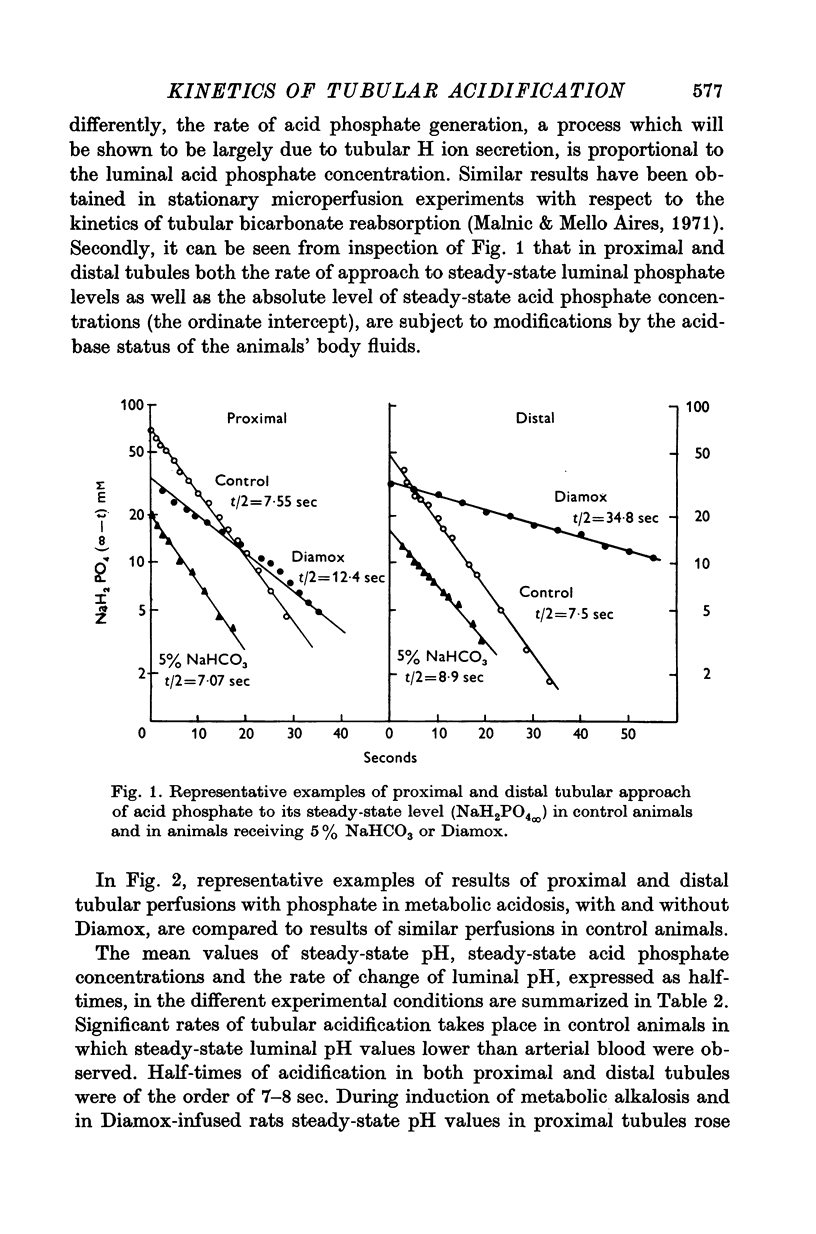
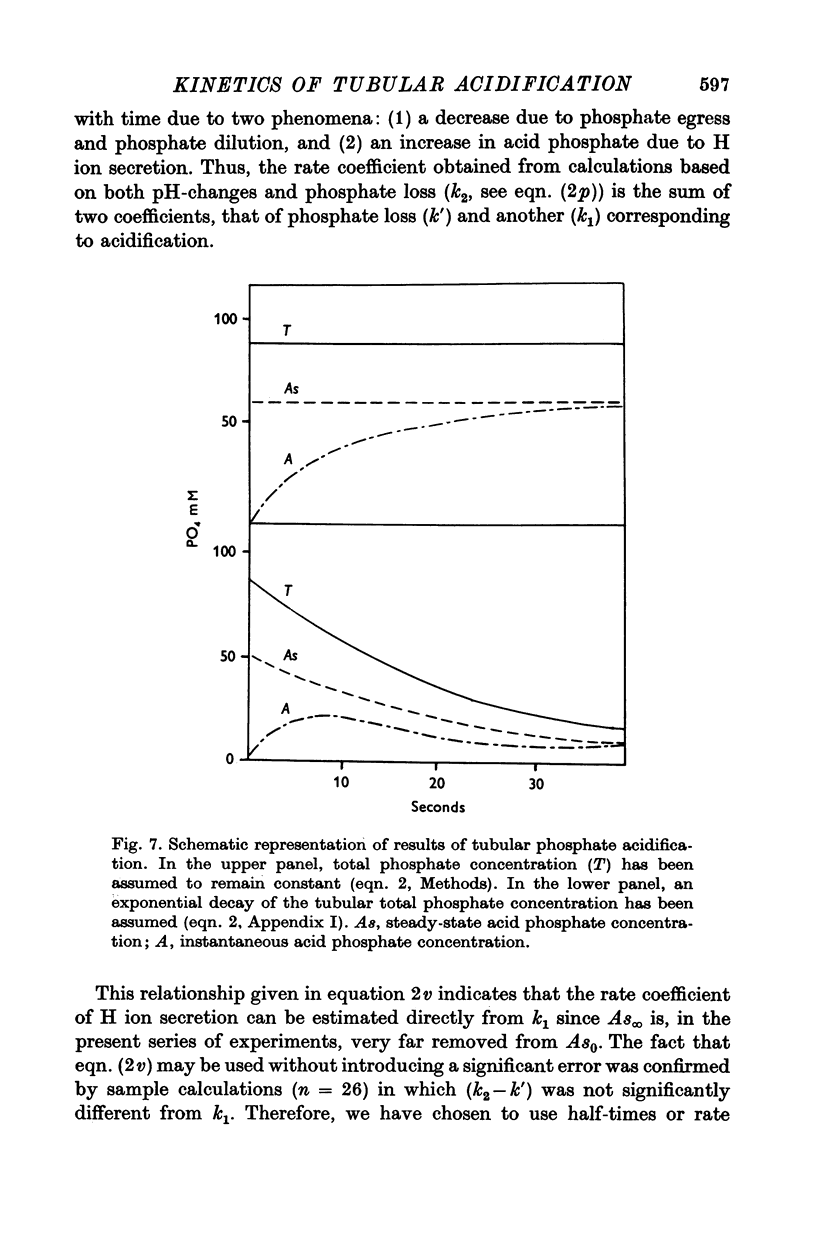
1. Some kinetic aspects of renal tubular acidification were studied in proximal and distal tubules of the rat kidney by combining stationary microperfusion methods and continuous measurements of luminal pH changes of phosphate or bicarbonate buffers by means of antimony electrodes. The analysis included the measurement of steady-state pH, steady-state buffer concentrations and acidification half-times. From these data, net rates of tubular bicarbonate reabsorption and of H ion secretion were obtained since it was shown that the rate of phosphate acidification provides a realistic estimate of H ion secretion. 2. Experiments were performed in control rats, in animals undergoing metabolic acidosis or alkalosis and in control and acidotic rats receiving the carbonic anydrase inhibitor Diamox. 3. In all experiments, the rates of tubular bicarbonate reabsorption and of phosphate acidification (H ion secretion) were proportional to luminal buffer levels. The changes of luminal acid concentrations followed first-order kinetics. 4. Steady-state transepithelial pH differences were reduced in metabolic alkalosis and after diamox but augmented during metabolic acidosis. 5. Acidification half-times were prolonged in metabolic acidosis and after Diamox but remained similar to control levels in metabolic alkalosis. 6. From the observation that both bicarbonate reabsorption and phosphate acidification are similarly affected by these experimental manoeuvres, it is concluded that H ion secretion plays a key role in both transport processes.
Full text
PDF


























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODSKY W. A., SATRAN R. Comparison of effects of acidosis and alkalosis on the renal action of diamox. Am J Physiol. 1959 Sep;197:585–594. doi: 10.1152/ajplegacy.1959.197.3.585. [DOI] [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S. A microperfusion study of bicarbonate accumulation in the proximal tubule of the rat kidney. J Clin Invest. 1967 Jan;46(1):95–102. doi: 10.1172/JCI105515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassola A. C., Giebisch G., Malnic G. Mechansims and components of renal tubular acidification. J Physiol. 1977 Jun;267(3):601–624. doi: 10.1113/jphysiol.1977.sp011828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., Gage P. W. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibers. J Gen Physiol. 1969 Mar;53(3):279–297. doi: 10.1085/jgp.53.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERTZ K. H. [Transtubular sodium chloride transport and permeability for nonelectrolytes in the proximal and distal convolution of the rat kidney]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;276:336–356. [PubMed] [Google Scholar]
- GOTTSCHALK C. W., LASSITER W. E., MYLLE M. Localization of urine acidification in the mammalian kidney. Am J Physiol. 1960 Mar;198:581–585. doi: 10.1152/ajplegacy.1960.198.3.581. [DOI] [PubMed] [Google Scholar]
- Garg L. C., Maren T. H. The rates of hydration of carbon dioxide and dehydration of carbonic acid at 37 degrees. Biochim Biophys Acta. 1972 Jan 28;261(1):70–76. doi: 10.1016/0304-4165(72)90315-7. [DOI] [PubMed] [Google Scholar]
- Green R., Giebisch G. Some problems with the antimony microelectrode. Adv Exp Med Biol. 1974;50(0):43–53. doi: 10.1007/978-1-4615-9023-1_5. [DOI] [PubMed] [Google Scholar]
- Karlmark B., Sohtell M. The determination of bicarbonate in nanoliter samples. Anal Biochem. 1973 May;53(1):1–11. doi: 10.1016/0003-2697(73)90401-6. [DOI] [PubMed] [Google Scholar]
- Kunau R. T., Jr The influence of the carbonic anhydrase inhibitor, benzolamide (CL-11,366), on the reabsorption of chloride, sodium, and bicarbonate in the proximal tubule of the rat. J Clin Invest. 1972 Feb;51(2):294–306. doi: 10.1172/JCI106814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman N. A. Regulation of renal bicarbonate reabsorption by extracellular volume. J Clin Invest. 1970 Mar;49(3):586–595. doi: 10.1172/JCI106269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine D. Z., Nash L. A. Effect of chronic NH4Cl acidosis on proximal tubular H2O and HCO3 reabsorption. Am J Physiol. 1973 Aug;225(2):380–384. doi: 10.1152/ajplegacy.1973.225.2.380. [DOI] [PubMed] [Google Scholar]
- MALNIC G., KLOSE R. M., GIEBISCH G. MICROPUNCTURE STUDY OF RENAL POTASSIUM EXCRETION IN THE RAT. Am J Physiol. 1964 Apr;206:674–686. doi: 10.1152/ajplegacy.1964.206.4.674. [DOI] [PubMed] [Google Scholar]
- MAREN T. H. Carbonic anhydrase inhibition. IV. The effects of metabolic acidosis on the response to diamox. Bull Johns Hopkins Hosp. 1956 Mar;98(3):159–183. [PubMed] [Google Scholar]
- Malnic G., Aires M. M., Cassola A. C. Kinetics analysis of renal tubular acidification by antimony microelectrodes. Adv Exp Med Biol. 1974;50(0):89–108. doi: 10.1007/978-1-4615-9023-1_8. [DOI] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972 Jan;222(1):147–158. doi: 10.1152/ajplegacy.1972.222.1.147. [DOI] [PubMed] [Google Scholar]
- Malnic G., Giebisch G. Symposium on acid-base homeostasis. Mechanism of renal hydrogenion secretion. Kidney Int. 1972 May;1(5):280–296. doi: 10.1038/ki.1972.41. [DOI] [PubMed] [Google Scholar]
- Malnic G., Mello Aires M. Microperfusion study of anion transfer in proximal tubules of rat kidney. Am J Physiol. 1970 Jan;218(1):27–32. doi: 10.1152/ajplegacy.1970.218.1.27. [DOI] [PubMed] [Google Scholar]
- Malnic G., Mello-Aires M., De Mello G. B., Giebisch G. Acidification of phosphate buffer in cortical tubules of rat kidney. Pflugers Arch. 1972;331(3):275–278. doi: 10.1007/BF00589134. [DOI] [PubMed] [Google Scholar]
- Malnic G., Steinmetz P. R. Transport processes in urinary acidification. Kidney Int. 1976 Feb;9(2):172–188. doi: 10.1038/ki.1976.19. [DOI] [PubMed] [Google Scholar]
- Malnic G., de Mello-Aires M. Kinetic study of bicarbonate reabsorption in proximal tubule of the rat. Am J Physiol. 1971 Jun;220(6):1759–1767. doi: 10.1152/ajplegacy.1971.220.6.1759. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Chemistry of the renal reabsorption of bicarbonate. Can J Physiol Pharmacol. 1974 Dec;52(6):1041–1050. doi: 10.1139/y74-138. [DOI] [PubMed] [Google Scholar]
- Mello Aires M., Malnic G. Peritubular pH and PCO'2 in renal tubular acidification. Am J Physiol. 1975 Jun;228(6):1766–1774. doi: 10.1152/ajplegacy.1975.228.6.1766. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Zurbach P. E. Re-evaluation of microelectrode methodology for the in vitro determination of pH and bicarbonate concentration. Kidney Int. 1974 Aug;6(2):81–91. doi: 10.1038/ki.1974.83. [DOI] [PubMed] [Google Scholar]
- STRICKLER J. C., THOMPSON D. D., KLOSE R. M., GIEBISCH G. MICROPUNCTURE STUDY OF INORGANIC PHOSPHATE EXCRETION IN THE RAT. J Clin Invest. 1964 Aug;43:1596–1607. doi: 10.1172/JCI105035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWAN R. C., PITTS R. F. Neutralization of infused acid by nephrectomized dogs. J Clin Invest. 1955 Feb;34(2):205–212. doi: 10.1172/JCI103073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiess W. A., Ayer J. L., Lotspeich W. D., Pitts R. F., Miner P. THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. II. FACTORS AFFECTING THE EXCRETION OF TITRATABLE ACID BY THE NORMAL HUMAN SUBJECT. J Clin Invest. 1948 Jan;27(1):57–64. doi: 10.1172/JCI101924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P. R., Lawson L. R. Effect of luminal pH on ion permeability and flows of Na+and H+ in turtle bladder. Am J Physiol. 1971 Jun;220(6):1573–1580. doi: 10.1152/ajplegacy.1971.220.6.1573. [DOI] [PubMed] [Google Scholar]
- Struyvenberg A., Morrison R. B., Relman A. S. Acid-base behavior of separated canine renal tubule cells. Am J Physiol. 1968 May;214(5):1155–1162. doi: 10.1152/ajplegacy.1968.214.5.1155. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Baumann K. Renal proximal tubular buffer-(glycodiazine) transport. Inhomogeneity of local transport rate, dependence on sodium, effect of inhibitors and chronic adaptation. Pflugers Arch. 1975 Jun 26;357(3-4):149–163. doi: 10.1007/BF00585971. [DOI] [PubMed] [Google Scholar]
- Vieira F. L., Malnic G. Hydrogen ion secretion by rat renal cortical tubules as studied by an antimony microelectrode. Am J Physiol. 1968 Apr;214(4):710–718. doi: 10.1152/ajplegacy.1968.214.4.710. [DOI] [PubMed] [Google Scholar]
- Wright F. S. Increasing magnitude of electrical potential along the renal distal tubule. Am J Physiol. 1971 Mar;220(3):624–638. doi: 10.1152/ajplegacy.1971.220.3.624. [DOI] [PubMed] [Google Scholar]


