Abstract
An immunodominant protein, P40, of Mycoplasma agalactiae was analyzed genetically and functionally. The gene encoding P40 was cloned from type strain PG2, sequenced, submitted to point mutagenesis in order to convert mycoplasma-specific TGATrp codon to the universal TGGTrp codon, and subsequently expressed in Escherichia coli. Nucleotide sequence-derived amino acid sequence comparisons revealed a similarity of P40 to the adhesin P50 of Mycoplasma hominis and to protein P89 of Spiroplasma citri, which is expected to be involved in adhesion. The amino acid sequence of P40 revealed a recognition site for a signal peptidase and strong antigenic and hydrophilic motifs in the C-terminal domain. Triton X-114 phase partitioning confirmed that P40 is a membrane protein. Fab fragments of antibodies directed against recombinant purified P40 significantly inhibited adherence of M. agalactiae strains PG2 to lamb joint synovial cells LSM 192. Sera taken sequentially from sheep infected with PG2 revealed that P40 induced a strong and persistent immune response that gave strong signals on immunoblots containing recombinant P40 even 3 months after infection. The gene encoding P40 was present in a single copy in all of the 26 field strains of M. agalactiae analyzed and was not detected in closely related mycoplasma species. P40 was expressed as a protein with an apparent molecular mass of 37 kDa on sodium dodecyl sulfate-acrylamide gels by all M. agalactiae strains except for serotype C strains, which showed nonsense mutations in their p40 genes.
Mycoplasma agalactiae is a major pathogen of goats and sheep. It is found particularly in Mediterranean countries but is also reported from many other areas in the world. M. agalactiae causes contagious agalactia, a syndrome that primarily affects the mammary gland, but it can also affect joints, eyes and, in some cases, the lungs (6, 13). Contagious agalactia rises to a chronic state usually explained by the capacity of mycoplasmas to evade the host immune system. Different strategies are used by mycoplasmas for this purpose, including the phase and/or size variation of the membrane surface proteins, which leads to a constantly changing surface structure and the capacity of some lipoproteins to induce the expression of up- and downmodulating cytokines (32). However, a prerequisite for colonization and infection is adhesion to the host cell (32). Adhesion involves particular proteins named adhesins. In some mycoplasmas, such as Mycoplasma pneumoniae, Mycoplasma genitalium, and Mycoplasma gallisepticum, adhesion results from the action of multiple proteins, leading to the movement and concentration of the adhesins at a specialized structure, the attachment tip organelle (7, 20, 21, 25, 35). In Mycoplasma hominis, adhesins are found dispersed over the cell surface and are directly involved in the attachment to the host cell (17, 31). The close contact between mycoplasma and the target cell membrane resulting from this adhesion is thought to favor the transfer or exchange of metabolic components (32). Moreover, adhesion may enable oxidative damage of host cells by facilitating the transfer of metabolites such as peroxides and superoxides from the mycoplasmas to the host cells (31, 38).
While the mechanisms of adhesion are currently well described, particularly for human pathogens, no information is available regarding the factors involved in adherence of Mycoplasma agalactiae to mammary epithelium cells. In the present study, we focused our work on the molecular characterization of a new immunodominant adhesin, named P40, and studied its impact on the adhesion of M. agalactiae to sheep synovial membrane cells of a low passage number.
MATERIALS AND METHODS
Strains, cultivation, construction, and screening of gene libraries and radiolabeling.
The mycoplasma strains used in the present study are listed in Table 1. They were cultured in standard mycoplasma medium (Axcell Biotechnologies, Lyon, France) at 37°C until the stationary growth phase (30). All M. agalactiae strains used in the present study were confirmed to belong to the species M. agalactiae by using a specific uvrC-based PCR as described previously (36).
TABLE 1.
Mycoplasma strains used
| Mycoplasma species | Straina | Origin | Yr isolated | Host | Serotype(s)b | p40c | P40 expressiond |
|---|---|---|---|---|---|---|---|
| M. agalactiae | PG2 | Spain | 1973 | Goat/type strain | A | + | ++ |
| 9 | Italy | Sheep | C | + | − | ||
| 190 | Rumania | 1951 | Sheep/vaccine strain | A | + | ++ | |
| 209 | France | 1983 | Goat | F | + | ++ | |
| 3990 | France | H | + | ++ | |||
| 4021 | France | 1988 | Sheep | A | + | ++ | |
| 4054-1 | France | 1986 | Goat | EFGH | + | + | |
| 4055 | France | 1987 | Goat | F | + | − | |
| 4210 | France | 1982 | Goat | D | + | + | |
| 4212 | France | 1984 | G | + | + | ||
| 4258 | France | Goat | AB | + | + | ||
| 5225 | Spain | 1991 | Goat | C | + | − | |
| 5632 | Spain | 1991 | Goat | F | + | ++ | |
| 5670 | Spain | 1991 | Sheep | ABCD | + | ++ | |
| 5725 | France | 1990 | Sheep | A | + | ++ | |
| 5826 | Spain | 1992 | Sheep | A | + | ++ | |
| 6833 | Italy | Goat | A | + | ++ | ||
| 6968 | Spain | 1993 | Sheep | D | + | + | |
| 7169 | Switzerland | Goat | C | + | − | ||
| 7314 | Greece | 1986 | Sheep | A | + | ++ | |
| 7327 | Greece | 1987 | Goat | A | + | ++ | |
| 7375 | Switzerland | Goat | C | + | − | ||
| 7784 | France | 1994 | Sheep | A | + | ++ | |
| 8064 | Ivory Coast | 1989 | Sheep | E | + | ++ | |
| 8750 | France | 1994 | Sheep | A | + | ++ | |
| 9385 | Portugal | Goat | A | + | ++ | ||
| 9600 | Portugal | Goat | A | + | ++ | ||
| M. bovis | PG45 | Cattle/type strain | − | − | |||
| M. capricolum subsp. capricolum | California kid | California | Goat/type strain | − | − | ||
| M. mycoides subsp. mycoides LC | Y-goat | Australia | Goat/type strain | − | − | ||
| Mycoplasma "bovine group 7' | PG50 | Australia | Cattle/reference strain | − | − | ||
| M. putrefaciens | KS1 | Goat/type strain | − | − | |||
| M. ovipneumoniae | Y98 | − | − | ||||
| M. mycoides subsp. capri | PG3 | Goat/type strain | − | − | |||
| Mycoplasma serogroup 11 | 2D | − | − | ||||
| M. bovigenitalium | PG11 | − | − | ||||
| M. conjunctivae | HRC/581 | − | − | ||||
| M. arginini | G230 | − | − |
Collections: strains were obtained from F. Poumarat, AFSSA, Lyon, France; F. Thiaucourt, CIRAD, Montpellier, France; M. Lambert, AFSSA, Sophia Antipolis, France; and S. Tola, Istituto Zooprofilattico Sperimentale, Sassari, Italy.
Presence of the p40 gene as analyzed by PCR and Southern blotting.
“++” indicates a strong signal, “+” indicates a moderate signal, and “−” indicates no amplification or detection.
For labeling of M. agalactiae cells, type strain PG2 was grown in standard mycoplasma medium (Axcell Biotechnologies) at 37°C with 5% CO2. Then, 9 ml of standard medium was inoculated with 1 ml of a 3-day culture. After an additional 2 days of growth at 37°C, this 10-ml mixture was added to 190 ml of standard mycoplasma medium containing 20 μCi of [U-14C]palmitic acid (Amersham Pharmacia Biotech, Uppsala, Sweden). Cells were grown for 3 days at 37°C until stationary growth phase. Labeled mycoplasmas were harvested by centrifugation at 6,000 × g for 20 min, washed twice with buffer A (0.05 M Tris-HCl, pH 7.2; 0.1 M NaCl; 1 mM CaCl2), and resuspended in ca. 2 ml of buffer A to reach a titer of 109 CFU/ml. Aliquots of 200 μl were used directly or frozen at −80°C until further use.
A phage expression gene library of M. agalactiae type strain was made by using the ZAP Express Predigested Vector Kit (Stratagene, La Jolla, Calif.) as described previously (14). The phage library was plated according to the manufacturer's protocol by using the Escherichia coli host strain XL1-Blue MRF′ (Stratagene). Screening of the library was performed by standard protocols with serum obtained from a naturally infected, convalescent sheep (serum PAL 97) and used at a dilution of 1:600. Positive clones were selected, amplified, and finally excised in vivo into phagemids pBK-CMV by using the filamentous helper phage ExAssist (Stratagene) as directed by the manufacturer. The pETHIS-1 expression vector (34) was used for polyhistidine fusion at both the N-terminal and C-terminal ends of the cloned proteins. The E. coli BL21(DE3) (Novagen, Madison, Wis.) was used for expression of the recombinant proteins. Plasmids were extracted by using the QIAprep Spin Miniprep Kit (Qiagen AG, Basel, Switzerland).
Nucleotide sequencing and sequence analysis.
DNA sequencing was performed with a DNA Sequenator AB 3100 genetic analyzer and the Taq dye deoxy terminator cycle sequencing kit (Applied Biosystems, Norwalk, Conn.), with oligonucleotide primers matching the T3 and T7 promoter sequences (Table 2) that flank the cloning site of the pBK-CMV vector. For the analysis of the complete cloned segment, the deletion technique was employed by using exonuclease III of the double-stranded nested deletion kit (Amersham Pharmacia Biotech). The DNA sequence was assembled by using the program Sequencher 3.0 (GeneCodes, Ann Arbor, Mich.). The DNA and deduced amino acid sequences were analyzed with the PC/Gene program PROSITE (3). Sequence comparisons with sequences in the GenBank and EMBL databases were made by using the BLAST programs BLASTN, BLASTX, and BLASTP (1). Alignments were performed with PILEUP from the GCG Wisconsin package (Genetics Computer Group, Inc., Madison, Wis.). Analysis of protein sequences for characteristic motifs was carried out by using the programs SignalIP (27), ProtScale (http://www.expasy.ch/cgi-bin/protscale.pl), and TMpred (http://www.ch.embnet.org/software/TMPRED_form.html).
TABLE 2.
Oligonucleotide primers
| Primer | Sequencea | Nucleotide positionb (range) | Annealing tempc (°C) |
|---|---|---|---|
| T3 (universal) | 5′-GCGCGCAATTAACCCTCACTAAAG-3′ | 53.0 | |
| T7 (universal) | 5′-GTAATACGACTCACTATAGGGC-3′ | 48.5 | |
| pBK13a-L1 | 5′-GCAACTAATGATAAAGAAGGC-3′ | 349-368 | 48.8 |
| pBK13a-R1 | 5′-GTAATGCCGCACTTTGCAGC-3′ | 3220-3229 | 51.8 |
| 13aMut-L | 5′-GAAAAATGgGAAGCAAATACATCA-3 | 1760-1783 | 51.3 |
| 13aMut-R | 5′-TGCTTCcCATTTTTCAATATTTTTTA-3 | 1774-1749 | 51.4 |
| 13aEcoR1-L | 5′-GCgaattcTGATGATAAGAACGAAAATTCAC-3′ | 1082-1112 | 49.5d |
| 13aNot1-R | 5′-AAgcggccgcACCCAGTGTCTTTTGATTTAAC-3′ | 2001-1970 | 49.2d |
| MagaP13a-L1 | 5′-AAACGGGGCTAAAGAAGCTG-3′ | 828-847 | 51.0 |
| MagaP13a-R1 | 5′-AAGCTGGTTATATTTTCCATATC-3′ | 2151-2173 | 48.9 |
| MagaP13a-L2 | 5′-TGTCAAAAATACAAATCTAGGTG-3′ | 1168-1190 | 48.8 |
| MagaP13a-R2 | 5′-CTTTAACTTGTGATGAGGTATC-3′ | 1823-1844 | 47.9 |
| MagaP13a-L3 | 5′-CGATATATGTAATTAGTGCTCTC-3′ | 47.9 | |
| MagaP13a-R3 | 5′-GTTCTTATCATCACATTTAGCAG-3′ | 48.6 |
Lowercase letters indicate nucleotides added to create restriction enzyme recognition sites and mutations for cloning.
Based on nucleotide sequence AJ315329.
Obtained with the PCR primer annealing temperature calculator developed by J. Boxall at http://www.iacr.bbsrc.ac.uk/res/depts/biochem/old-or-to-move/tcalculator.html by using the parameters 30% as the target GC content and 1,000 bp as the target size.
Nucleotides added to create the restriction enzyme recognition sites were not considered.
PCR amplification and Southern blot analysis.
PCRs were performed by using a DNA thermal cycler Gene Amp 9600 (Applied Biosystems) in a 50-μl reaction mixture [50 mM Tris-HCl, pH 9.2; 1.75 mM MgCl2; 16 mM (NH4)2SO4, and 350 μM concentrations of each deoxynucleoside triphosphate (dNTP)] containing a 300 nM concentration of each primer (Table 2), 1.75 U of Taq DNA polymerase (Roche Diagnostics, Rotkreuz, Switzerland), and 50 ng of genomic DNA as a template. The DNA samples were heated for 2 min at 94°C to ensure denaturation prior to amplification for 35 cycles (30 s of denaturation at 94°C, 30 s at the optimal annealing temperature of the primers [Table 2], and a 1-min extension at 68°C). A mixture of Taq and Pwo DNA polymerase (expand long template PCR system kit; Roche Diagnostics) was used when PCR products were to be cloned. Digoxigenin-11-dUTP (DIG)-labeled probes were produced by PCR as described above in the presence of 50 μM DIG (Roche Diagnostics).
For Southern blotting, genomic mycoplasmal DNA was digested with HindIII, and the fragments were separated by electrophoresis on a 0.7% agarose gel and then transferred onto a positively charged nylon membrane (Roche Diagnostics) (2). Hybridization was performed with DIG-labeled probes and by detection by anti-digoxigenin antibody conjugated to alkaline phosphatase, followed by CDP-Star (Roche Diagnostics) detection as described by the manufacturer.
Site-directed mutagenesis.
For the expression of a mature P40 protein in E. coli host strains, the p40 gene from genomic DNA of M. agalactiae strain PG2 was first amplified with the primers 13aEcoRI-L and 13aNotI-R (Table 2). The PCR product was cloned into the pGEM-T vector (Promega Corp., Madison, Wis.) for site-directed mutagenesis. In order to replace the mycoplasma specific TGATrp codon with the universal TGGTrp codon, we used the overlap extension-PCR method (11). For this purpose, PCR was performed in a 50-μl reaction mixture [10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris-HCl (pH 8.8), 2.0 mM MgSO4, 0.1% Triton X-100, 0.1 mg of nuclease-free bovine serum albumin/ml, and a 350 μM concentration of each dNTP] containing 2 to 3 U of Pfu DNA polymerase (Promega Corp.), 40 ng of Miniprep DNA as a template and 15 pmol of overlapping mutagenesis primers (carrying the appropriate nucleotide substitution) (Table 2). After one step at 94°C for 2 min, the plasmid and the DNA insert were amplified for 16 cycles (30 s of denaturation at 94°C, 30 s at the optimal annealing temperature of the primers [Table 2], and an 8-min extension at 72°C], followed by a final step at 72°C for 7 min). The PCR product was digested with DpnI for 1 h at 37°C to eliminate the methylated (parental) DNA (26) and subsequently used to transform E. coli XL1-Blue.
Expression and purification of recombinant protein P40.
The products of the site-directed mutagenesis were sequenced. One positive clone was then digested by EcoRI and NotI for cloning into pETHIS-1. The construct was analyzed by restriction enzyme digestion and DNA sequencing, and then introduced by transformation into E. coli BL21(DE3) (Novagen) for expression of the fusion protein. Expression of this recombinant protein was induced by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at mid-exponential phase, and incubation continued at 37°C for 3 h. The cells were sedimented by centrifugation at 3,000 × g for 10 min, resuspended in 5 ml of PN buffer (50 mM NaH2PO4, pH 8; 300 mM NaCl), sonicated with a Microtip for 4 min with a duty cycle of 50% (1-s pulses) in a Branson Sonifier 250 (Branson Ultrasonics, Danbury, Conn.), and then centrifuged at 15,000 × g for 20 min. The supernatant containing the cytosolic fraction was kept, and the pelleted cell debris was resuspended in 5 ml of PN buffer (insoluble fraction). Analysis of the sonicated fraction on sodium dodecyl sulfate (SDS)-12% acrylamide gels (23) showed that the induced protein was contained in the pellet. Guanidine hydrochloride was added to a final concentration of 6 M to the insoluble fraction, and the mixture was loaded onto a prewashed 2.5-ml bed volume nickel-nitrilotriacetic acid-agarose column (Qiagen AG) and washed once more with 30 ml of PNG buffer (50 mM NaH2PO4, pH 8.0; 300 mM NaCl; 6 M guanidine hydrochloride). Step elution was performed with 10 ml of PNG buffer (50 mM NaH2PO4, pH 8.0; 300 mM NaCl; 6 M guanidine hydrochloride) at pH 7.0, 6.0, 5.5, 5.0, and 4.5, and fractions of 1 ml were collected. The fractions were dialyzed against PN buffer (50 mM NaH2PO4, pH 8; 300 mM NaCl) and analyzed on SDS-12% acrylamide gels. The purified fusion protein eluted at pH 5.0 and was dialyzed overnight against PN buffer.
Sera and polyclonal antibodies.
Serum PAL 97 from a convalescent, naturally infected ewe that developed a chronic infection was used to screen the expression library and as positive control for immunoblots. Serum samples taken sequentially after experimental infections of ewes by subcutaneous inoculation with type strain PG2 or strain 5725 as described previously (14) were used to study the course of the immune response against P40 during an infection with M. agalactiae. Polyclonal monospecific serum directed against P40 was obtained by subcutaneous immunization of rabbits with 160 μg of purified recombinant polyhistidine-tailed protein P40 in 500 μl of PN buffer mixed with 500 μl of Adjuvant 10 (Gerbu Biotechnik GmbH, Gaiberg, Germany), followed by booster immunizations with 40 and 20 μg of protein in Adjuvant 10 at 2 and 4 weeks, respectively, after the first immunization. The rabbits were bled 10 days after the last booster immunization. Antisera were prepared from the blood samples and stored at −20°C.
Immunoglobulin purification and Fab preparation.
Immunoglobulin G (IgG) fractions from rabbit monospecific anti-P40-His serum and from preserum of the same rabbit were purified with the HiTrap Protein G kit (Amersham Pharmacia Biotech) as indicated by the manufacturer. Preparation of the Fab fragments was performed with the ImmunoPure Fab Preparation kit (Pierce, Rockford, Ill.) according to the manufacturer's instruction by incubating 10 mg of IgG anti-P40-His from an immunized rabbit and from preserum of the same rabbit with immobilized papain. The crude digest was then applied to a column of immobilized protein A. Separation of Fab fragments from the Fc fragments bound to protein A was obtained by washing the column. The Fab fragments were then dialyzed overnight against phosphate-buffered saline buffer (140 mM NaCl, 2.7 mM KCl, 15 mM KH2PO4, 8 mM Na2HPO4; pH 7.4). Protein concentrations were determined by using the method of Bradford (10).
Immunoblot analysis and Triton X-114 partitioning.
Total antigen from mycoplasmas was treated as previously described (14). Immunoblotting was carried out with the sheep PAL 97 serum at a dilution of 1:600, the rabbit monospecific serum anti-P40 at a dilution of 1:1000, and sheep experimental serial sera at a dilution of 1:100 (2).
M. agalactiae total antigen was separated into hydrophobic and hydrophilic fractions by the Triton X-114 partitioning method (8) with a 10-ml culture of PG2 grown until the stationary phase. Samples from the Triton X-114 detergent phase and from the aqueous phase and whole mycoplasma cells were mixed with protein sample buffer, analyzed on SDS-12% acrylamide gels, and blotted onto nitrocellulose. The filter was subsequently used for immunoblotting with the monospecific, polyclonal antibodies directed against P40.
Culture of lamb joint synovial cells.
Preparation of lamb membrane cell culture (LSM 192) from carpal joint synovial tissue was explanted into six-well tissue culture plates. The tissue was mechanically minced and subsequently incubated at 37°C in tissue culture medium (minimal essential medium [Biochrom, Berlin, Germany] supplemented with 10% fetal calf serum, 2.5 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml) in a CO2 incubator. The medium was changed every 3 days. After they reached confluence, the cells were treated with trypsin and transferred to 75-cm2 tissue culture flasks. Cells were passaged at weekly intervals and transferred to 24-well plates. Synovial cells were used in adherence assays when they had reached confluence, with 105 cells per well of 2 cm2. Nonspecific sites of eukaryotic membrane proteins were blocked by incubation of the cells with a solution of 0.1% bovine serum albumin for 15 min at 37°C prior to the addition of the mycoplasma suspensions for adherence assays.
Adherence and inhibition assays.
To determine the optimal concentration of M. agalactiae type strain PG2 cultures for the adhesion assay, dilutions (1:2 to 1:128) of the 109 CFU/ml of culture (either fresh or stored at −80°C) were prepared in buffer A (33). After this, 200 μl of each dilution was transferred to each LSM 192 monolayer and incubated for 2 h at 37°C with shaking. In each case, three parallel trials were carried out. After the removal of excess liquid, cells were washed twice with 500 μl of buffer A and then solubilized with 100 μl of 1% (wt/vol) SDS solution plus 500 μl of buffer A for 2 h at 37°C with shaking. The lysed suspension of each well was then transferred into a vial containing 3 ml of scintillation cocktail Emulsifier Scintillator Plus (Packard Instrument Company, Meriden, Conn.), and the decays per minute were measured with a scintillation spectrometer (Wallac 1410 Liquid Scintillation Counter; Perkin-Elmer, Regensdorf, Switzerland). The relative adherence (i.e., the percentage of mycoplasmas attached to LSM 192 cells) was expressed as the ratio of decays per minute in the washed and lysed samples divided by the decays per minute measured in 200 μl of mycoplasma suspension at the corresponding dilution.
A mycoplasma concentration of 3.1 × 107 CFU/ml which resulted in 22.4% of the mycoplasmas adhering to the LSM 192 cells was used for inhibition assays. Inhibition of adhesion by P40 antibodies was measured as the percentage of the reduction of adhesion after exposure of the mycoplasmas to 130 μg of purified IgG/ml from monospecific rabbit antiserum directed against recombinant P40. This IgG concentration gave optimal inhibition of adhesion and corresponded to the IgG concentration in serum diluted 1:20. This corresponds to the serum dilution used by Washburn et al. (39). As controls, purified IgG antibodies from preserum of the same rabbit were used at the same concentration as used for the anti-P40 IgG antibodies. Fab fragments directed against P40-His and from preserum were used at a concentration of 90 μg/ml. Inhibition assays were performed by adding 100 μl of each purified IgG (260 μg/ml) or Fab fragments (180 μg/ml) to 100 μl of PG2 mycoplasmas at 6.2 × 107 CFU/ml to reach a final PG2 concentration of 3.1 × 107 CFU/ml. The samples were incubated for 2 h at room temperature with shaking. The mixture was then transferred into the 2-cm2 cavities of the LSM 192 culture plate and incubated for additional 2 h at 37°C with shaking. Cells were then washed and harvested as described above and adhering mycoplasmas measured with the scintillation spectrometer.
Nucleotide sequence accession number.
The EMBL/GenBank accession number for the nucleotide sequence of the 3,495-bp fragment in plasmid pJFFBF13a is AJ315329. The sequences of the p40 genes from 11 M. agalactiae strains are deposited in EMBL/GenBank under accession numbers AJ344229 to AJ344239.
RESULTS
Cloning the gene encoding a 40-kDa antigen of M. agalactiae.
Screening for immunodominant proteins of the expression library of M. agalactiae type strain PG2 containing ca. 5 × 107 recombinant phage clones with serum PAL 97 revealed several positive phage plaques, one of which was used in this study. The DNA from this plaque was converted into phagemid by in vivo excision and the resulting plasmid, named pJFFBF13a, contained a 3.5-kb insert. Total antigen preparation of the Escherichia coli clone containing the selected plasmid pJFFBF13a revealed on immunoblots a few protein bands with apparent molecular masses between 25 and 35 kDa reacting weakly with serum PAL 97, and a strongly reacting band at ca. 28 kDa (data not shown).
Analysis of the 3.5-kb insert in plasmid pJFFBF13a.
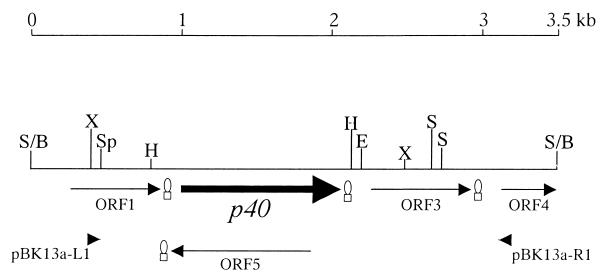
The 3.5-kb insert of plasmid pJFFBF13a was sequenced. PCR amplifications by using genomic DNA of PG2 as a template and primers pBK13a-L1 and pBK13a-R1 (Table 2 and Fig. 1) derived from the nucleotide sequence at the extremities of the insert confirmed that the cloned segment was contiguous and not composed of nonadjacent Sau3AI fragments. Sequence analysis of the insert revealed four open reading frames (ORFs) and the N-terminal part of a fifth ORF (Fig. 1). The 200-amino-acid (aa) protein coded by ORF1 (nucleotides 271 to 873 of sequence AJ315329) presents 27% identical and 50% similar amino acids to a protein with repeat motifs, bdrA9, of Borrelia turicatae (12) and 25% identical and 41% similar amino acids to the P50 adhesin of M. hominis (19). It does not possess a signal sequence and appears to be highly immunogenic and surface exposed. ORF1 is followed by a putative transcription termination site (Fig. 1). The second ORF, p40 (nucleotides 1010 to 2089), encodes a 359-aa polypeptide, named P40, with a predicted molecular mass of 40 kDa. The putative promoter region is composed of a −10 box at positions 963 to 968 (9, 32). ORF p40 is followed by a putative transcription termination site at positions 2104 to 2133 with a ΔG of −9.0 kcal/mol. ORF p40 presents a consensus sequence for a potential ribosome-binding site at positions 990 to 997. It contains one TGATrp codon at position 1766. The predicted amino acid sequence of P40 contains a potential signal peptidase recognition site at aa 24 to 28 (AAKCD), a consensus sequence that was also found in other M. agalactiae membrane proteins (37). The protein shows a strong antigenic and hydrophilic C-terminal region. The amino acid sequence of the mature protein P40 shows 23% identical and 41% similar amino acids with the above-mentioned P50 adhesin of M. hominis (19) and 31% identical and 53% similar amino acids with the P89 putative adhesin of Spiroplasma citri (4). Furthermore, it revealed 27% identical and 42% similar amino acids with the variable surface lipoprotein AvgD of M. agalactiae (15). ORF3 (nucleotides 2254 to 2952 of AJ315329) shows 22% identical and 41% similar amino acids with the variable surface lipoprotein from gene vspJ of Mycoplasma bovis (24) and 21% identical and 43% similar amino acids with the variable surface-exposed membrane protein P120 of M. hominis (22). ORF3 is also followed by a putative transcription termination site (Fig. 1). Further downstream we detected a partial ORF, ORF4, showing 36% identical and 50% similar amino acids with the variable surface lipoprotein encoded by vspF of M. bovis (24). Finally, a fifth ORF was found on the minus strand (Fig. 1), presenting no significant homology with any protein known in the databases.
FIG. 1.
Physical map of the 3.5-kb insert in clone pJFFBF13a. The different ORFs are indicated with arrowheads showing the direction of translation. The p40 gene is represented by a black arrow. Putative transcriptional termination sites are indicated by hairpins. The restriction sites are indicated by the following abbreviations: B, BamHI; E, EcoRI; H, HindIII; S, Sau3AI; Sp, SpeI; S, and X, XbaI. The EMBL/GenBank accession no. of the DNA sequence is AJ315329.
Presence of the p40 gene in different mycoplasma species.
To study the species specificity of the p40 gene, we looked for its presence in several field strains of M. agalactiae with the primer pair 13aEcoRI-L-13aNotI-R, which was designed for the production of the recombinant protein. The expected fragment of 920 bp was amplified from the genomic DNA of the type strain and all field strains of M. agalactiae used in the present study (the results are summarized in Table 1). Strain 4212 showed a weak signal (not shown) that was later explained to be due to three nucleotide modifications of this strain in the binding site of primer 13aEcoRI-L. These modifications were verified by sequence analysis of the PCR product obtained with the primers MagaP13a-L1 and MagaP13a-R1 (Table 2) amplifying a 1,346-bp fragment. No amplification from genomic DNA of any other related mycoplasma species tested was obtained with the primers 13aEcoRI-L and 13aNotI-R. All strains listed in Table 1 were subjected to Southern blot analysis. Genomic DNA was cut with HindIII and hybridized with the DIG-labeled probe specific for p40. Type strain PG2 and most of the field strains of M. agalactiae presented a single 1.3-kb band (Fig. 2), whereas strains 209, 5632, 3990, 4054-1, 4055, 4212, and 8064 showed single hybridization bands of larger sizes (data not shown). In some strains, additional bands reacted faintly with the probe, a result probably due to nonspecific cross-hybridizations. No hybridization signal was observed with strains of the other mycoplasma species (Fig. 2). These results imply that the gene encoding P40 is specific for M. agalactiae species (Table 1).
FIG. 2.
Presence of p40 in different mycoplasma species and M. agalactiae strains. Detection of the p40 gene in different species of mycoplasmas (upper panel) and strains of M. agalactiae (lower panel) was performed by Southern blot; 300 ng of genomic DNA was digested with HindIII, and digestion products were probed with the p40 DIG probe. The strain numbers refer to Table 1. The arrows indicate the fragments containing p40. Std, molecular mass standards (23.1, 9.4, 6.6, 4.4, 2.3, 2.0, and 0.6 kb).
Expression of P40 in M. agalactiae strains and analysis of the p40 gene.
The total antigens of 27 strains of M. agalactiae and other mycoplasma species listed in Table 1 were probed with the monospecific rabbit anti-P40 serum directed against purified recombinant polyhistidine-tailed P40. The serum reacted with a protein of an apparent molecular mass of 37 kDa in the type strain PG2 and in 22 out of 26 field strains, with variable intensity. In four strains (9, 5225, 7169, and 7375), the serum anti-P40 did not recognize any P40 protein (Fig. 3). All strains that were devoid of expression of P40 belonged to serotype C (5) (Table 1).
FIG. 3.
P40 expression in M. agalactiae. Immunoblot analysis was carried with 10 μg of total antigen in each lane. Total antigen of all of the 27 M. agalactiae strains tested was separated by SDS-12% acrylamide gels, transferred onto a nitrocellulose membrane, and probed with antiserum to P40. The letter following each strain name indicates the serotype.
To determine the genetic basis of the observed differences of expression and size of P40 among the different strains of M. agalactiae, we sequenced the p40 gene of 10 field strains, including 4 strains that strongly expressed P40 under culture conditions (209, 3990, 4021, and 8064), 3 strains that moderately expressed P40 (4212, 4258, and 6968) and 3 strains of serotype C that were devoid of P40 expression (9, 5225, and 7375). The differences in strength of expression of P40 could be correlated to variations in the −10 sequences of the potential promoters (strain 4258) or upstream regions (strain 4212), as has also been observed for protein P30 (14). The p40 gene of strain 6968 has a completely different translation initiation codon which is far from the predicted ribosome-binding site. The p40 genes from the three strains 9, 5225, and 7375 that were devoid of P40 expression showed nucleotide deletions or insertions at the beginning of the P40 coding sequence, causing frameshifts that lead to aberrant peptide formation and a premature stop in translation. Small size variations were observed among some P40 bands (Fig. 3) that could be due to differences observed in the nucleotide and amino acid sequences of the protein. Most of the strains, such as PG2, 4021, and 8064, which have identical P40 nucleotide sequences, presented P40 bands at 37 kDa, whereas strains 209 and 3990, which carry several variations in their p40 gene sequences, both presented P40 bands of smaller sizes.
Identification of P40 as a membrane protein involved in adherence.
To determine the cellular location of P40, the total antigen of M. agalactiae type strain PG2 was subjected to a Triton X-114 phase partitioning. Monospecific anti-P40 serum reacted strongly with a 37-kDa protein (P40) in the Triton X-114 phase but not with proteins found in the aqueous phase. This indicates that P40 is an integral membrane protein (Fig. 4A). TMpred identified a unique and significant transmembrane region (score 1762) spanning aa 7 to 25 in the amino-terminal region of the protein corresponding to the putative signal sequence. To show the immunodominance of P40, a Western blot of the Triton X-114 extract was performed with sheep antiserum PAL 97. One band of ca. 37 kDa that reacted with the sheep antiserum was shown to migrate on the gel to the same position as P40 (Fig. 4B).
FIG. 4.
Triton X-114 phase partitioning of M. agalactiae type strain PG2. (A) Mycoplasmas at the end of exponential growth were subjected to Triton X-114 partitioning; 15 μl of fractions and total antigen were separated on SDS-12% acrylamide gels, transferred to a nitrocellulose filter, and probed with monospecific P40 antiserum. Lane 1, total antigen; lane 2, detergent phase; lane 3, aqueous phase. (B) Western blot analysis of Triton X-114 extract from PG2 with serum PAL 97 (lane 1) and serum anti-P40 (lane 2).
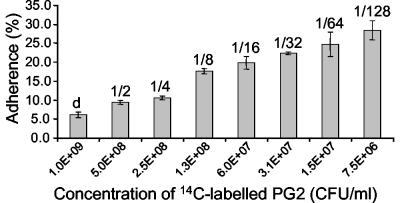
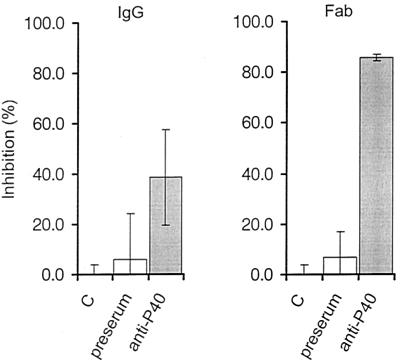
Considering the significant similarity of P40 to other mycoplasmal adhesins, we studied its impact on the adhesion process of M. agalactiae. An adhesion assay of type strain PG2 to lamb synovial membrane cells was developed. The relative adherence of M. agalactiae to LSM 192 cells increased with increasing dilutions of the mycoplasmas, indicating that saturation of adherence was achieved over the whole range of mycoplasma concentrations used (Fig. 5). The results were the same if fresh cultures or frozen stocks were used. Inhibition assays showed that monospecific anti-P40 IgG antibodies significantly reduced adhesion to lamb LSM 192 cells by 38.7% compared to purified IgG from preserum of the same rabbit, which reduced the number of M. agalactiae adhering to the lamb cells by 6.0% (Fig. 6). Fab fragments directed against P40 dramatically reduced adhesion by 85.7%, while those from preserum had an inhibitory effect of 6.7% (Fig. 6).
FIG. 5.
Adherence assay. Histogram representing the relative adherence of serial dilutions of 14C-labeled PG2 to lamb joint synovial membrane-derived cells LSM 192 in culture after 2 h of incubation at 37°C. Values above the columns indicate the dilutions. d, nondiluted. The data are the means of three independent measurements. Error bars indicate the standard errors.
FIG. 6.
Inhibition of adherence. The attachment of 14C-labeled PG2 to LSM 192 was assessed in the absence of antibodies (C), by preincubation for 2 h at 37°C with IgG (130 μg/ml) from rabbit preserum and anti-P40 serum (left panel), and with Fab fragments (90 μg/ml) from preserum and directed against P40 (right panel). The data are the means of three independent measurements. Error bars indicate the standard errors.
Immune response of ewes infected with M. agalactiae against recombinant P40.
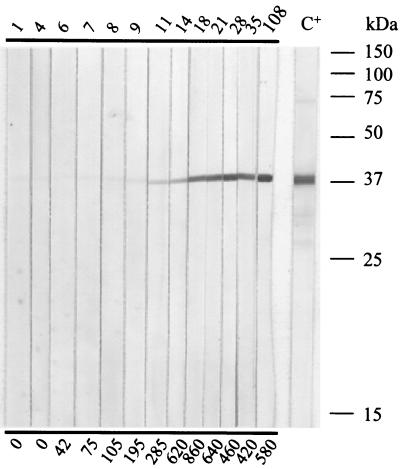
In order to examine the immune response to the recombinant P40 of sera from experimentally infected ewes, immunoblots were performed with serial serum samples collected during the first 3 to 4 months after inoculation. The immunoblot results were compared to enzyme-linked immunosorbent assay (ELISA) titers of an assay that is routinely used for the control of sheep and goats for potential infection by M. agalactiae. The immune response was comparable for all ewes. Typically, the serum from a ewe (e.g., no. 6286) infected with M. agalactiae PG2 reacted with recombinant P40 at day 11 after inoculation. The reaction with P40 was highest at day 18 after inoculation and persistent until the end of the experiment at day 108 after infection, whereas the ELISA titers dropped at day 21 after infection (Fig. 7).
FIG. 7.
Immune response to P40 of ewe 6286 artificially infected with type strain PG2. An SDS-15% acrylamide gel was run and blotted onto nitrocellulose with ca. 1 μg of P40-His per strip. Serial serum samples were used at a dilution of 1/100. The numbers above the immunoblot strips indicate the days postinoculation. C+, control serum PAL 97 (anti-M. agalactiae) from a naturally infected ewe (diluted 1/600). The numbers below the panel indicate the titers in international units as determined by ELISA.
DISCUSSION
Functional analysis of the P40 protein by means of monospecific, polyclonal antibodies directed against mature P40 revealed that it is involved in the adhesion of M. agalactiae to lamb synovial membrane cells. These cells were chosen because (i) sheep are natural hosts of M. agalactiae; (ii) arthritis is a manifestation of infection with this pathogen; and (iii) direct adhesion analysis and inhibition experiments of the purified recombinant polyhistidine-tailed protein P40 by means of monospecific, polyclonal antibodies directed against P40 revealed that it is involved in adhesion to sheep cells and that this adhesion is not influenced by the presence of polyhistidine tails on the recombinant protein (data not shown). Other appropriate cells for the adhesion assays, such as cells from the udder or from the digestive tract, were not available. Adherence experiments were performed with viable lamb synovial membrane cells that formed monolayers in order to exclude artifacts from dead cells. Lamb cells of passage numbers 4 through 15 were used in the experiments as the adherence of M. agalactiae decreased with higher passage level. Moreover, cells of the passage number that was optimal for the adherence experiments also replicated Maedi visna virus to high titers (not shown). This virus is known to cause pneumonia, mastitis, encephalitis, and arthritis in sheep (28). Together with the results of the adherence experiments, this indicates that lamb synovial membrane cells permit the study of host-pathogen interactions that closely reflect the situation in vivo. Inhibition experiments with purified IgG antibodies from monospecific rabbit serum directed against recombinant P40 inhibited attachment of M. agalactiae PG2 to lamb synovial membrane cells by 38.7%. Fab fragments from anti-P40 IgG inhibited adhesion of M. agalactiae by 85.7%, confirming that P40 plays a crucial role in cytadherence of M. agalactiae. The low inhibition (6.0 to 6.7%) obtained with IgG and Fab fragments from preserum suggested that clumping of the mycoplasmas did not interfere with the adhesion assay.
The similarity of P40 with the P50 adhesin of M. hominis was shown by BLAST search. Analysis of the P40 sequence showed that amino acid composition, especially for the lysine (16.2% in P40 and 16.7% in P50) and the leucine and isoleucine (16.9% in P40 and 17% in P50), was similar for both proteins (18). These properties, together with the possible presence of a leucine zipper motif in P40, as was found in P50, suggest a functional analogy of P40 to the adhesin P50 of M. hominis.
Protein P40 was shown to be located in the membrane by Triton X-114 partitioning experiments. The membrane location of P40 is an inherent property related to its function as an adhesin (32). The TMpred program identified a single transmembrane region in P40 matching the signal sequence. This information, together with the fact that p40 was found in a phage plaque strongly reacting with serum PAL 97 and the fact that P40 fractionated in the Triton X-114 phase, suggest that this lipoprotein is an outer membrane protein. P40 does not seem to be subject to high-frequency phase variations since DNA sequence analysis revealed no loci that would be involved in such mechanisms (16). The reason why certain strains of M. agalactiae, all belonging to the serotype C as serotyped by Bergonier et al. (5), were devoid of P40 production was shown to be due to one point mutation found in the 5′ half of the coding region of P40, but no correlation was found with virulence strength of the different strains. Mycoplasma species other than M. agalactiae, including the phylogenetically closely related M. bovis (29), do not produce the P40 antigen, nor do they contain the p40 gene. Although it is not clear why strains of serotype C fail to express P40, the finding that the protein is characteristic to all other M. agalactiae strains tested may support the functional feature of P40 being an adhesin.
The analysis of the humoral response to P40 from ewes artificially infected with the M. agalactiae type strain PG2 indicated that P40 is strongly immunogenic, inducing an early antibody response showing the first signal 11 days postinfection and persisting for at least 3 months after infection when ELISA titers had decreased. The same effect was observed for P30 (14) and other lipoproteins (37). These experimental infections mimicked both a natural infection, since the inoculations induced a persistent disease with mycoplasmas found in the milk, and typical clinical aspects and seroconversion (6). Considering the strong and persistent antibody response against P40 and its role in adhesion, a central mechanism of virulence of mycoplasmas, P40 represents an interesting antigen for further studies on the virulence of M. agalactiae and for new attempts in the development of future diagnostics and prophylactics.
Acknowledgments
We thank F. Poumarat (AFSSA, Lyon, France), F. Thiaucourt (CIRAD-EMVT, Montpellier, France), and S. Tola (Istituto Zooprofilattico Sperimentale, Sassari, Italy) for the gift of strains. We are also grateful to Y. Schlatter (Institute for Veterinary Bacteriology, Berne, Switzerland) and E. Schläfli and L. Nebel (Institute for Veterinary Virology, Berne, Switzerland) for their technical help. We thank A. Blanchard (Institut Pasteur, Paris, France) for scientific advice.
We thank Walter Bommeli (Bommeli Diagnostics, Berne-Liebefeld, Switzerland) for supporting this project with a grant to B. Fleury to visit the Institute of Veterinary Bacteriology, Berne, Switzerland. This study was part of the European COST Action 826 on “ruminant's mycoplasmoses.” It was supported by a Ph.D. fellowship from AFSSA and the French Ministry of Agriculture and Fishery, by grant C96.0073 of the Swiss Ministry of Education and Science, and by the Swiss Federal Veterinary Office.
Editor: J. T. Barbieri
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1999. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Bairoch, A., P. Bucher, and K. Hofmann. 1995. The PROSITE database: its status in 1995. Nucleic Acids Res. 24:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, M., U. Melcher, and J. Fletcher. 2001. Characterization of Spiroplasma citri adhesion related protein SARP1, which contains a domain of a novel family designated sarpin. Gene 275:57-64. [DOI] [PubMed] [Google Scholar]
- 5.Bergonier, D., F. De Simone, P. Russo, M. Solsona, M. Lambert, and F. Poumarat. 1996. Variable expression and geographic distribution of Mycoplasma agalactiae surface epitopes demonstrated with monoclonal antibodies. FEMS Microbiol. Lett. 143:159-165. [DOI] [PubMed] [Google Scholar]
- 6.Bergonier, D., and F. Poumarat. 1996. Contagious agalactia of small ruminants: epidemiology, diagnosis and control. Rev. Sci. Technol. 15:1431-1475. [PubMed] [Google Scholar]
- 7.Boguslavsky, S., D. Menaker, I. Lysnyansky, T. Liu, S. Levisohn, R. Rosengarten, M. Garcia, and D. Yogev. 2000. Molecular characterization of the Mycoplasma gallisepticum pvpA gene which encodes a putative variable cytadhesin protein. Infect. Immun. 68:3956-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. [PubMed] [Google Scholar]
- 9.Bové, J. M. 1993. Molecular features of mollicutes. Clin. Infect. Dis. 17(Suppl. 1):S10-S31. [DOI] [PubMed] [Google Scholar]
- 10.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 11.Braman, J., C. Papworth, and A. Greener. 1996. Site-directed mutagenesis using double-stranded plasmid DNA templates. Methods Mol. Biol. 57:31-44. [DOI] [PubMed] [Google Scholar]
- 12.Carlyon, J. A., D. M. Roberts, M. Theisen, C. Sadler, and R. T. Marconi. 2000. Molecular and immunological analyses of the Borrelia turicatae Bdr protein family. Infect. Immun. 68:2369-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DaMassa, A. J., P. S. Wakenell, and D. L. Brooks. 1992. Mycoplasmas of goats and sheep. J. Vet. Diagn. Investig. 4:101-113. [DOI] [PubMed] [Google Scholar]
- 14.Fleury, B., D. Bergonier, X. Berthelot, Y. Schlatter, J. Frey, and E. M. Vilei. 2001. Characterization and analysis of a stable serotype-associated membrane protein (P30) of Mycoplasma agalactiae. J. Clin. Microbiol. 39:2814-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flitman-Tene, R., S. Levisohn, I. Lysnyansky, E. Rapoport, and D. Yogev. 2000. A chromosomal region of Mycoplasma agalactiae containing vsp-related genes undergoes in vivo rearrangement in naturally infected animals. FEMS Microbiol. Lett. 191:205-212. [DOI] [PubMed] [Google Scholar]
- 16.Glew, M. D., L. Papazisi, F. Poumarat, D. Bergonier, R. Rosengarten, and C. Citti. 2000. Characterization of a multigene family undergoing high-frequency DNA rearrangements and coding for abundant variable surface proteins in Mycoplasma agalactiae. Infect. Immun. 68:4539-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henrich, B., R. C. Feldmann, and U. Hadding. 1993. Cytoadhesins of Mycoplasma hominis. Infect. Immun. 61:2945-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henrich, B., A. Kitzerow, R. C. Feldmann, H. Schaal, and U. Hadding. 1996. Repetitive elements of the Mycoplasma hominis adhesin P50 can be differentiated by monoclonal antibodies. Infect. Immun. 64:4027-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henrich, B., K. Lang, A. Kitzerow, C. MacKenzie, and U. Hadding. 1998. Truncation as a novel form of variation of the p50 gene in Mycoplasma hominis. Microbiology 144:2979-2985. [DOI] [PubMed] [Google Scholar]
- 20.Hu, P. C., R. M. Cole, Y. S. Huang, J. A. Graham, D. E. Gardner, A. M. Collier, and W. A. Clyde, Jr. 1982. Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science 216:313-315. [DOI] [PubMed] [Google Scholar]
- 21.Krause, D. C. 1998. Mycoplasma pneumoniae cytadherence: organization and assembly of the attachment organelle. Trends Microbiol. 6:15-18. [DOI] [PubMed] [Google Scholar]
- 22.Ladefoged, S. A., and G. Christiansen. 1998. Mycoplasma hominis expresses two variants of a cell-surface protein, one a lipoprotein and one not. Microbiology 144:761-770. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Lysnyansky, I., K. Sachse, R. Rosenbusch, S. Levisohn, and D. Yogev. 1999. The vsp locus of Mycoplasma bovis: gene organization and structural features. J. Bacteriol. 181:5734-5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison-Plummer, J., A. Lazzell, and J. B. Baseman. 1987. Shared epitopes between Mycoplasma pneumoniae major adhesin protein P1 and a 140-kilodalton protein of Mycoplasma genitalium. Infect. Immun. 55:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson, M., and M. McClelland. 1992. Use of DNA methyltransferase/endonuclease enzyme combinations for megabase mapping of chromosomes. Methods Enzymol. 216:279-303. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von-Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 28.Pépin, M., C. Vitu, P. Russo, J. F. Mornex, and E. Peterhans. 1998. Maedi-visna virus infection in sheep: a review. Vet. Res. 29:341-367. [PubMed] [Google Scholar]
- 29.Pettersson, B., M. Uhlen, and K. E. Johansson. 1996. Phylogeny of some mycoplasmas from ruminants based on 16S rRNA sequences and definition of a new cluster within the hominis group. Int. J. Syst. Bacteriol. 46:1093-1098. [DOI] [PubMed] [Google Scholar]
- 30.Poumarat, F., B. Perrin, and D. Longchambon. 1991. Identification of ruminant mycoplasmas by dot immunobinding on membrane filtration (MF dot). Vet. Microbiol. 29:329-338. [DOI] [PubMed] [Google Scholar]
- 31.Razin, S., and E. Jacobs. 1992. Mycoplasma adhesion. J. Gen. Microbiol. 138:407-422. [DOI] [PubMed] [Google Scholar]
- 32.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachse, K. 1998. Detection and analysis of mycoplasma adhesins. Methods Mol. Biol. 104:299-307. [DOI] [PubMed] [Google Scholar]
- 34.Schaller, A., R. Kuhn, P. Kuhnert, J. Nicolet, T. J. Anderson, J. I. MacInnes, R. P. A. M. Segers, and J. Frey. 1999. Characterization of apxIVA, a new RTX determinant of Actinobacillus pleuropneumoniae. Microbiology 145:2105-2116. [DOI] [PubMed] [Google Scholar]
- 35.Seto, S., G. Layh-Schmitt, T. Kenri, and M. Miyata. 2001. Visualization of the attachment organelle and cytadherence proteins of Mycoplasma pneumoniae by immunofluorescence microscopy. J. Bacteriol. 183:1621-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramaniam, S., D. Bergonier, F. Poumarat, S. Capaul, Y. Schlatter, J. Nicolet, and J. Frey. 1998. Species identification of Mycoplasma bovis and Mycoplasma agalactiae based on the uvrC genes by PCR. Mol. Cell. Probes 12:161-169. [DOI] [PubMed] [Google Scholar]
- 37.Tola, S., S. Crobeddu, G. Chessa, S. Uzzau, G. Idini, B. Ibba, and S. Rocca. 2001. Sequence, cloning, expression and characterisation of the 81-kDa surface membrane protein (P80) of Mycoplasma agalactiae. FEMS Microbiol. Lett. 202:45-50. [DOI] [PubMed] [Google Scholar]
- 38.Vilei, E. M., and J. Frey. 2001. Genetic and biochemical characterization of glycerol uptake in Mycoplasma mycoides subsp. mycoides SC: its impact on H2O2 production and virulence. Clin. Diagn. Lab. Immunol. 8:85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Washburn, L. R., S. Hirsch, and L. L. Voelker. 1993. Mechanisms of attachment of Mycoplasma arthritidis to host cells in vitro. Infect. Immun. 61:2670-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]