Abstract
1. Responses were recorded in decereberate, unanaesthetized cats from individual cuneate neurones in order to determine firstly, the afferent sources of inhibition on cuneate neurones and secondly, the influence of afferent-induced inhibition on those response features of dynamically sensitive tactile neurones which determine their capacity to code information about parameters of tactile stimuli.
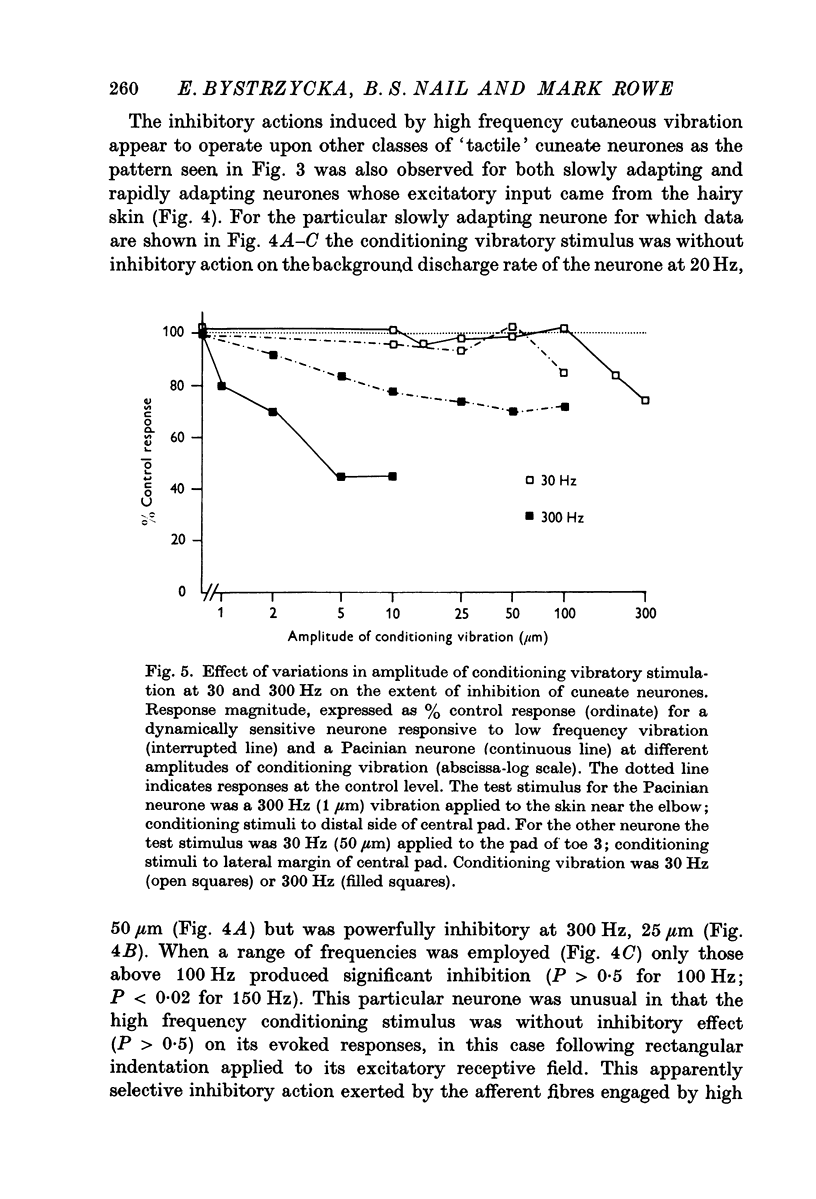
2. For all cuneate neurones which displayed afferent-induced inhibition from areas surrounding or within their excitatory receptive field (71% of the sample) it was consistently found that 300 Hz vibration at low amplitudes (< 25-50 μm) which selectively engages Pacinian corpuscles was an effective source of inhibition. In contrast, steady indentation which activates slowly adapting tactile afferents was quite ineffective, as was low frequency vibration (30 Hz) at amplitudes of < 50-100 μm. The latter stimulus can be used to engage rapidly adapting receptors either within glabrous skin (presumed to be Meissners corpuscles) or in association with hair follicles. It is concluded that afferents from Pacinian corpuscles are the dominant or exclusive source of afferent-induced inhibition of cuneate neurones.
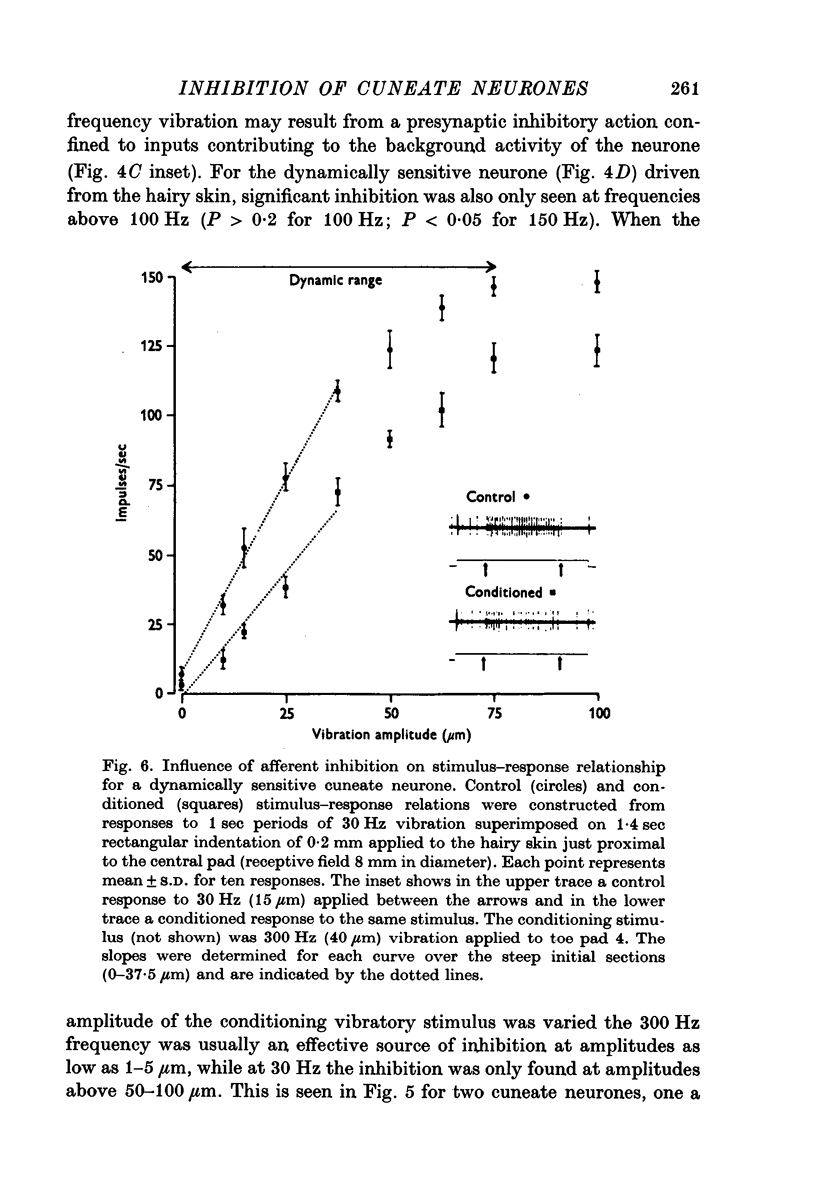
3. For dynamically sensitive neurones responsive to low frequency cutaneous vibration (30 Hz) there was a reduction in the slope of stimulus—response relations with afferent-induced inhibition, but no expansion of the range of stimulus amplitudes over which the neurone responded.
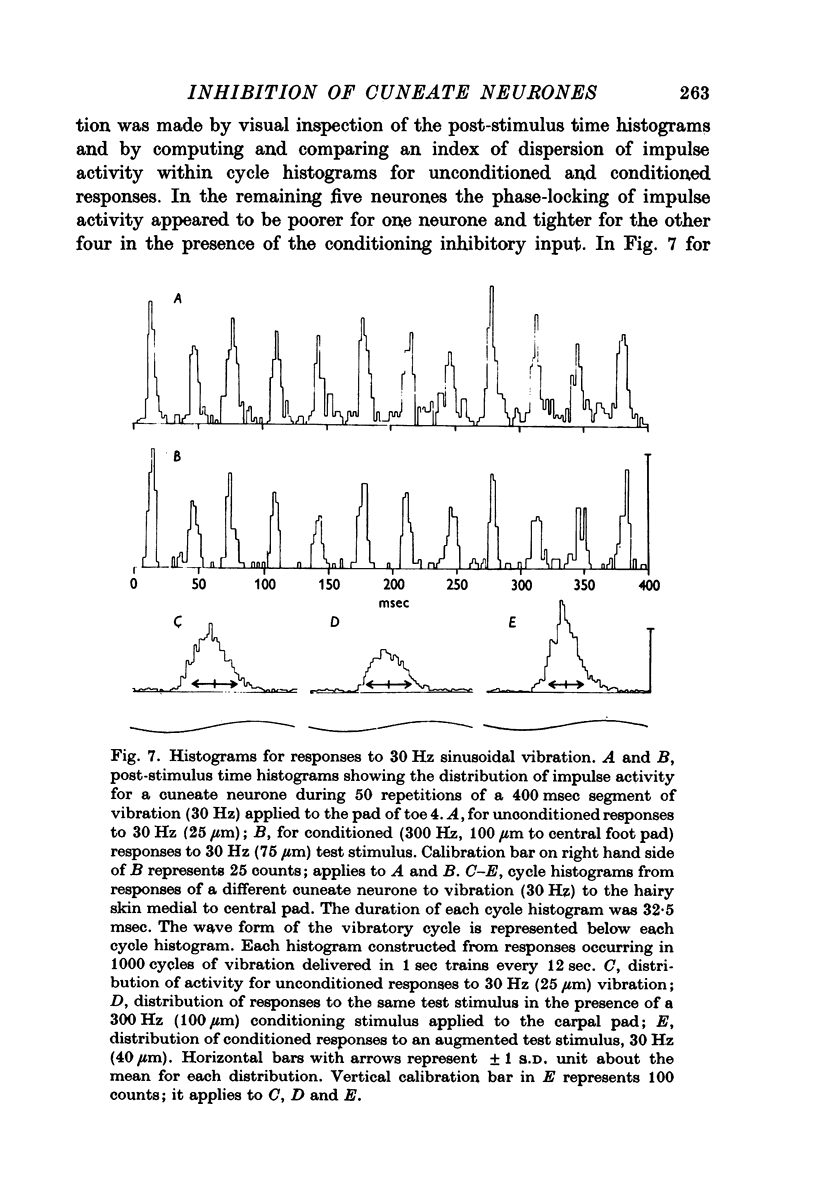
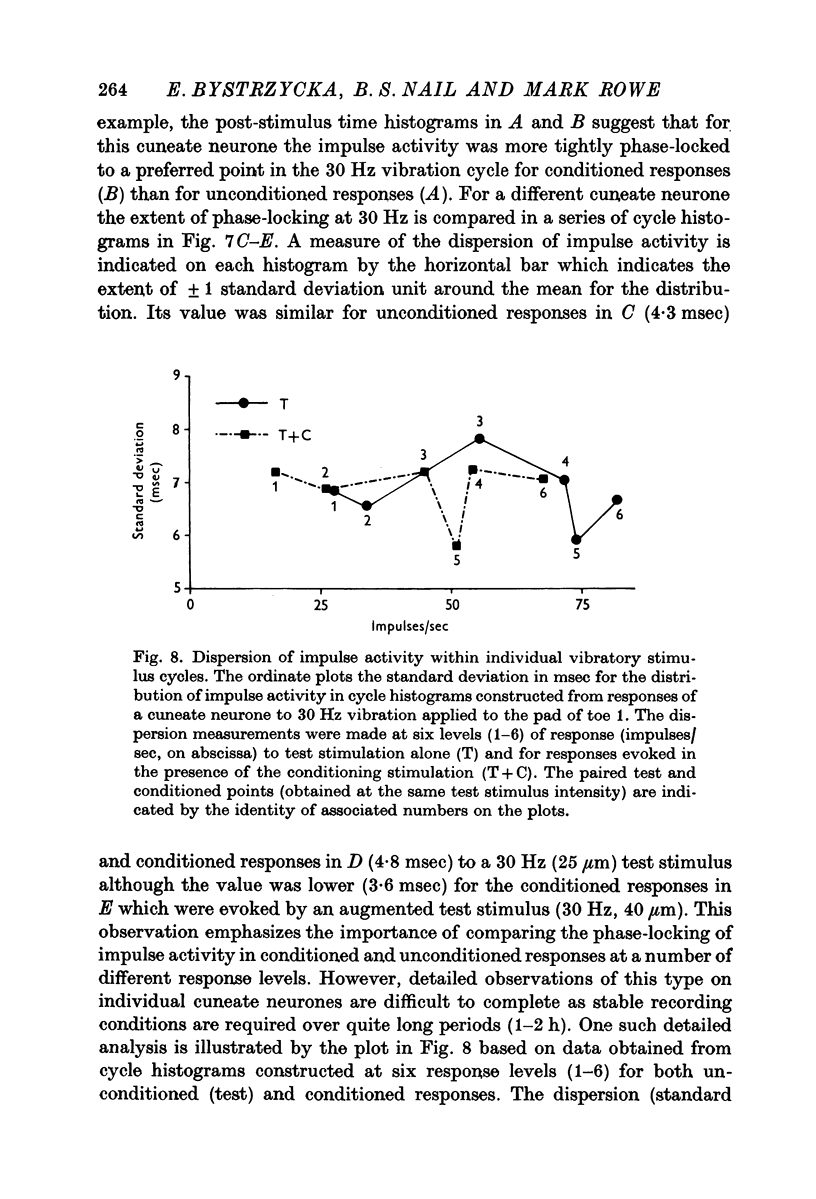
4. The influence of afferent-induced inhibition on the phase-locking of impulse activity to a cutaneous vibratory wave form was examined by constructing post-stimulus time histograms and cycle histograms. Measures of dispersion of impulse activity around the preferred point of firing in the vibratory waveform indicated that the capacity of individual cuneate neurones to code information about the frequency of the cutaneous vibration was not systematically changed in the presence of afferent-induced inhibition.
Full text
PDF



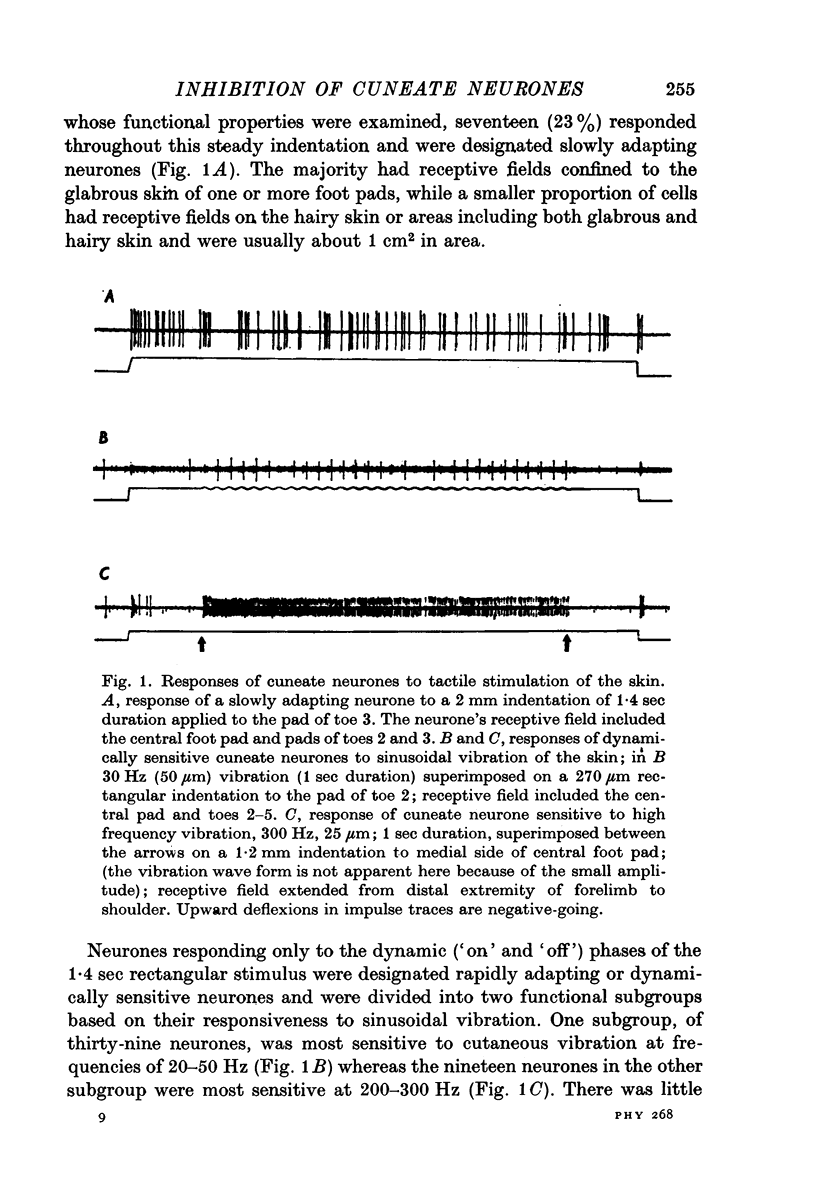
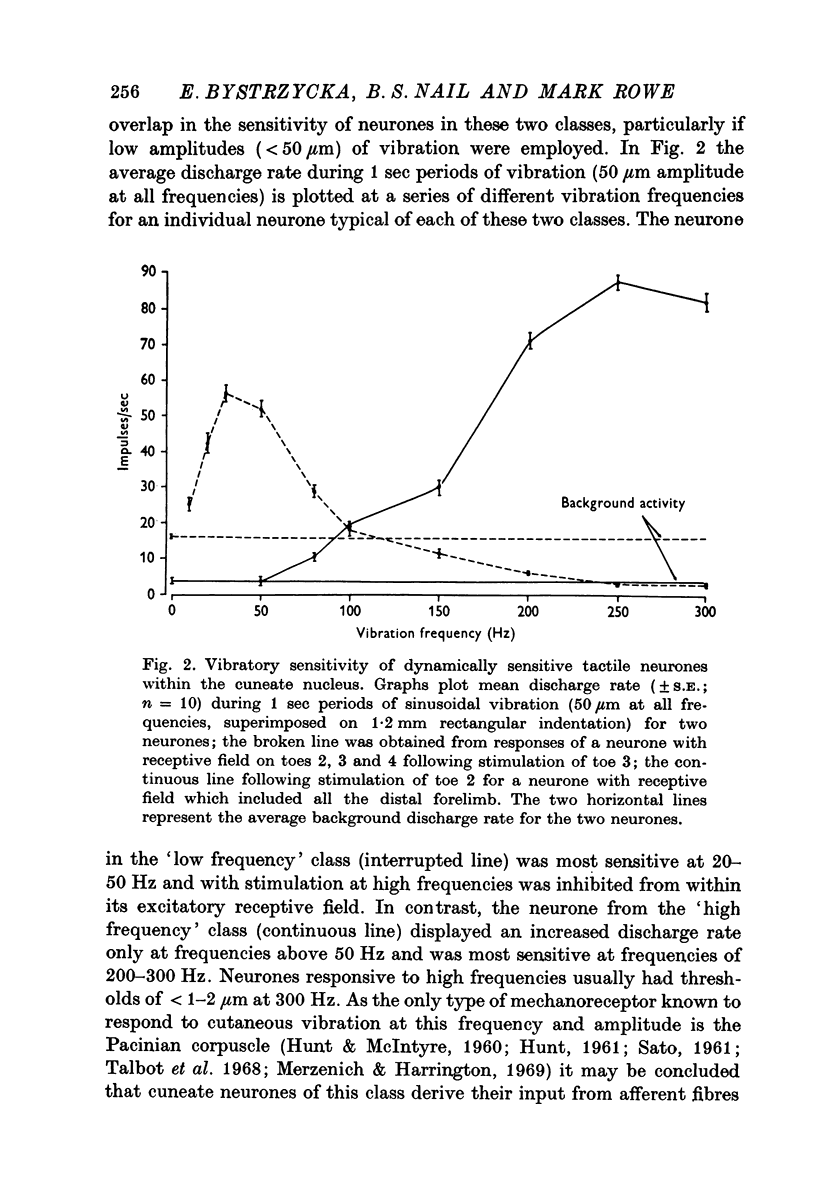

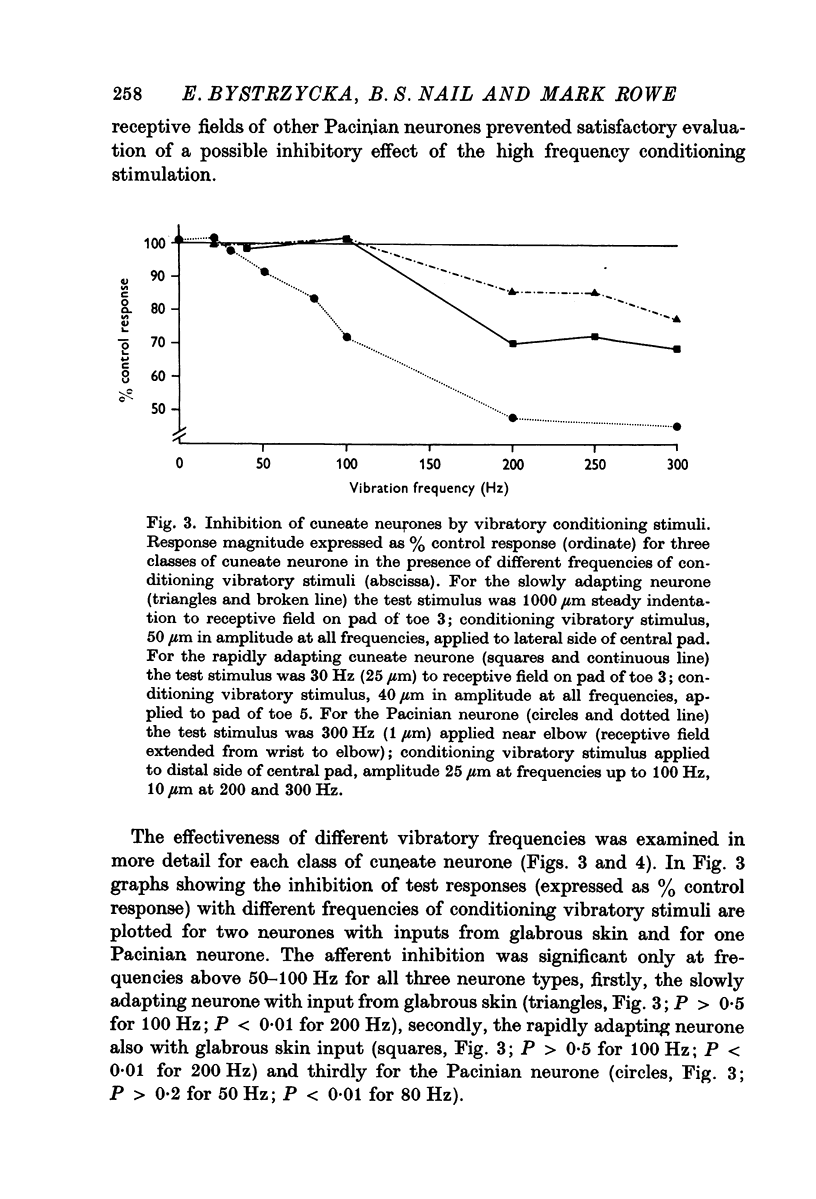
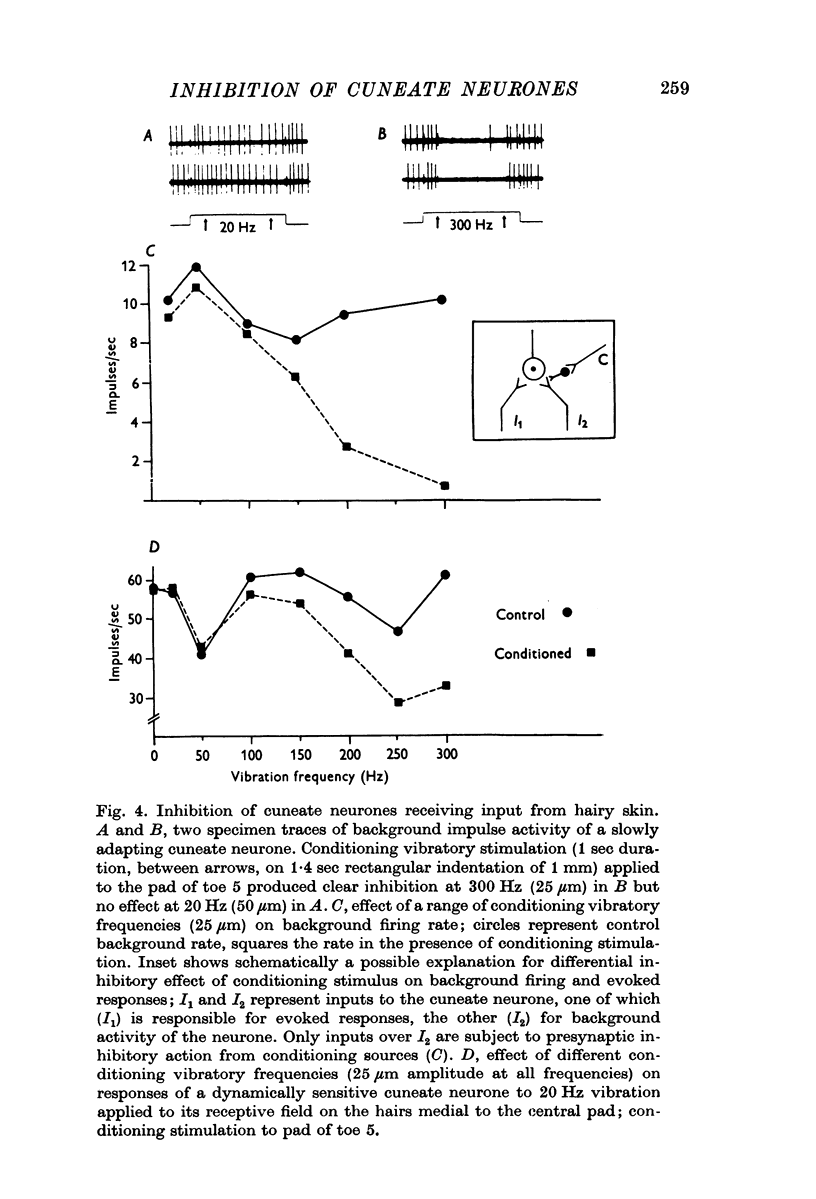







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. SLOW POTENTIAL WAVES PRODUCED IN THE CUNEATE NUCLEUS BY CUTANEOUS VOLLEYS AND BY CORTICAL STIMULATION. J Neurophysiol. 1964 Jan;27:78–91. doi: 10.1152/jn.1964.27.1.78. [DOI] [PubMed] [Google Scholar]
- Andersen P., Etholm B., Gordon G. Presynaptic and post-synaptic inhibition elicited in the cat's dorsal column nuclei by mechanical stimulation of skin. J Physiol. 1970 Sep;210(2):433–455. doi: 10.1113/jphysiol.1970.sp009219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Etholm B., Gordon G. Presynaptic depolarization of dorsal column fibres by adequate stimulation. J Physiol. 1968 Feb;194(2):83–4P. [PubMed] [Google Scholar]
- Brown A. G. Cutaneous afferent fibre collaterals in the dorsal columns of the cat. Exp Brain Res. 1968;5(4):293–305. doi: 10.1007/BF00235904. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Gordon G., Kay R. H. A study of single axons in the cat's medial lemniscus. J Physiol. 1974 Jan;236(1):225–246. doi: 10.1113/jphysiol.1974.sp010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Petit D., Warren R. M. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- Carmody J., Rowe M. Inhibition within the trigeminal nucleus induced by afferent inputs and its influence on stimulus coding by mechanosensitive neurones. J Physiol. 1974 Nov;243(1):195–210. doi: 10.1113/jphysiol.1974.sp010749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I., Rowe M. J., Sessle B. J. "Tactile" stimulus intensity: information transmission by relay neurons in different trigeminal nuclei. Science. 1968 May 17;160(3829):791–794. doi: 10.1126/science.160.3829.791. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DUAL ORGANIZATION OF THE EXTEROCEPTIVE COMPONENTS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. An analysis of fibre diameter and receptor characteristics of myelinated cutaneous afferent fibres in cat. J Physiol. 1960 Aug;153:99–112. doi: 10.1113/jphysiol.1960.sp006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. On the nature of vibration receptors in the hind limb of the cat. J Physiol. 1961 Jan;155:175–186. doi: 10.1113/jphysiol.1961.sp006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. O. Reconstruction of population response to a vibratory stimulus in quickly adapting mechanoreceptive afferent fiber population innervating glabrous skin of the monkey. J Neurophysiol. 1974 Jan;37(1):48–72. doi: 10.1152/jn.1974.37.1.48. [DOI] [PubMed] [Google Scholar]
- Jänig W. Morphology of rapidly and slowly adapting mechanoreceptors in the hairless skin of the cat's hind foot. Brain Res. 1971 May 7;28(2):217–231. doi: 10.1016/0006-8993(71)90656-1. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Two specific feedback pathways to the central afferent terminals of phasic and tonic mechanoreceptors. Exp Brain Res. 1968;6(2):116–129. doi: 10.1007/BF00239166. [DOI] [PubMed] [Google Scholar]
- Leicht R., Rowe M. J., Schmidt R. F. Cutaneous convergence on to the climbing fibre input to cerebellar Purkyne cells. J Physiol. 1973 Feb;228(3):601–618. doi: 10.1113/jphysiol.1973.sp010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B. The form and distribution of the receptive fields of Pacinian corpuscles found in and around the cat's large foot pad. J Physiol. 1971 Sep;217(3):755–771. doi: 10.1113/jphysiol.1971.sp009598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L. Inhibitory and facilitatory spatial interactions in retinal receptive fields. Vision Res. 1968 Sep;8(9):1187–1194. doi: 10.1016/0042-6989(68)90026-6. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Harrington T. H. The sense of flutter-vibration evoked by stimulation of the hairy skin of primates: comparison of human sensory capacity with the responses of mechanoreceptive afferents innervating the hairy skin of monkeys. Exp Brain Res. 1969;9(3):236–260. doi: 10.1007/BF00234457. [DOI] [PubMed] [Google Scholar]
- PERL E. R., WHITLOCK D. G., GENTRY J. R. Cutaneous projection to second-order neurons of the dorsal column system. J Neurophysiol. 1962 May;25:337–358. doi: 10.1152/jn.1962.25.3.337. [DOI] [PubMed] [Google Scholar]
- Petit D., Burgess P. R. Dorsal column projection of receptors in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):849–855. doi: 10.1152/jn.1968.31.6.849. [DOI] [PubMed] [Google Scholar]
- Ratliff F., Knight B. W., Toyoda J., Hartline H. K. Enhancement of flicker by lateral inhibition. Science. 1967 Oct 20;158(3799):392–393. doi: 10.1126/science.158.3799.392. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Rowe M. J., Carmody J. J. Afferent inhibition over the response range of secondary trigeminal neurones. Brain Res. 1970 Mar 3;18(2):371–374. doi: 10.1016/0006-8993(70)90338-0. [DOI] [PubMed] [Google Scholar]
- SATO M. Response of Pacinian corpuscles to sinusoidal vibration. J Physiol. 1961 Dec;159:391–409. doi: 10.1113/jphysiol.1961.sp006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. F., Senges J., Zimmermann M. Presynaptic depolarization of cutaneous mechanoreceptor afferents after mechanical skin stimulation. Exp Brain Res. 1967;3(3):234–247. doi: 10.1007/BF00235587. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]


