Abstract
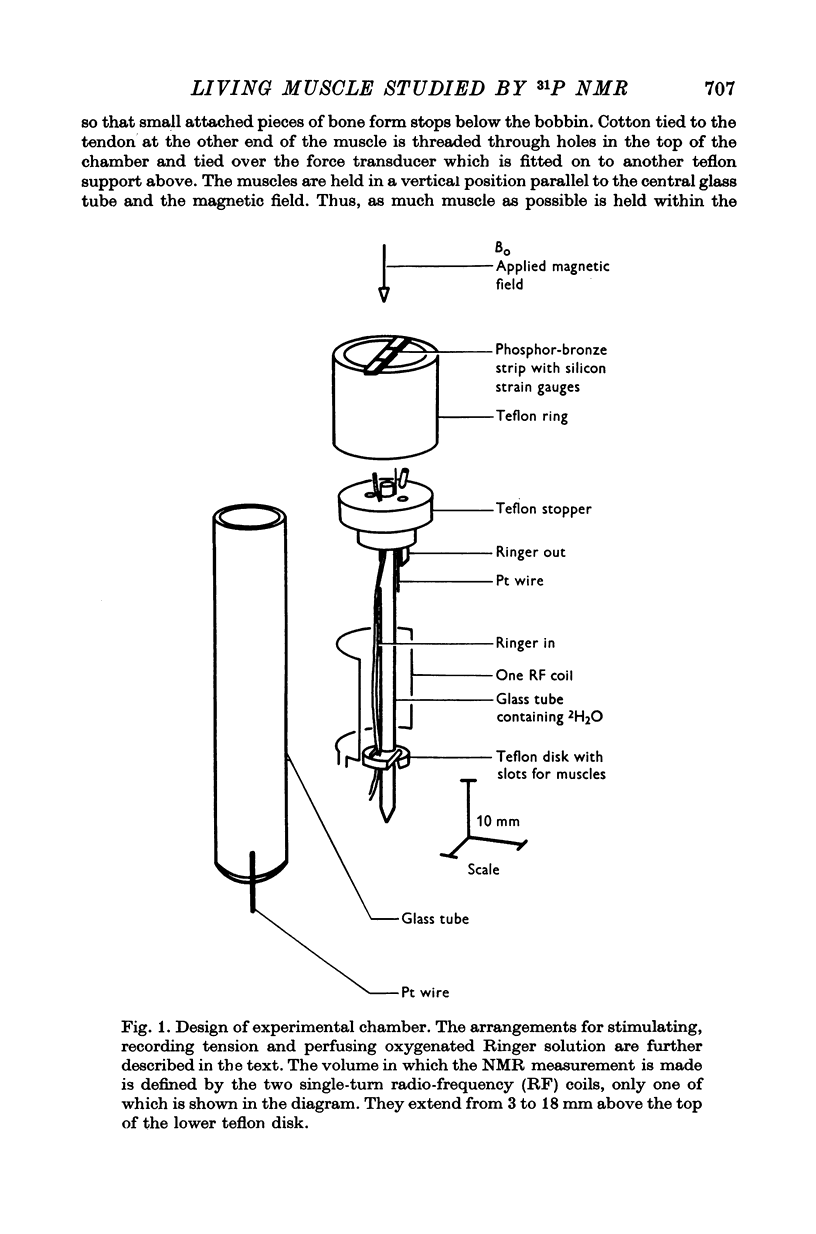
1. Phosphorus nuclear magnetic resonance (31P NMR) can be used to measure the concentrations of phosphorus-containing metabolites within living tissue. We have developed methods for maintaining muscles in physiological condition, stimulating them and recording tension while at the same time accumulating their 31P NMR spectra. Experiments were performed on frog sartorii and frog and toad gastrocnemii at 4° C.
2. The NMR signals from 31P (the naturally occurring phosphorus) is weak, and signal averaging is required. In order to follow the time course of reactions it is necessary to maintain the muscles in a steady state for many hours while they are undergoing repeated contractions. Signals were accumulated in separate computer bins according to time after initiation of contraction. By these means spectra were obtained which corresponded to the different intervals during the contraction and recovery cycle.
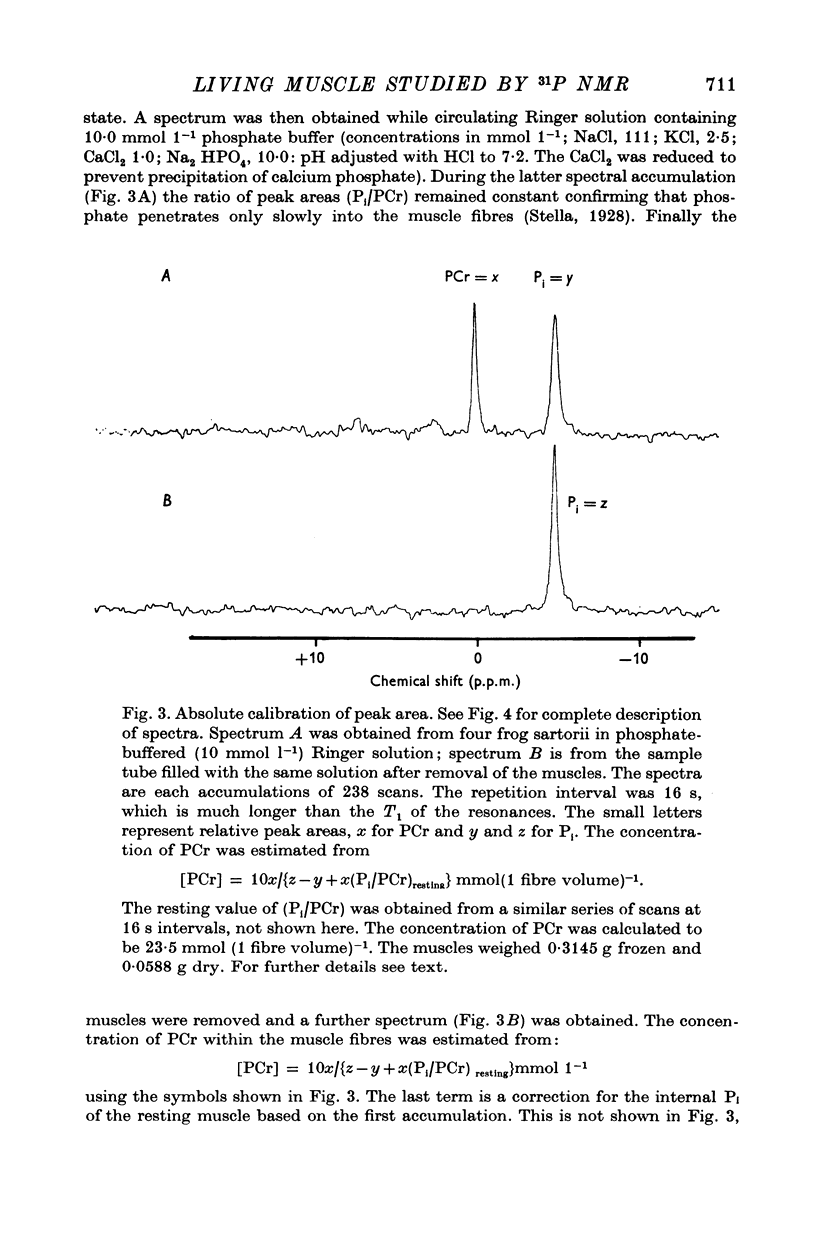
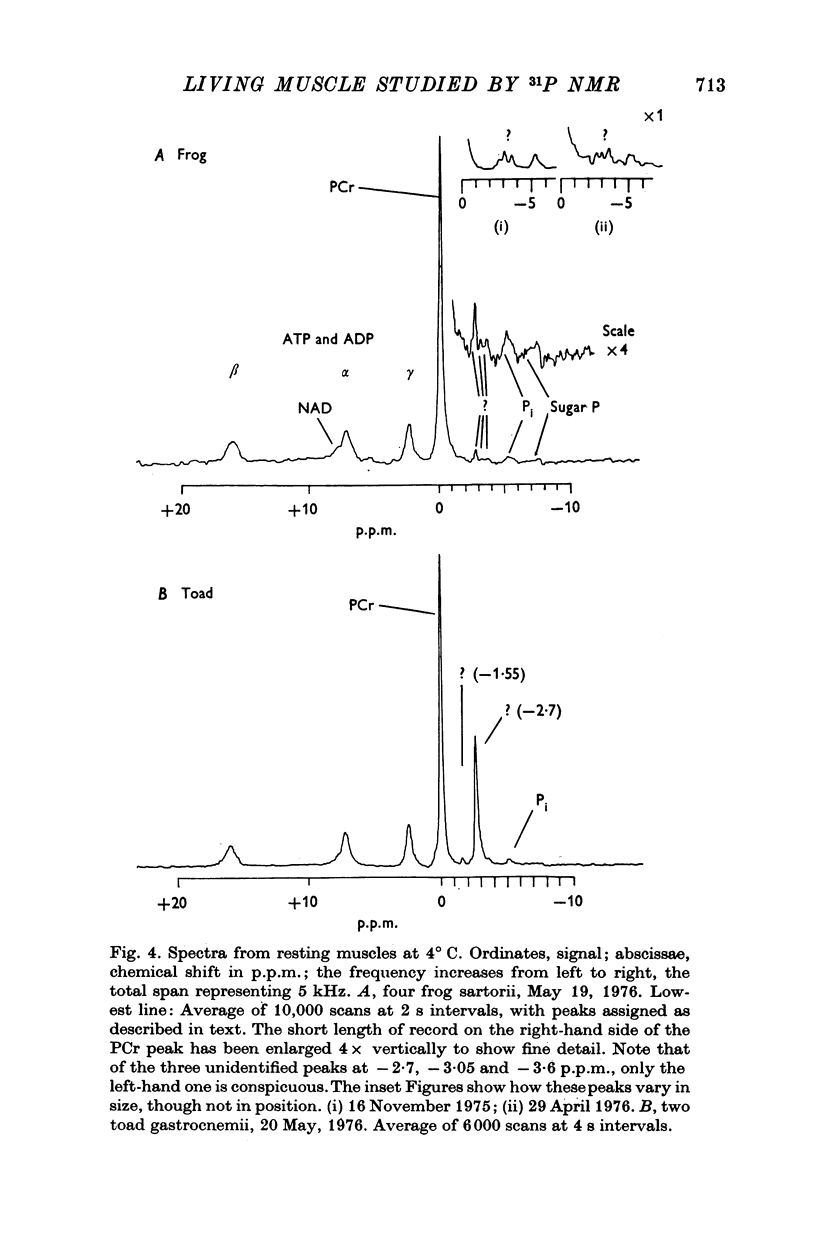
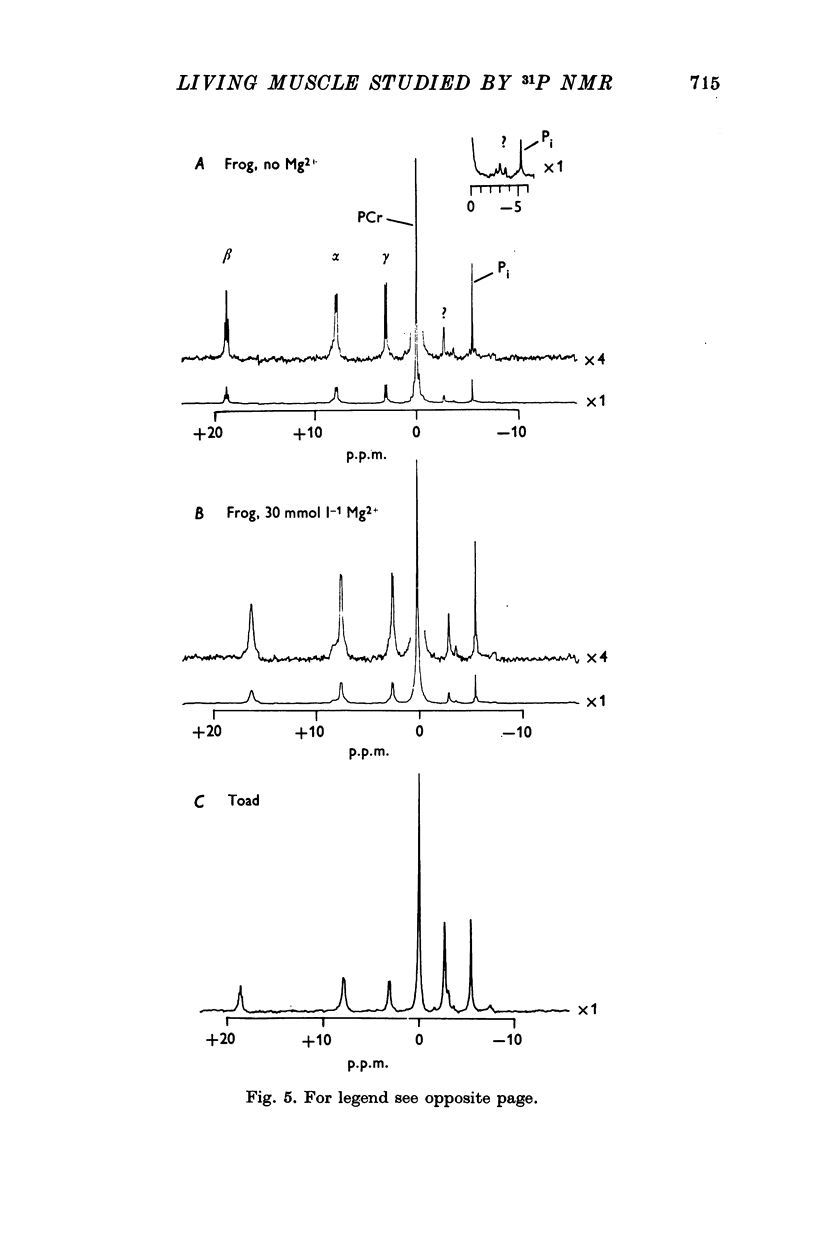
3. In the absence of stimulation, the spectra of frog sartorius muscles and of their extracts indicated concentrations of adenosine triphosphate (ATP), phosphoryl creatine (PCr), inorganic orthophosphate (Pi) and sugar phosphates (sugar P) which are in reasonable agreement with the values obtained by chemical analysis.
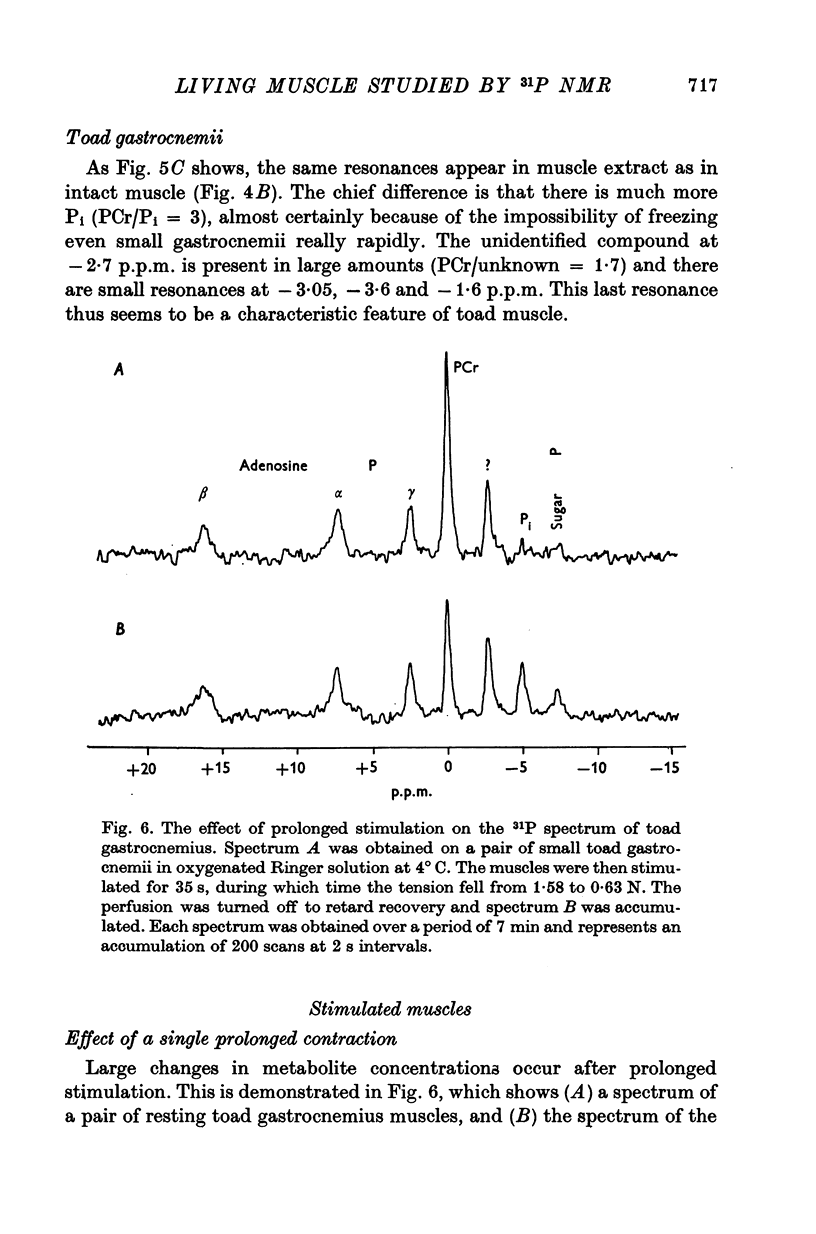
4. We have confirmed that unidentified resonances representing unknown compounds appear in the spectra of both frog and toad muscle; one of these is much larger in spectra from toad than from frog. We have found an additional small, unidentified resonance which appears to be specific to toad muscle.
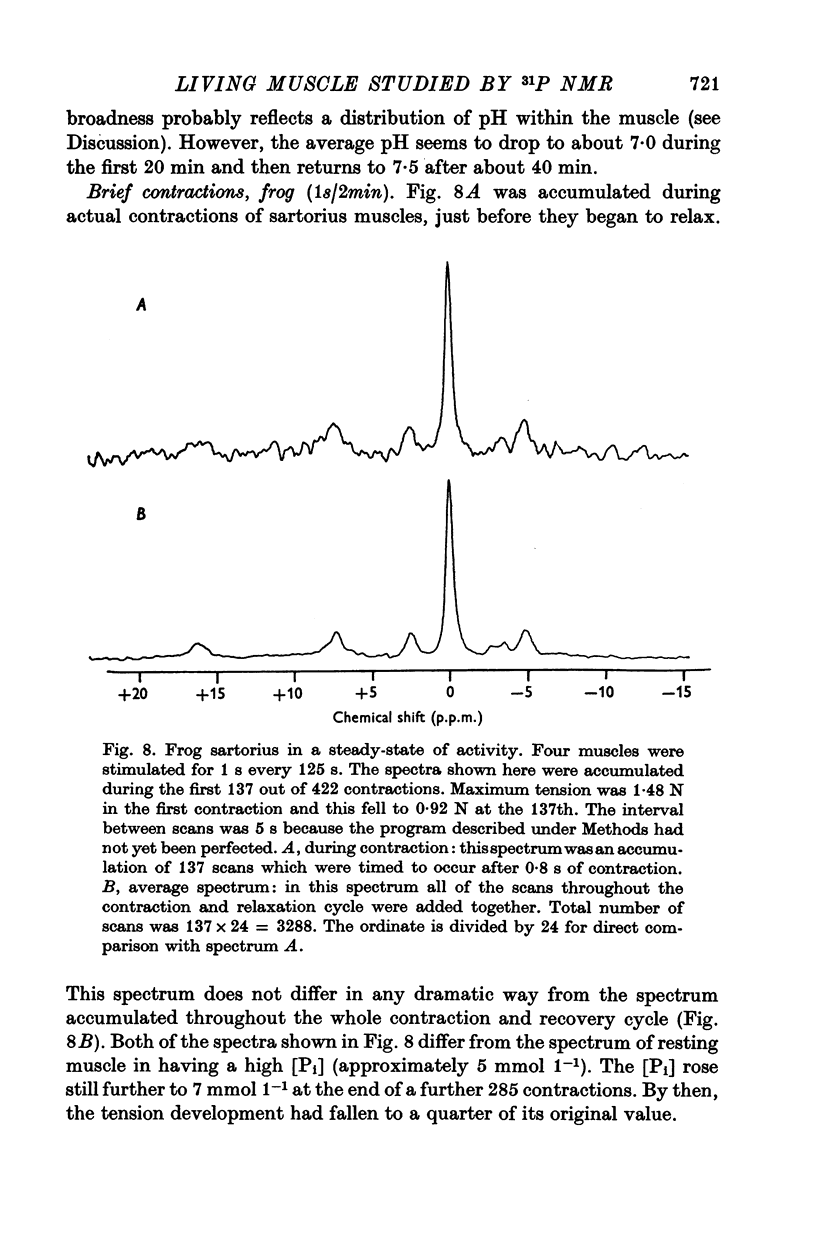
5. Spectra accumulated during actual contractions (1 s tetani every 2 min) did not differ dramatically from those accumulated throughout the 2 min cycle of contraction and partial recovery.
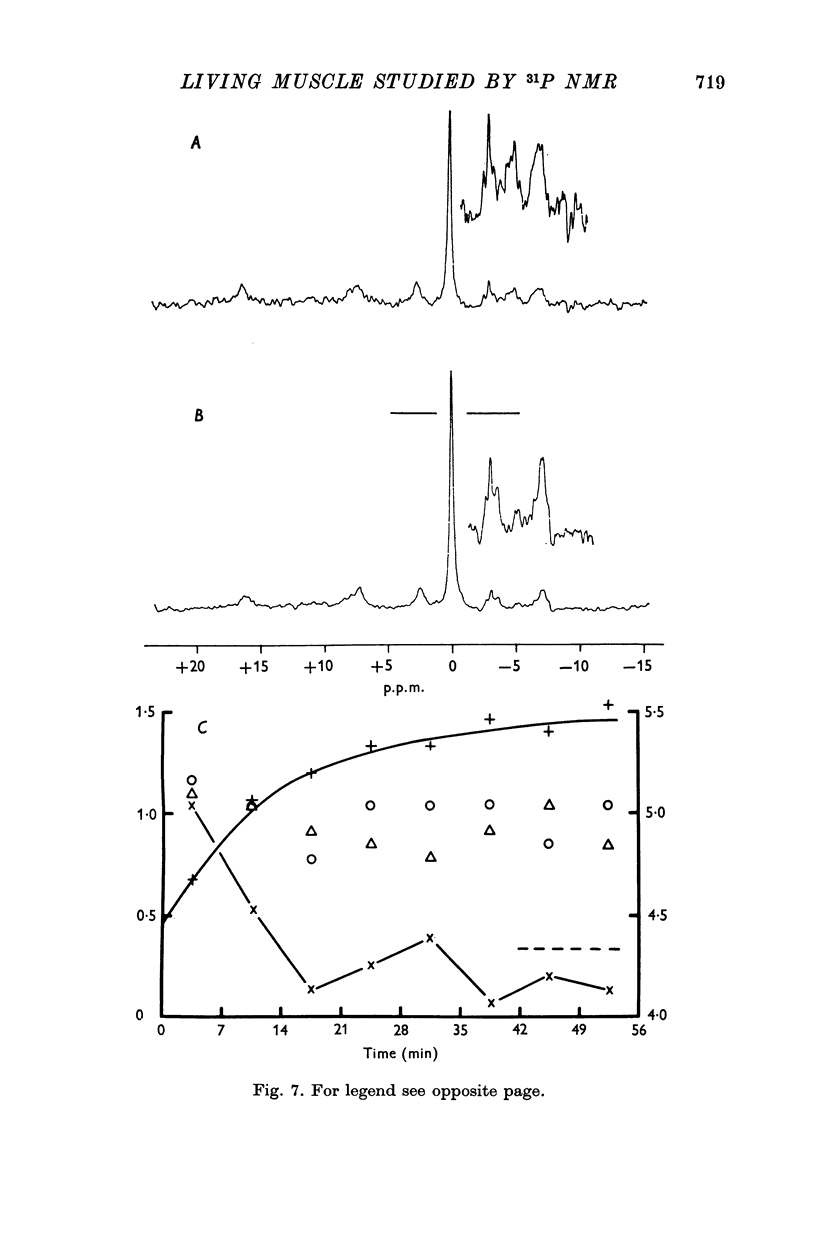
6. Following 25 s tetanii, approximately 20% of the PCr had been hydrolysed; it was then rebuilt exponentially with a half-time of about 10 min. The increase in [Pi] immediately after contraction and the time course of its disappearance corresponded to the changes in [PCr]. During the later half of the recovery period the concentration of Pi was reduced to below that in resting muscle. The [sugar P] remained very high (≃ 4 mmol kg-1) throughout the 56 min interval between contractions.
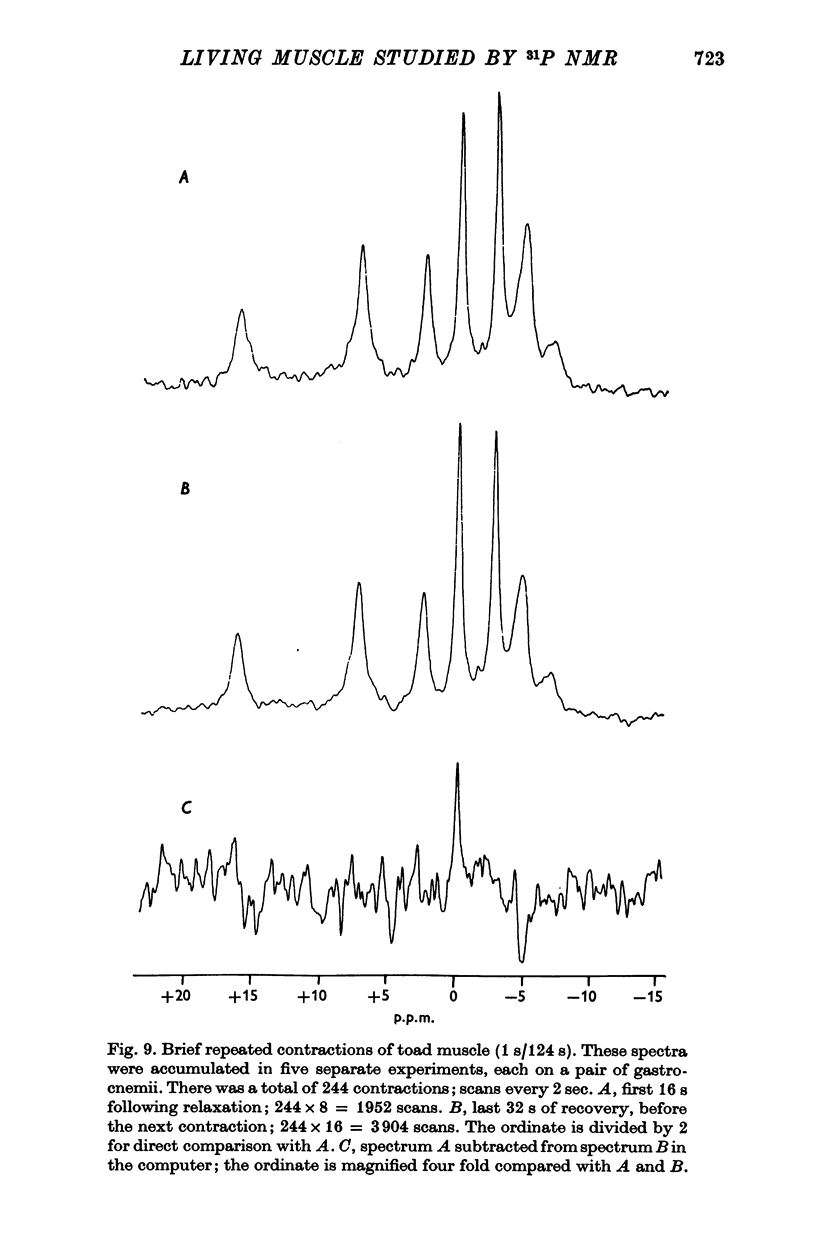
7. When frog sartorii were tetanized for 1 s every 2 min, the changes in [PCr] and [Pi] between contractions could not be observed because too little signal was obtained from these small muscles. However, when toad gastrocnemii were similarly stimulated, the changes in these compounds could be readily detected and were even greater than expected.
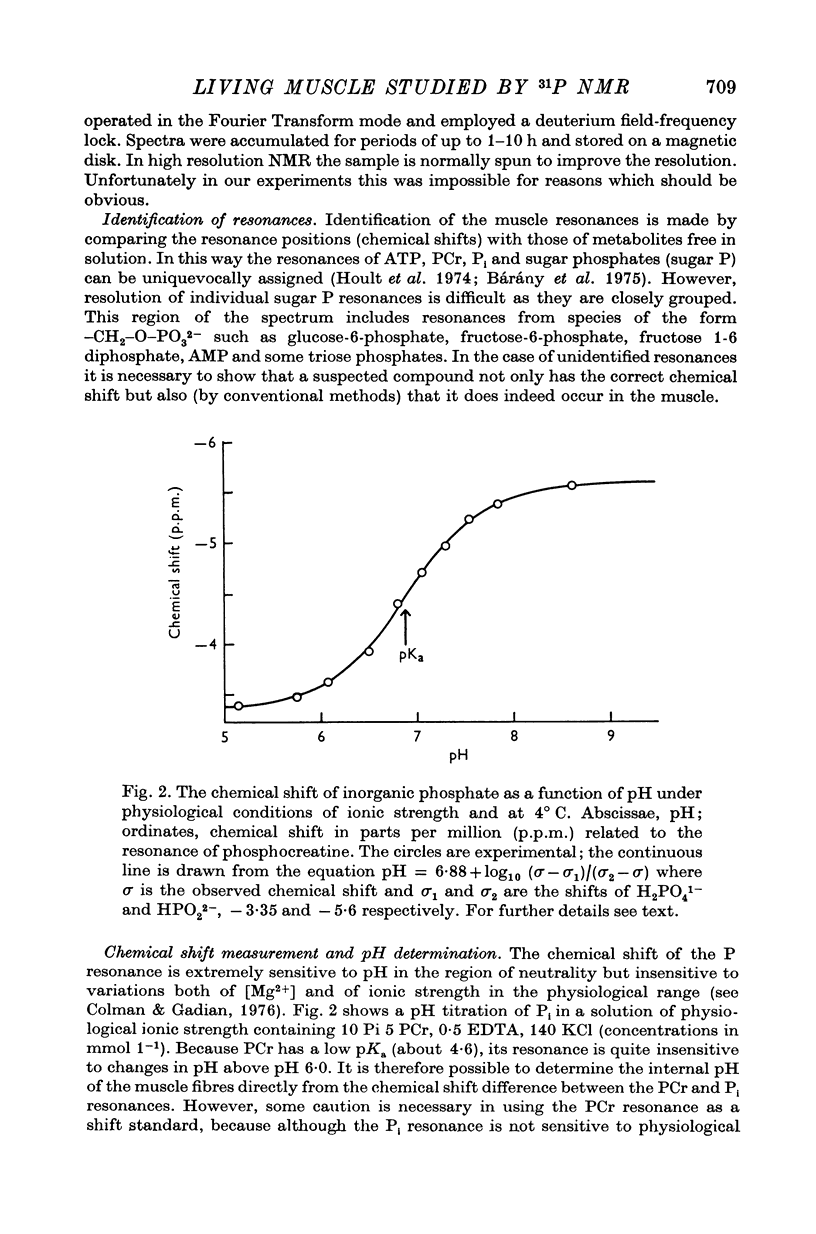
8. The position of the Pi resonance can be used to monitor intracellular pH and changes in pH. Under the conditions of our experiments the average intracellular pH in unstimulated frog sartorius muscles was 7·5. After a 25 s tetanus this was observed to move in the acid direction by a few tenths of a pH unit and to return to its pre-stimulation value before the end of the recovery period. After a 1 s contraction of toad gastrocnemius the environment of Pi became slightly more alkaline for the first few seconds.
Full text
PDF























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. Intracellular pH of mouse soleus muscle [proceedings]. J Physiol. 1976 Sep;260(2):25P–26P. [PMC free article] [PubMed] [Google Scholar]
- Aljure E. F., Borrero L. M. The evolution of fatigue associated with isometric contraction in toad sartorius. J Physiol. 1968 Feb;194(2):289–303. doi: 10.1113/jphysiol.1968.sp008408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Phosphorus-31 nuclear magnetic resonance detection of unexpected phosphodiesters in muscle. Biochemistry. 1976 Nov 2;15(22):4850–4853. doi: 10.1021/bi00667a015. [DOI] [PubMed] [Google Scholar]
- Bárány M., Bárány K., Burt C. T., Glonek T., Myers T. C. Structural changes in myosin during contraction and the state of ATP in the intact frog muscle. J Supramol Struct. 1975;3(2):125–140. doi: 10.1002/jss.400030205. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C., PRANKERD T. A. Phosphate esters in myotonic human muscle. J Neurol Neurosurg Psychiatry. 1954 May;17(2):127–128. doi: 10.1136/jnnp.17.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. The separation of the phosphate esters of muscle by paper chromatography. Biochem J. 1953 Oct;55(3):458–467. doi: 10.1042/bj0550458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAPO A., WILKIE D. R. The dynamics of the effect of potassium on frog's muscle. J Physiol. 1956 Dec 28;134(3):497–514. doi: 10.1113/jphysiol.1956.sp005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A., Gadian D. G. 31P nuclear-magnetic-resonance studies on the developing embryos of Xenopus laevis. Eur J Biochem. 1976 Jan 15;61(2):387–396. doi: 10.1111/j.1432-1033.1976.tb10032.x. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Energy balance in DNFB-treated and untreated frog muscle. J Physiol. 1975 Apr;246(3):737–752. doi: 10.1113/jphysiol.1975.sp010913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANFORTH W. H., HELMREICH E., CORICF The effect of contraction and of epinephrine on the phosphorylase activity of frog sartorius muscle. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1191–1199. doi: 10.1073/pnas.48.7.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J., Gadian D. G., Wilkie D. R. Proceedings: Living muscle studied by 31P nuclear magnetic resonance. J Physiol. 1976 Jun;258(2):82P–83P. [PubMed] [Google Scholar]
- Dawson J., Gower D., Kretzschmar M. K., Wilkie D. R. Proceedings: Heat production and chemical change in frog sartorius; a comparison of R. pipiens with R. temporaria. J Physiol. 1976 Jan;254(1):41P–42P. [PubMed] [Google Scholar]
- Dubuisson M. Studies on the chemical processes which occur in muscle before, during and after contraction. J Physiol. 1939 Jan 14;94(4):461–482. doi: 10.1113/jphysiol.1939.sp003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C., Kretzschmar K. M., Wilkie D. R., Woledge R. C. Chemical change and energy output during muscular contraction. J Physiol. 1971 Oct;218(1):163–193. doi: 10.1113/jphysiol.1971.sp009609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower D., Kretzschmar K. M. Heat production and chemical change during isometric contraction of rat soleus muscle. J Physiol. 1976 Jul;258(3):659–671. doi: 10.1113/jphysiol.1976.sp011439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. Autoradiographic localization of adenine nucleotide in frog's striated muscle. J Physiol. 1959 Jan 28;145(1):132–174. doi: 10.1113/jphysiol.1959.sp006133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. Preferred sites of adenine nucleotide in frog's striated muscle. J Physiol. 1960 Oct;153:433–446. doi: 10.1113/jphysiol.1960.sp006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. The location of adenine nucleotide in frog's striated muscle. J Physiol. 1960 Feb;150:347–373. doi: 10.1113/jphysiol.1960.sp006392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. The location of creatine phosphate in frog's striated muscle. J Physiol. 1962 Oct;164:31–50. doi: 10.1113/jphysiol.1962.sp007000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson T. O., Costello A. J., Omachi A. Phosphate metabolism in intact human erythrocytes: determination by phosphorus-31 nuclear magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2487–2490. doi: 10.1073/pnas.71.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Rall J. A., Wallner A., Ricchiuti N. V. Energy liberation and chemical change in frog skeletal muscle during single isometric tetanic contractions. J Gen Physiol. 1975 Jan;65(1):1–21. doi: 10.1085/jgp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoult D. I., Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974 Nov 22;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar K. M., Wilkie D. R. A new approach to freezing tissues rapidly. J Physiol. 1969 Jun;202(2):66P–67P. [PubMed] [Google Scholar]
- Kushmerick M. J., Paul R. J. Aerobic recovery metabolism following a single isometric tetanus in frog sartorius muscle at 0 degrees C. J Physiol. 1976 Jan;254(3):693–709. doi: 10.1113/jphysiol.1976.sp011253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Paul R. J. Relationship between initial chemical reactions and oxidative recovery metabolism for single isometric contractions of frog sartorius at 0 degrees C. J Physiol. 1976 Jan;254(3):711–727. doi: 10.1113/jphysiol.1976.sp011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwood G. W., Worsley-Brown P. The effects of extracellular pH and buffer concentration on the efflux of lactate from frog sartorius muscle. J Physiol. 1975 Aug;250(1):1–22. doi: 10.1113/jphysiol.1975.sp011040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS E., LOWE I. P. Occurrence of the O-phosphodiester of L-serine and ethanolamine in turtle tissue. J Biol Chem. 1954 Nov;211(1):1–12. [PubMed] [Google Scholar]
- Rall J. A., Homsher E., Wallner A., Mommaerts W. F. A temporal dissociation of energy liberation and high energy phosphate splitting during shortening in frog skeletal muscles. J Gen Physiol. 1976 Jul;68(1):13–27. doi: 10.1085/jgp.68.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and distribution of weak acids across cell membranes. A study of D- and L-lactate and of DMO in rat diaphragm. J Physiol. 1975 Jul;249(1):1–25. doi: 10.1113/jphysiol.1975.sp011000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley P. J., Busby S. J., Gadian D. G., Radda G. K., Richards R. E. A new approach to metabolite compartmentation in muscle. Biochem Soc Trans. 1976;4(1):62–64. doi: 10.1042/bst0040062. [DOI] [PubMed] [Google Scholar]
- Stella G. The concentration and diffusion of inorganic phosphate in living muscle. J Physiol. 1928 Sep 18;66(1):19–31. doi: 10.1113/jphysiol.1928.sp002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]


