Abstract
1. Single fusimotor fibres were stimulated repetitively to test their action on the responsiveness of muscle spindle primary endings in the cat soleus to sinusoidal stretching of both large and small amplitude. Frequencies of 0·06-4 Hz were used at amplitudes from 10 μm to 3 mm.
2. The response was assessed by fitting a sinusoid to the cycle histogram of the afferent firing throughout the course of the cycle; this linear approximation measures the fundamental of the response and ignores any harmonics. The sine was allowed to project to negative values and any empty bins in the histogram were ignored when fitting.
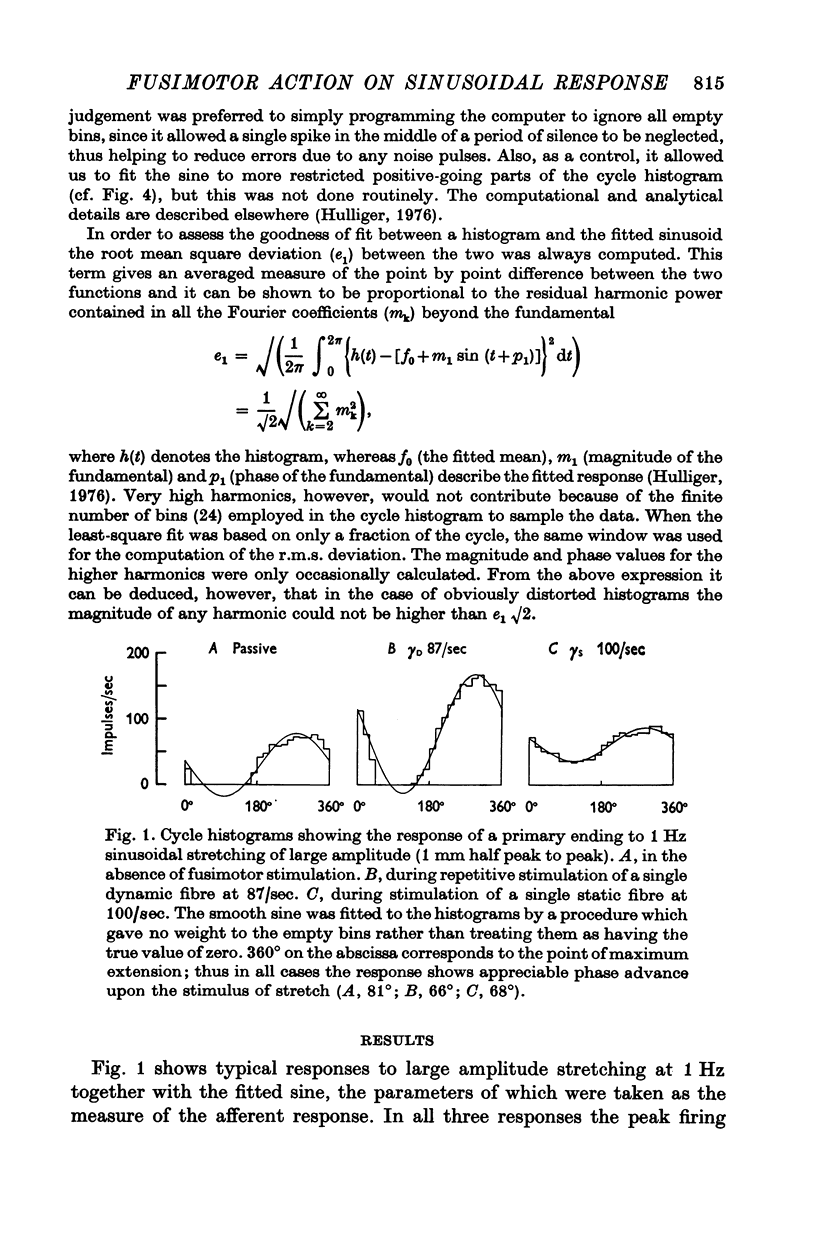
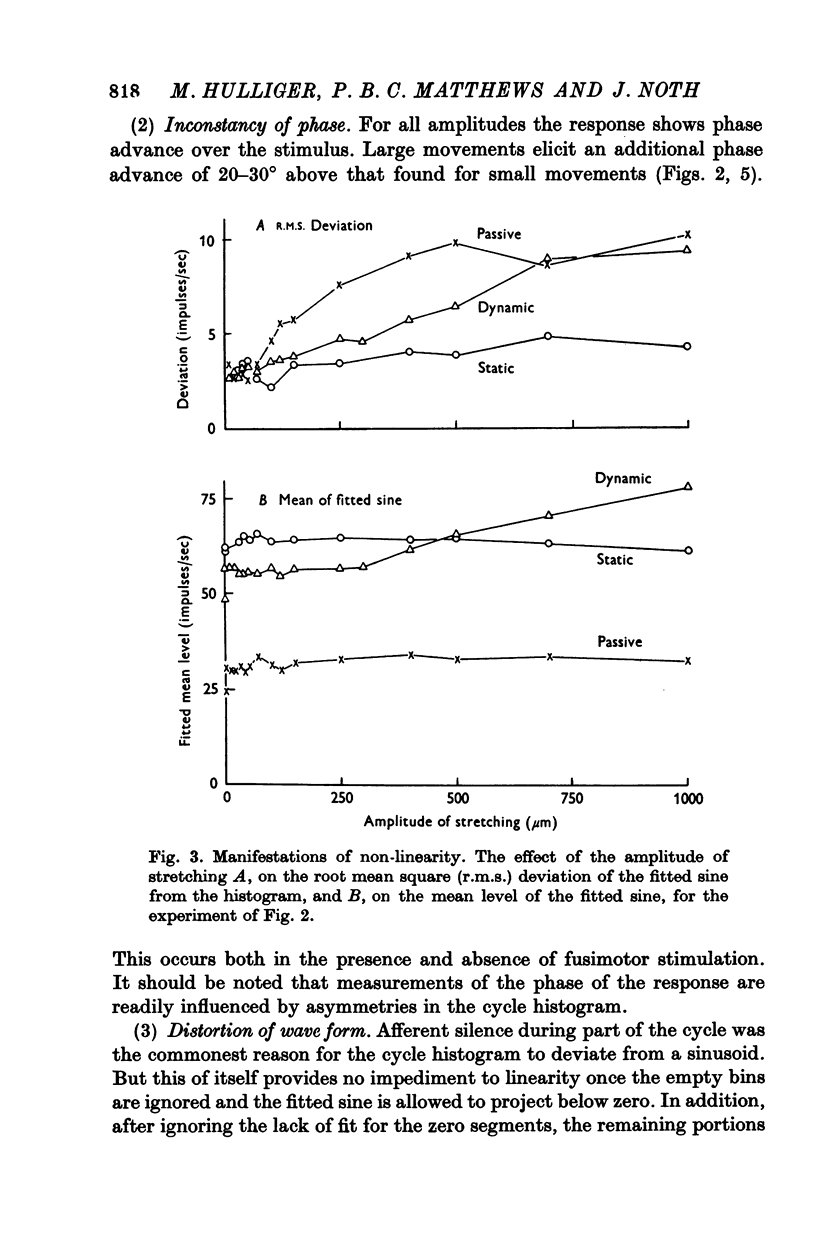
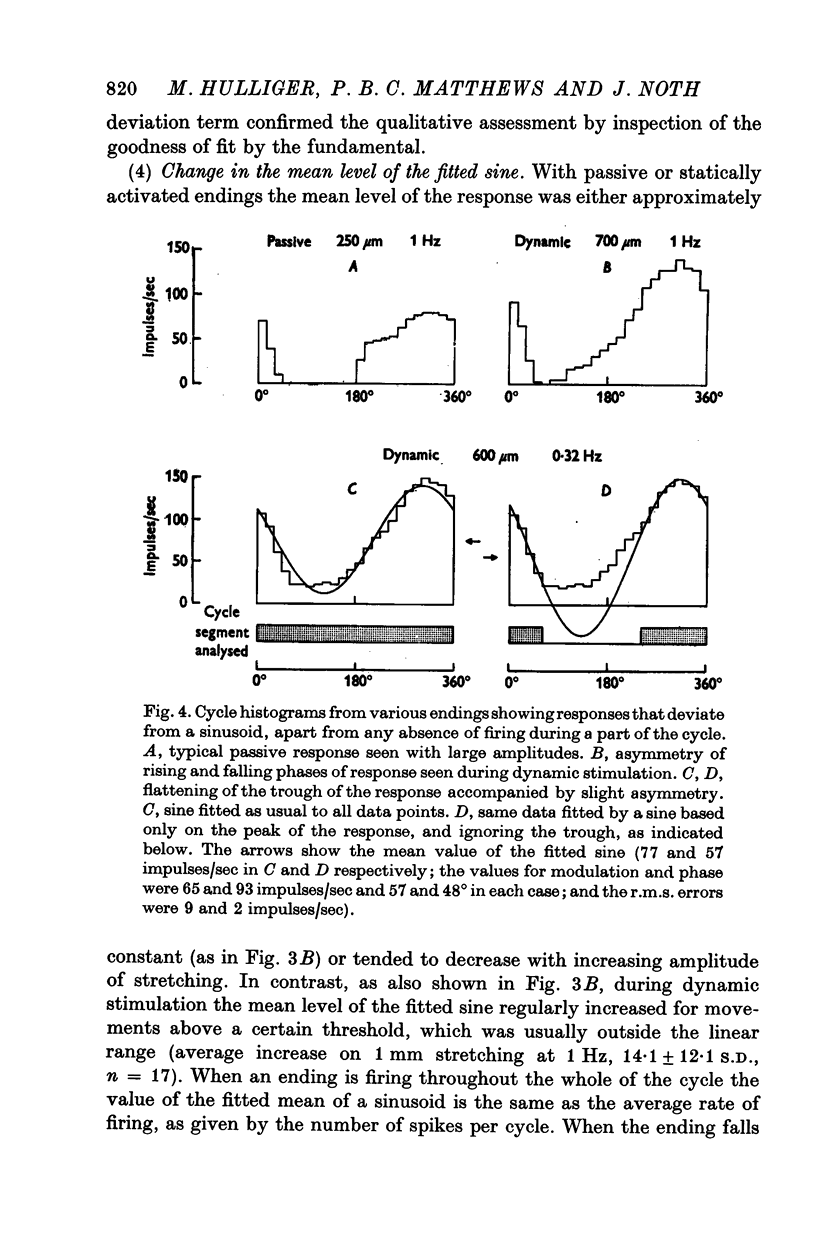
3. With small amplitudes of stretching the histograms were reasonably sinusoidal, but with large amplitudes they showed appreciable distortion of the wave form for the passive ending and during dynamic fusimotor stimulation. Non-linearity of response manifested itself also, with increasing amplitude of stretching, by an increase in the phase advance of the response, by increasing r.m.s. deviation of the histogram points from the fitted sine and (for dynamic stimulation) by an increase in the mean value of the fitted sine.
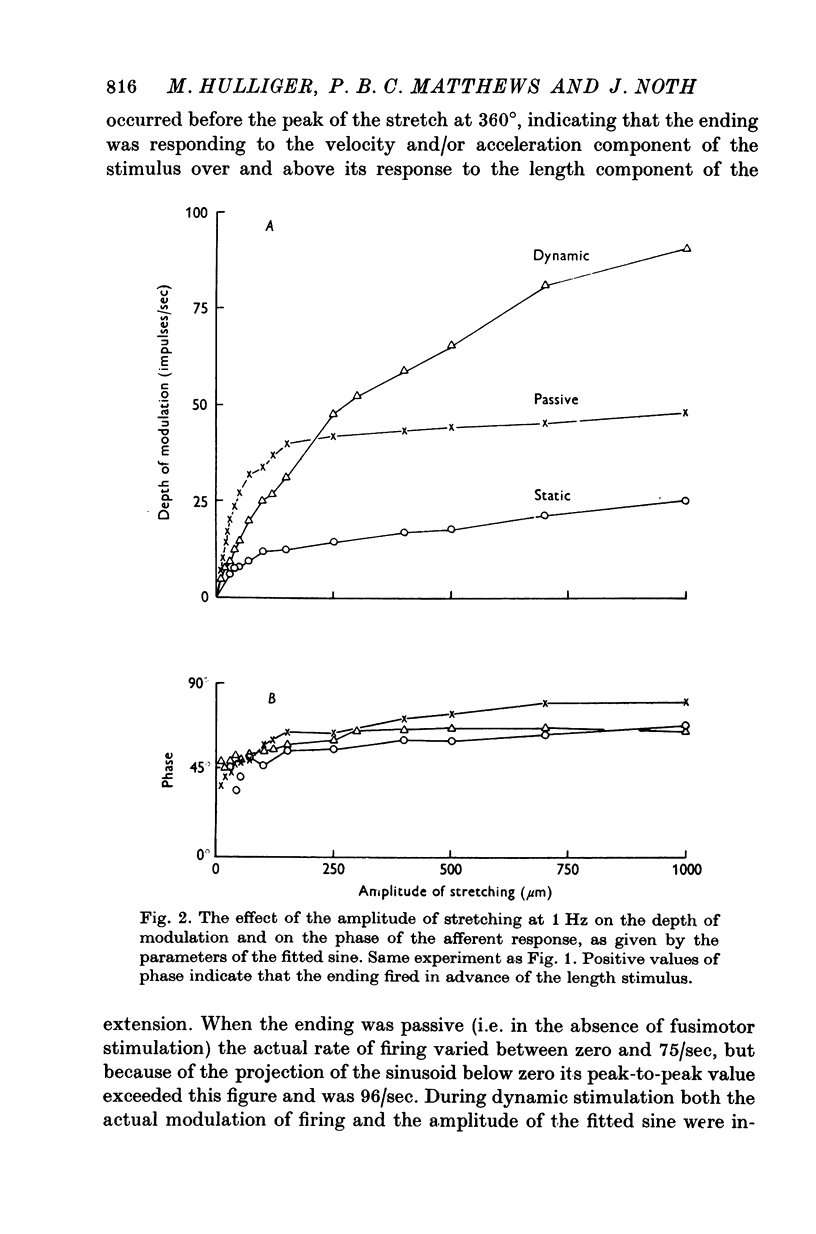
4. With increasing amplitude the response modulation ceased to increase proportionately with the stimulus, so that the sensitivity of the ending to a large stretch (defined as afferent modulation/stretch amplitude) was appreciably less than for a small stretch. This effect was most pronounced for the passive ending.
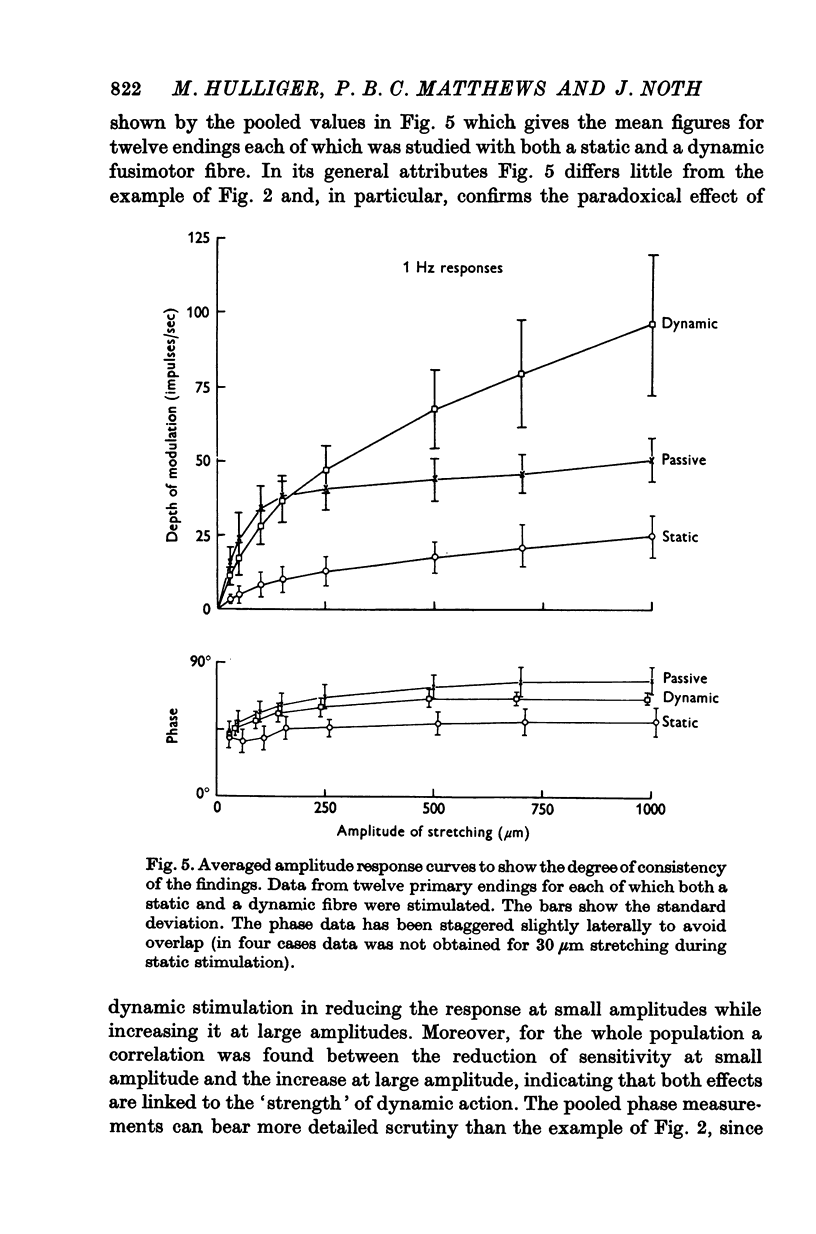
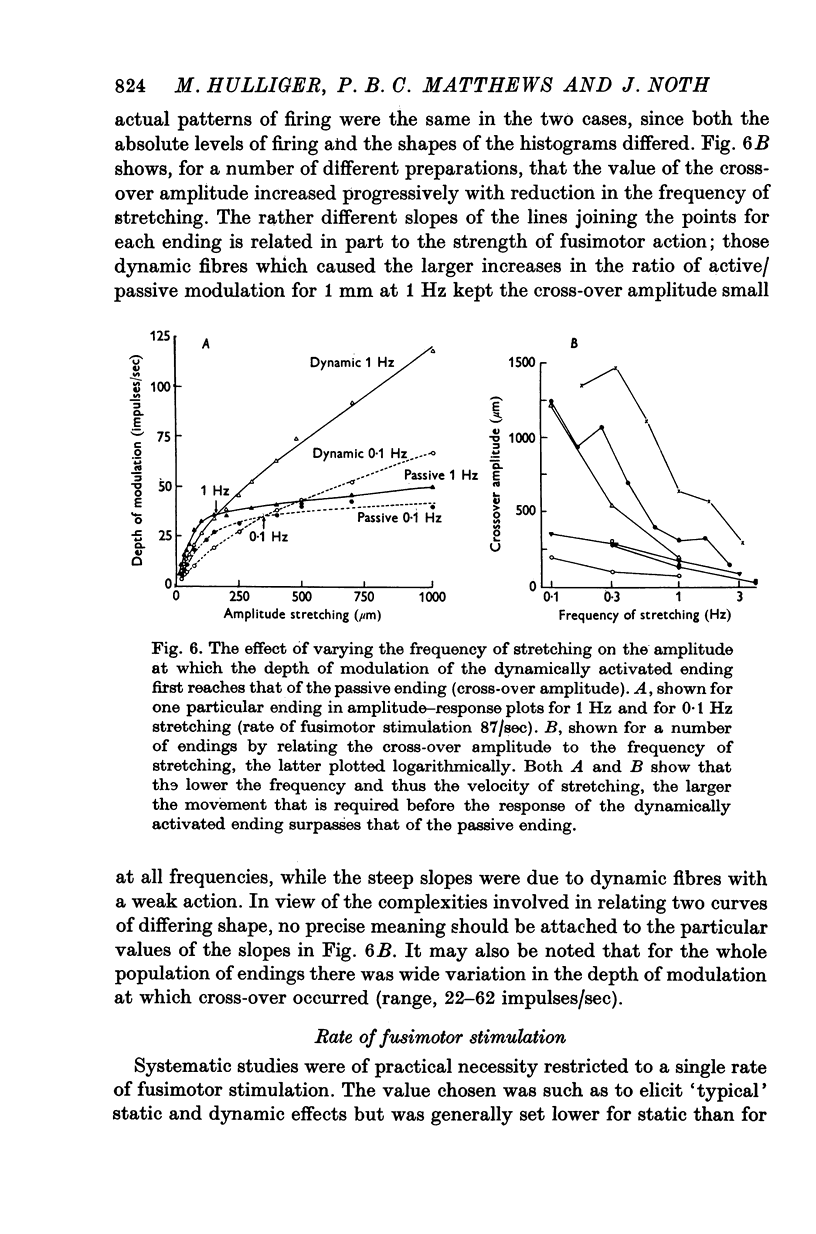
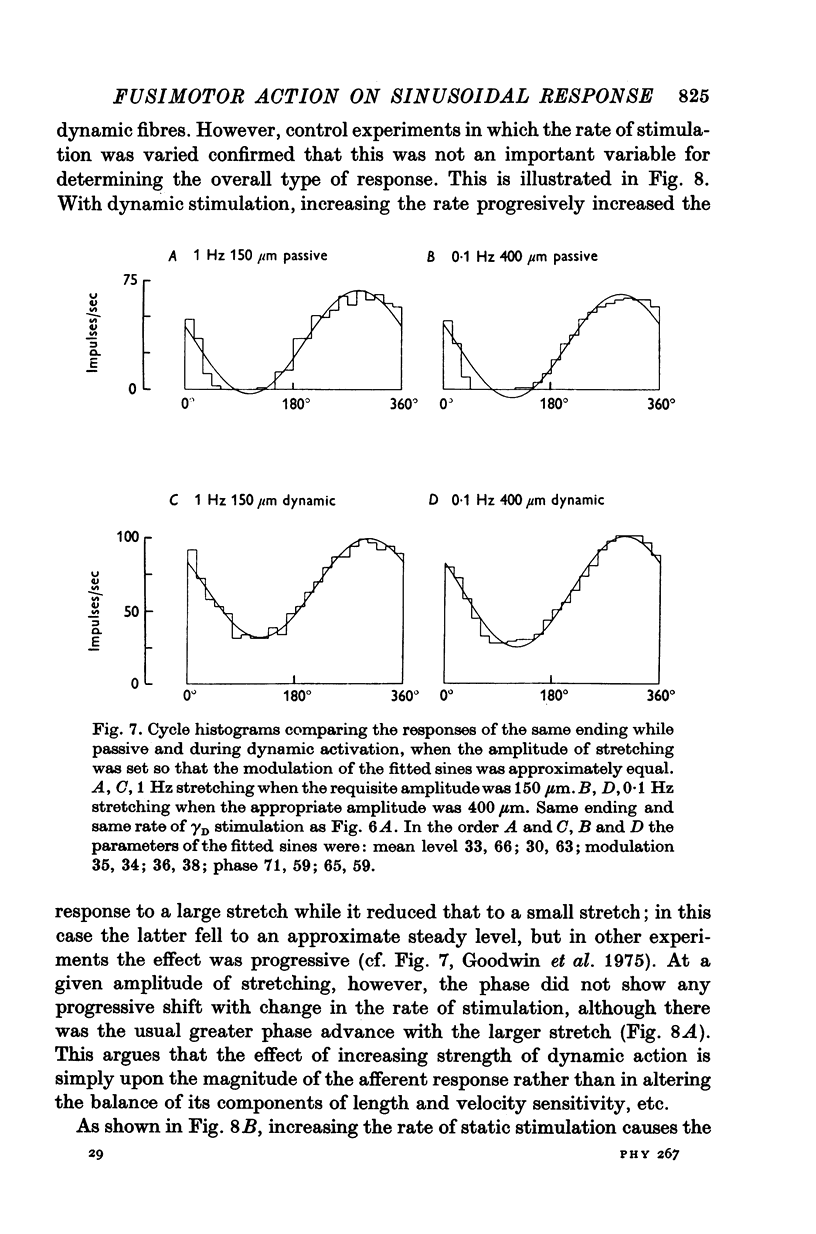
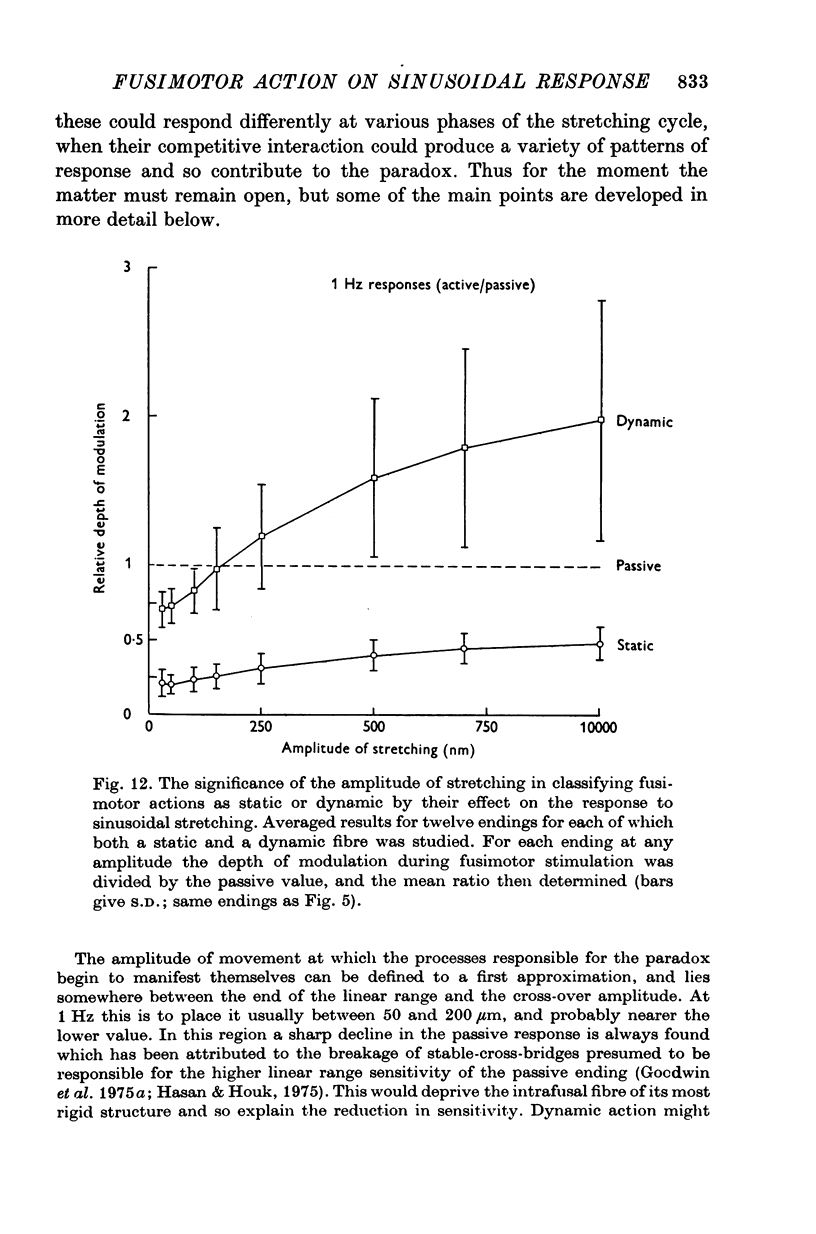
5. Whatever the amplitude of movement the modulation during static stimulation was less than that for the passive or during dynamic stimulation. For small amplitudes the response during dynamic stimulation was less than that of the passive, but for large amplitudes the response during dynamic stimulation was always the greater. At some intermediate cross-over amplitude the two responses were the same size, though still differing slightly in other respects. The value of the cross-over amplitude was usually about 200 μm at 1 Hz, and increased on lowering the frequency. Thus dynamic fusimotor action does not uniformly produce either an increase or a decrease in the sensitivity of the ending in relation to the passive.
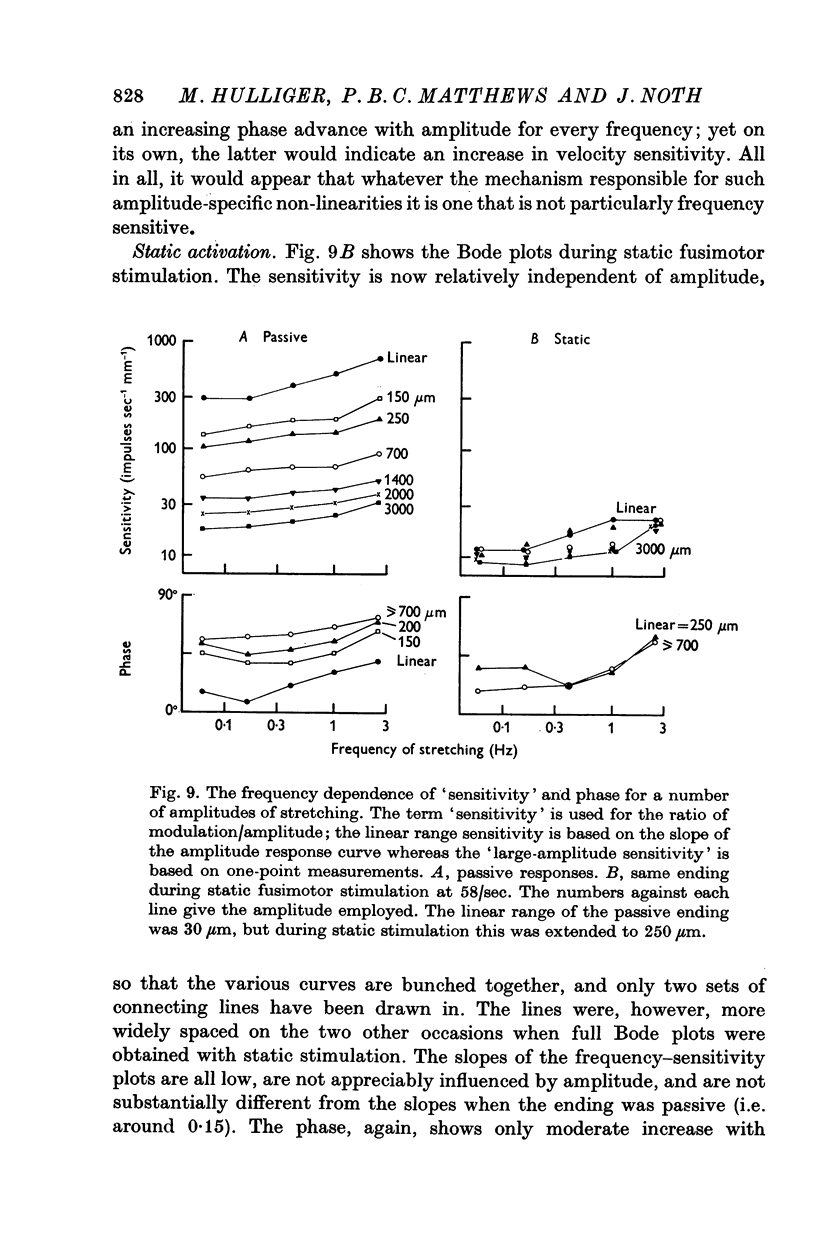
6. Bode plots, for each amplitude, of sensitivity and phase against frequency suggested that
(a) under all conditions the ending is relatively insensitive to frequency in the range studied, for the slope of the log-log sensitivity lines was only 0·15-0·2 (3·5-6 db/decade);
(b) the mechanism which makes for non-linearity is not particularly frequency sensitive;
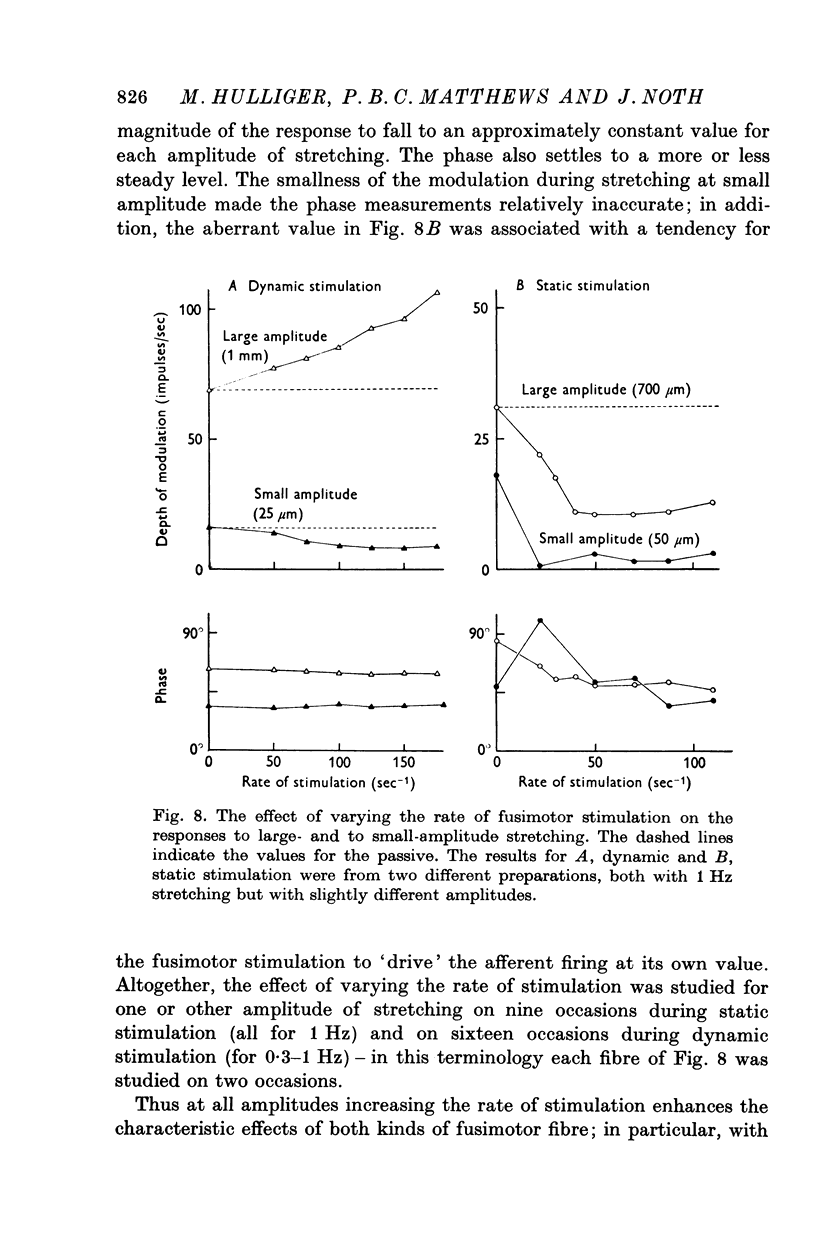
(c) static fusimotor stimulation does not change the frequency sensitivity of the ending;
(d) dynamic fusimotor stimulation very slightly increases the frequency sensitivity of the ending for large amplitudes.
In reaching these conclusions more attention was paid to the slope of the sensitivity lines than to the values of phase.
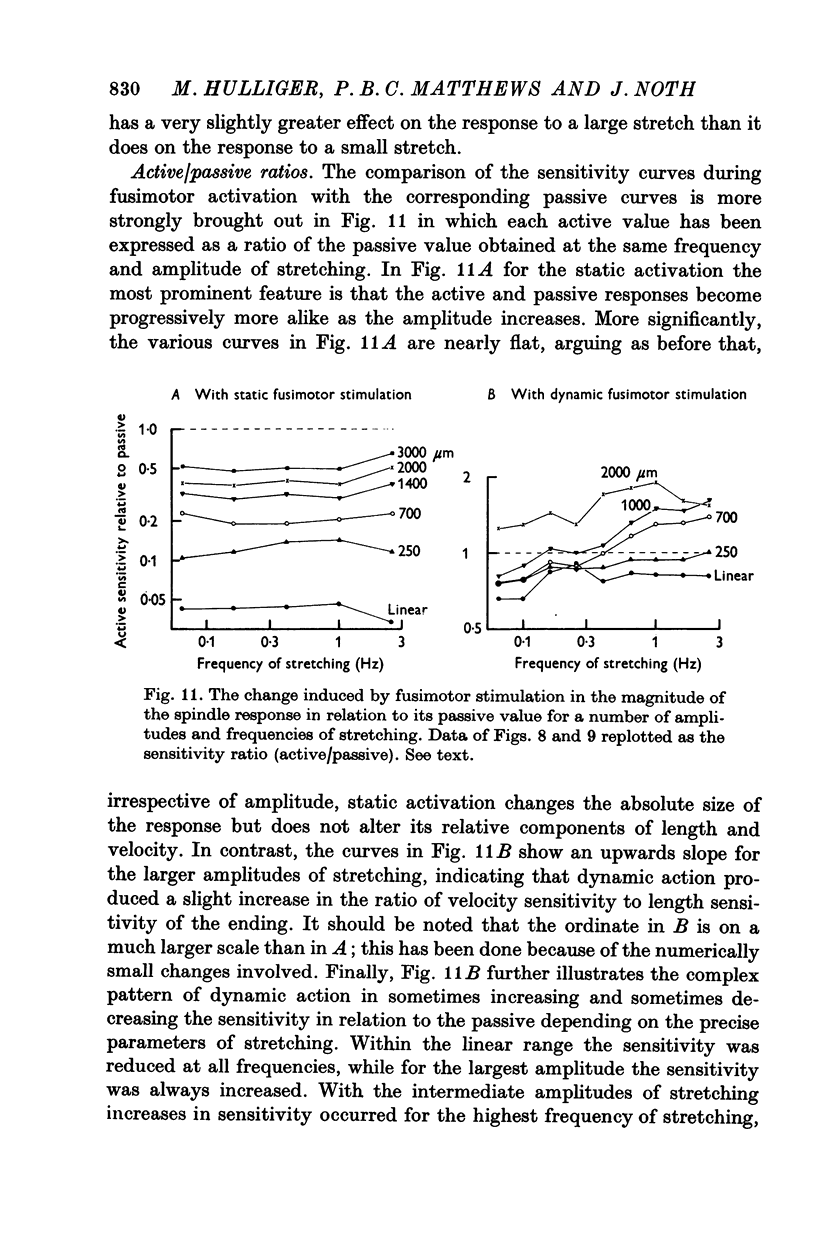
7. It appears that the major effect of fusimotor action, whether static or dynamic, is to regulate the sensitivity of the primary ending to stretching for all amplitudes of movement (i.e. gain) rather than to control the relative values of its sensitivity to length and to velocity (i.e. crudely, the damping in a feed-back loop).
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd I. A. The response of fast and slow nuclear bag fibres and nuclear chain fibres in isolated cat muscle spindles to fusimotor stimulation, and the effect of intrafusal contraction on the sensory endings. Q J Exp Physiol Cogn Med Sci. 1976 Jul;61(3):203–254. doi: 10.1113/expphysiol.1976.sp002354. [DOI] [PubMed] [Google Scholar]
- Brokensha G., Westbury D. R. Adaptation of the discharge of frog muscle spindles following a stretch. J Physiol. 1974 Oct;242(2):383–403. doi: 10.1113/jphysiol.1974.sp010713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Goodwin G. M., Matthews P. B. After-effects of fusimotor stimulation on the response of muscle spindle primary afferent endings. J Physiol. 1969 Dec;205(3):677–694. doi: 10.1113/jphysiol.1969.sp008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. FURTHER STUDIES OF STATIC AND DYNAMIC FUSIMOTOR FIBRES. J Physiol. 1964 Oct;174:132–151. doi: 10.1113/jphysiol.1964.sp007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. THE EFFECTS OF STIMULATION OF STATIC AND DYNAMIC FUSIMOTOR FIBRES ON THE RESPONSE TO STRETCHING OF THE PRIMARY ENDINGS OF MUSCLE SPINDLES. J Physiol. 1964 Oct;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Poppele R. E. Static fusimotor effect on the sensitivity of mammalian muscle spindles. Brain Res. 1973 Jul 16;57(1):244–247. doi: 10.1016/0006-8993(73)90585-4. [DOI] [PubMed] [Google Scholar]
- Eysel U. T., Grüsser O. J. The impulse pattern of muscle spindle afferents. A statistical analysis of the response to static and sinusoidal stimulation. Pflugers Arch. 1970;315(1):1–26. doi: 10.1007/BF00587233. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., Hulliger M., Matthews P. B. The effects of fusimotor stimulation during small amplitude stretching on the frequency-response of the primary ending of the mammalian muscle spindle. J Physiol. 1975 Dec;253(1):175–206. doi: 10.1113/jphysiol.1975.sp011186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser O. J., Thiele B. Reaktionen primärer und sekundärer Muskelspindelafferenzen auf sinusförmige mechanische Reizung. I. Variation der Sinusfrequenz. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968 Apr 23;300(3):161–184. [PubMed] [Google Scholar]
- Hasan Z., Houk J. C. Analysis of response properties of deefferented mammalian spindle receptors based on frequency response. J Neurophysiol. 1975 May;38(3):663–672. doi: 10.1152/jn.1975.38.3.663. [DOI] [PubMed] [Google Scholar]
- Hasan Z., Houk J. C. Transition in sensitivity of spindle receptors that occurs when muscle is stretched more than a fraction of a millimeter. J Neurophysiol. 1975 May;38(3):673–689. doi: 10.1152/jn.1975.38.3.673. [DOI] [PubMed] [Google Scholar]
- Hulliger M., Matthews P. B., Noth J. Effects of combining static and dynamic fusimotor stimulation on the response of the muscle spindle primary ending to sinusoidal stretching. J Physiol. 1977 Jun;267(3):839–856. doi: 10.1113/jphysiol.1977.sp011840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M., Matthews P. B., Noth J. The response of spindle primary afferents to 1 Hz sinusoidal stretching during paired fusimotor stimulation [proceedings]. J Physiol. 1976 Dec;263(1):182P–183P. [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The central control of the dynamic response of muscle spindle receptors. J Physiol. 1962 May;161:357–378. doi: 10.1113/jphysiol.1962.sp006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerstrand G., Thoden U. Position and velocity sensitivity of muscle spindles in the cat. II. Dynamic fusimotor single-fibre activation of primary endings. Acta Physiol Scand. 1968 Sep-Oct;74(1):16–29. doi: 10.1111/j.1748-1716.1968.tb04211.x. [DOI] [PubMed] [Google Scholar]
- Mann D. W., Chapman K. M. Component mechanisms of sensitivity and adaptation in an insect mechanoreceptor. Brain Res. 1975 Oct 31;97(2):331–336. doi: 10.1016/0006-8993(75)90454-0. [DOI] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969 Feb;200(3):723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U. Stretch-evoked potentiation of responses of muscle spindles in the cat. Brain Res. 1975 May 2;88(2):378–383. doi: 10.1016/0006-8993(75)90403-5. [DOI] [PubMed] [Google Scholar]
- STUART D., OTT K., ISHIKAWA K., ELDRED E. MUSCLE RECEPTOR RESPONSES TO SINUSOIDAL STRETCH. Exp Neurol. 1965 Sep;13:82–95. doi: 10.1016/0014-4886(65)90007-5. [DOI] [PubMed] [Google Scholar]


