Abstract
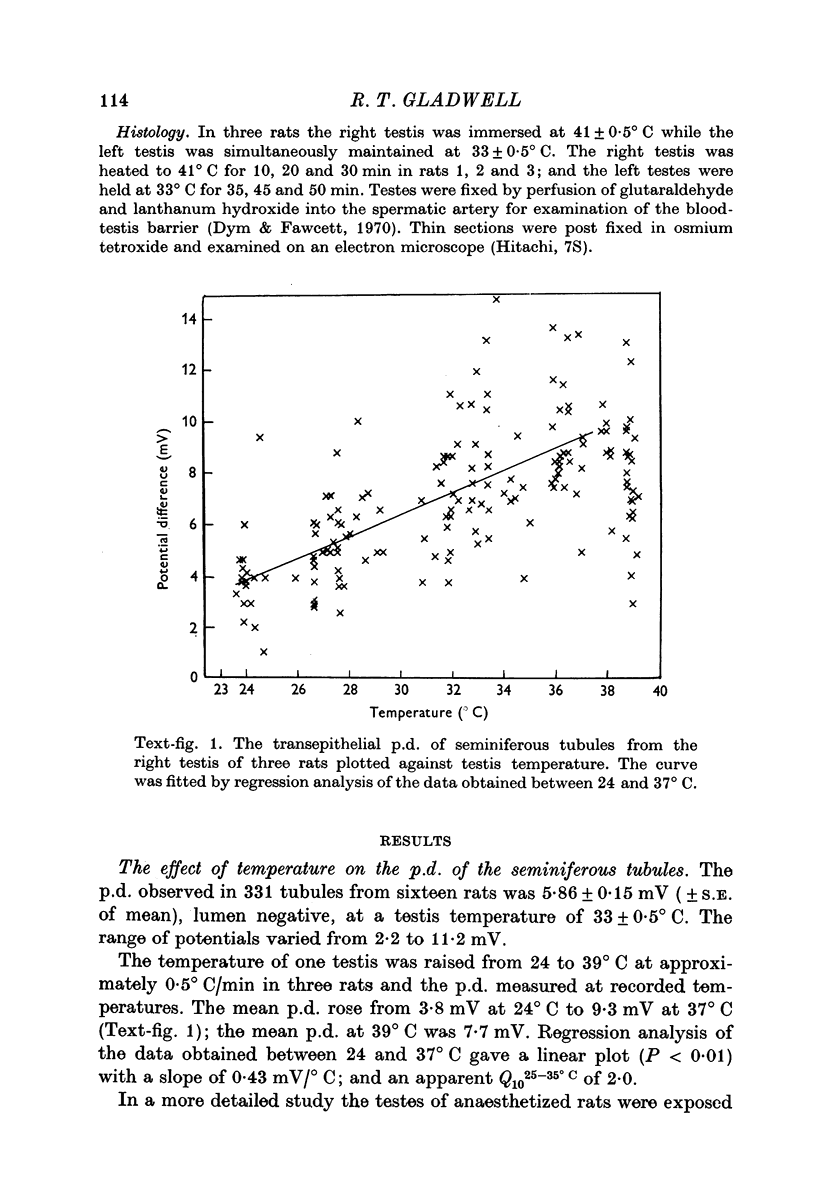
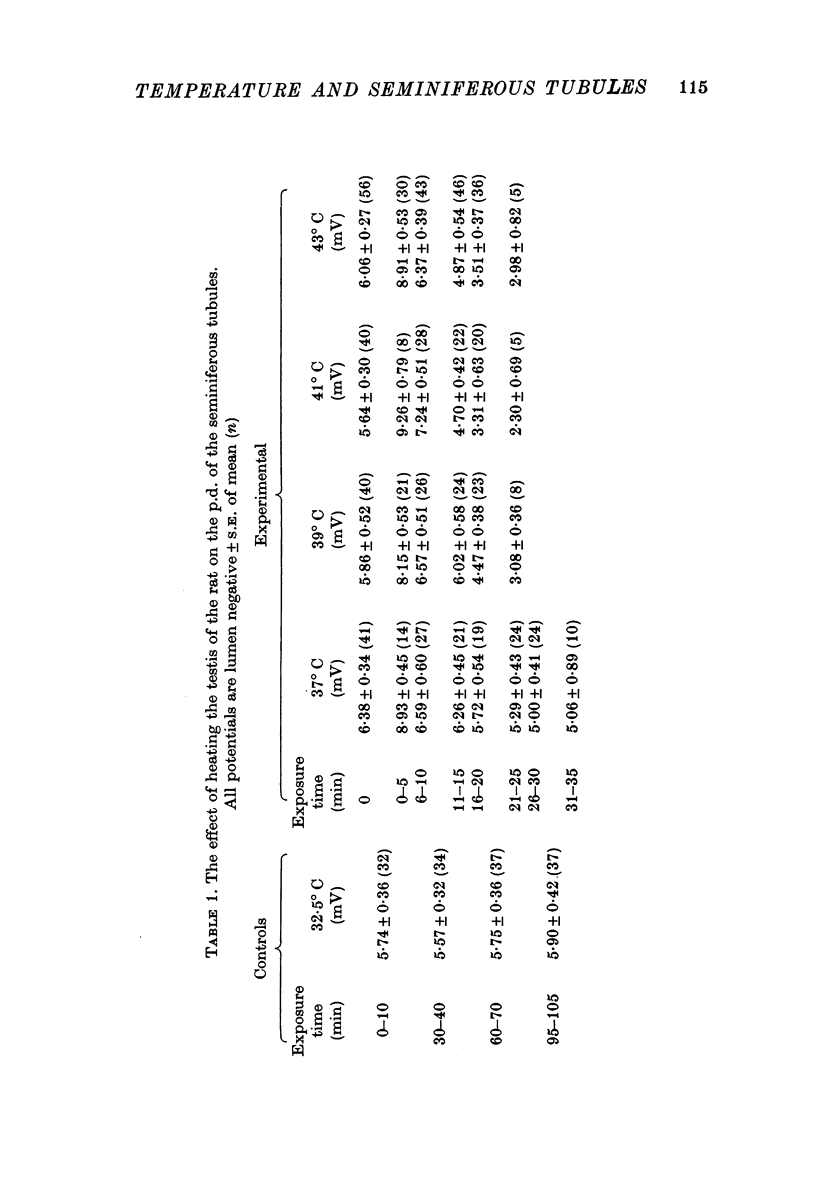
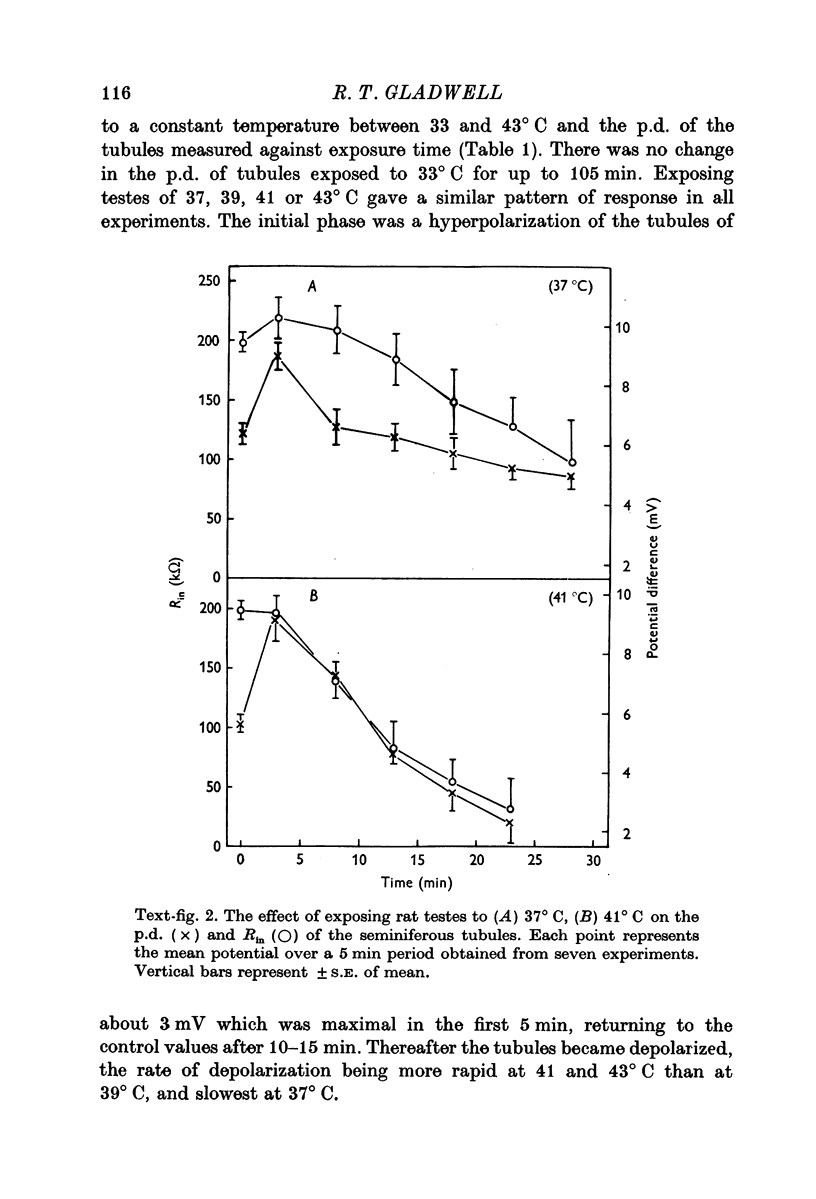
1. The p.d. of rat seminiferous tubules was 5·86 ± 0·15 mV, lumen negative, at 33° C and varied linearly with temperature between 24 and 37° C, exhibiting an apparent Q1025-35° C of 2·0 with a slope of 0·43 mV/° C. Exposing testes to a temperature of 37-43° C resulted in an initial hyperpolarization followed by depolarization of the tubules. These changes were more rapid in testes exposed to 41-43° C than in testes exposed to 37-39° C.
2. The Rin of seminiferous tubules was 198 ± 7·8 kΩ at a testis temperature of 33° C. The Rin decreased when testes were maintained at 37 and 41° C, the rate of decrease being similar to the rate of depolarization.
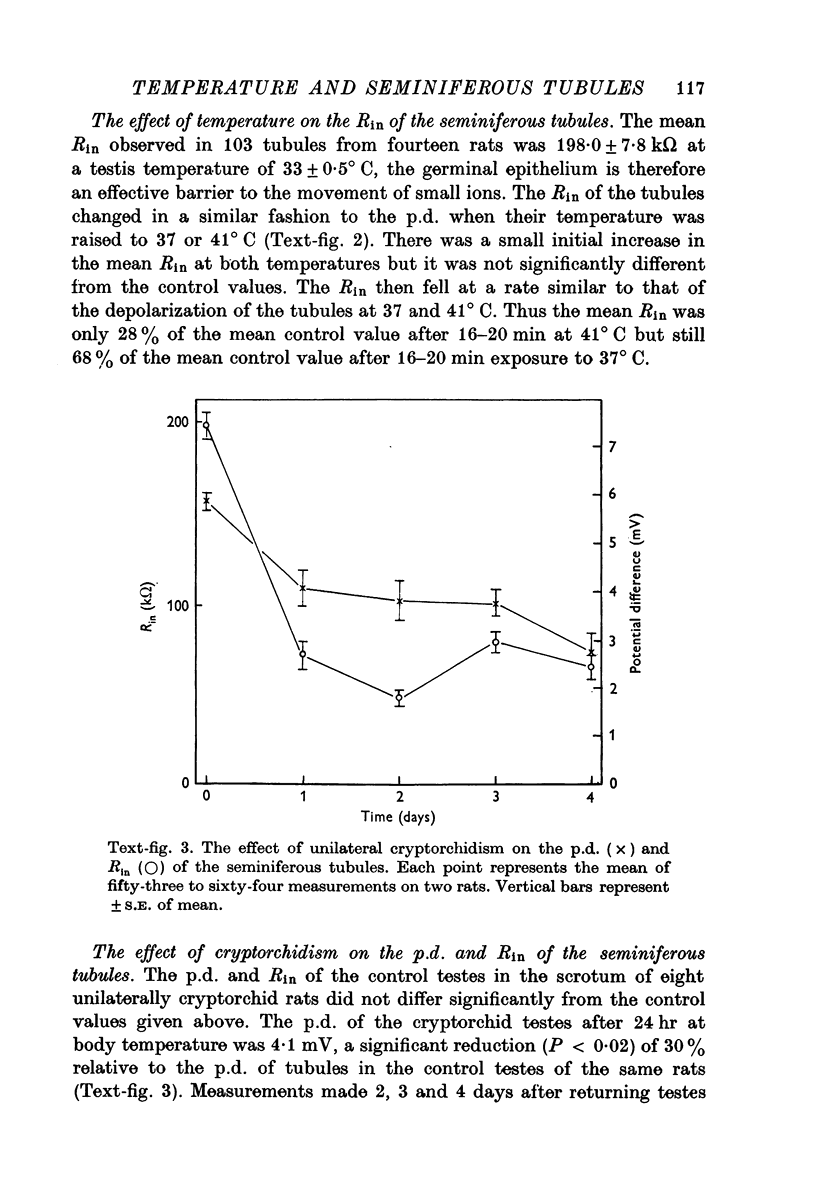
3. Exposing testes to deep body temperature by unilateral surgical cryptorchidism caused a reduction of 30 and 64% in tubular p.d. and Rin respectively when measured 24 hr after surgery. Exposure to deep body temperature for up to 4 days did not cause any further change in either parameter.
4. There was no evidence that lanthanum penetrated through the Sertoli cell tight junctions after exposing testes to 41° C for up to 30 min.
5. The results indicate that the seminiferous tubule p.d. is maintained by a temperature-sensitive, cellular mechanism. Exposing testes to deep body temperature or above depolarized the tubules and increased their permeability.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackshaw A. W., Hamilton D., Massey P. F. Effect of scrotal heating on testicular enzymes and spermatogenesis in the rat. Aust J Biol Sci. 1973 Dec;26(6):1395–1407. doi: 10.1071/bi9731395. [DOI] [PubMed] [Google Scholar]
- Blackshaw A. W., Hamilton D. The effect of heat on hydrolytic enzymes and spermatogenesis in the rat testis. J Reprod Fertil. 1970 Aug;22(3):569–571. doi: 10.1530/jrf.0.0220569. [DOI] [PubMed] [Google Scholar]
- CHOWDHURY A. K., STEINBERGER E. A QUANTITATIVE STUDY OF THE EFFECT OF HEAT ON GERMINAL EPITHELIUM OF RAT TESTES. Am J Anat. 1964 Nov;115:509–524. doi: 10.1002/aja.1001150307. [DOI] [PubMed] [Google Scholar]
- Chowdhury A. K., Steinberger E. Early changes in the germinal epithelium of rat testes following exposure to heat. J Reprod Fertil. 1970 Jul;22(2):205–212. doi: 10.1530/jrf.0.0220205. [DOI] [PubMed] [Google Scholar]
- Dym M., Fawcett D. W. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970 Dec;3(3):308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Levine N., Marsh D. J. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol. 1971 Mar;213(3):557–570. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Setchell B. P. Metabolism, sperm and fluid production of the isolated perfused testis of the sheep and goat. J Physiol. 1969 Mar;201(1):129–143. doi: 10.1113/jphysiol.1969.sp008747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main S. J., Waites G. M. Proceedings: Studies on some effects of temperature on the blood-testis barrier in rats. J Reprod Fertil. 1973 Dec;35(3):587–588. doi: 10.1530/jrf.0.0350587. [DOI] [PubMed] [Google Scholar]
- Schanne O. F., De Ceretti E. R. Measurement of input impedance and cytoplasmic resistivity with a single microelectrode. Can J Physiol Pharmacol. 1971 Jul;49(7):713–716. doi: 10.1139/y71-096. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. Electrical potential differences and electromotive forces in epithelial tissues. J Gen Physiol. 1972 Jun;59(6):794–798. doi: 10.1085/jgp.59.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell B. P. The secretion of fluid by the testes of rats, rams and goats with some observations on the effect of age, cryptorchidism and hypophysectomy. J Reprod Fertil. 1970 Oct;23(1):79–85. doi: 10.1530/jrf.0.0230079. [DOI] [PubMed] [Google Scholar]
- Setchell B. P., Voglmayr J. K., Waites G. M. A blood-testis barrier restricting passage from blood into rete testis fluid but not into lymph. J Physiol. 1969 Jan;200(1):73–85. doi: 10.1113/jphysiol.1969.sp008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell B. P., Waites G. M. The exocrine secretion of the testis and spermatogenesis. J Reprod Fertil Suppl. 1971 May;13(Suppl):15–27. [PubMed] [Google Scholar]
- TASAKI I., POLLEY E. H., ORREGO F. Action potentials from individual elements in cat geniculate and striate cortex. J Neurophysiol. 1954 Sep;17(5):454–474. doi: 10.1152/jn.1954.17.5.454. [DOI] [PubMed] [Google Scholar]
- Tuck R. R., Setchell B. P., Waites G. M., Young J. A. The composition of fluid collected by micropuncture and catheterization from the seminiferous tubules and rete testis of rats. Pflugers Arch. 1970;318(3):225–243. doi: 10.1007/BF00593663. [DOI] [PubMed] [Google Scholar]
- WAITES G. M., SETCHELL B. P. EFFECT OF LOCAL HEATING ON BLOOD FLOW AND METABOLISM IN THE TESTIS OF THE CONSCIOUS RAM. J Reprod Fertil. 1964 Dec;8:339–349. doi: 10.1530/jrf.0.0080339. [DOI] [PubMed] [Google Scholar]
- Waites G. M., Jones A. R., Main S. J., Cooper T. G. The entry of antifertility and other compounds into the testis. Adv Biosci. 1973;10:101–116. [PubMed] [Google Scholar]
- Waites G. M., Setchell B. P., Quinlan D. Effect of local heating of the scrotum, testes and epididymides of rats on cardiac output and regional blood flow. J Reprod Fertil. 1973 Jul;34(1):41–49. doi: 10.1530/jrf.0.0340041. [DOI] [PubMed] [Google Scholar]