Abstract
1. Implanted dorsal root electrodes were used to record discharge trains of single spindle primary afferents (Ia's) of the cat's hind limb during different types of movement.
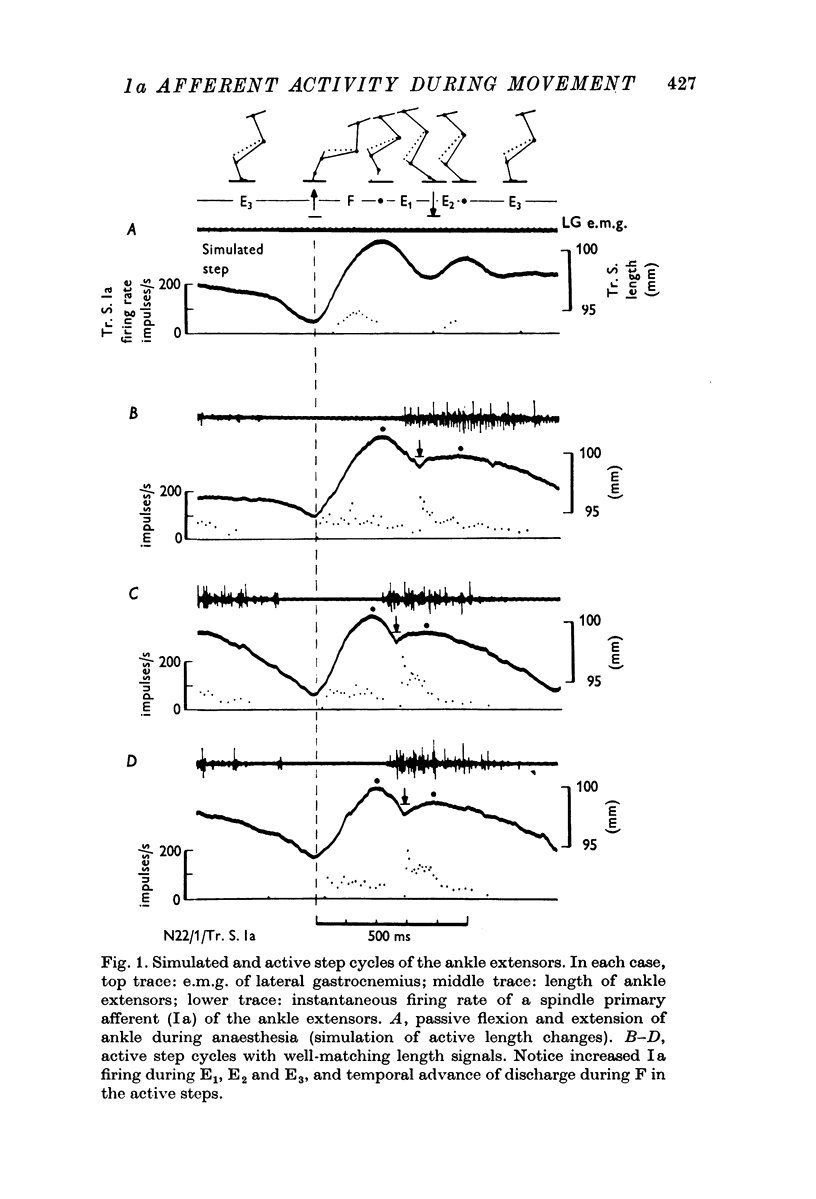
2. The length of the ipsilateral ankle extensors was continuously monitored by an implanted length gauge. Length changes occurring during active stepping were subsequently passively reproduced during brief anaesthesia.
3. A comparison of the Ia responses in active and simulated step cycles revealed that moderate fusimotor drive to ankle extensor spindles probably occurred mainly, if not exclusively, during the E1, E2 and E3 phases of active stepping.
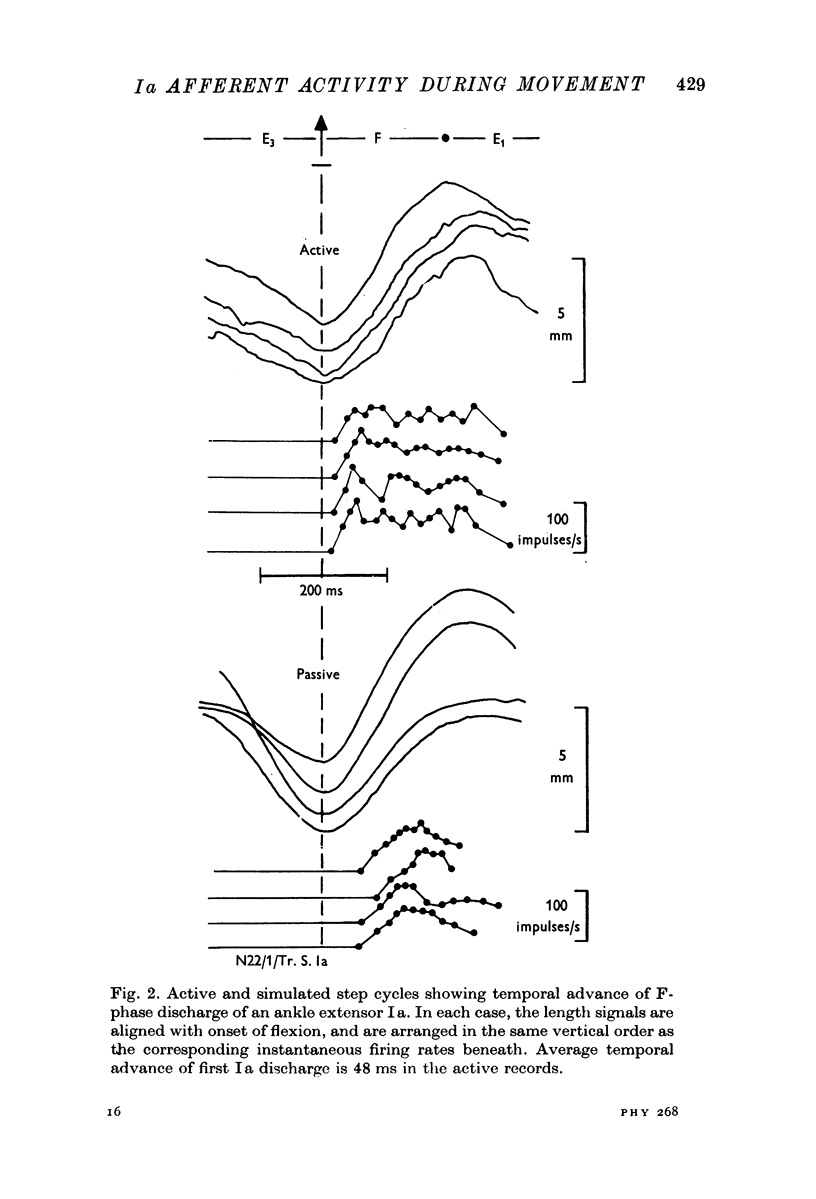
4. A temporal advance in the Ia response to passive stretching in the F-phase was attributed to the after-effects of fusimotor activity in the extension phases.
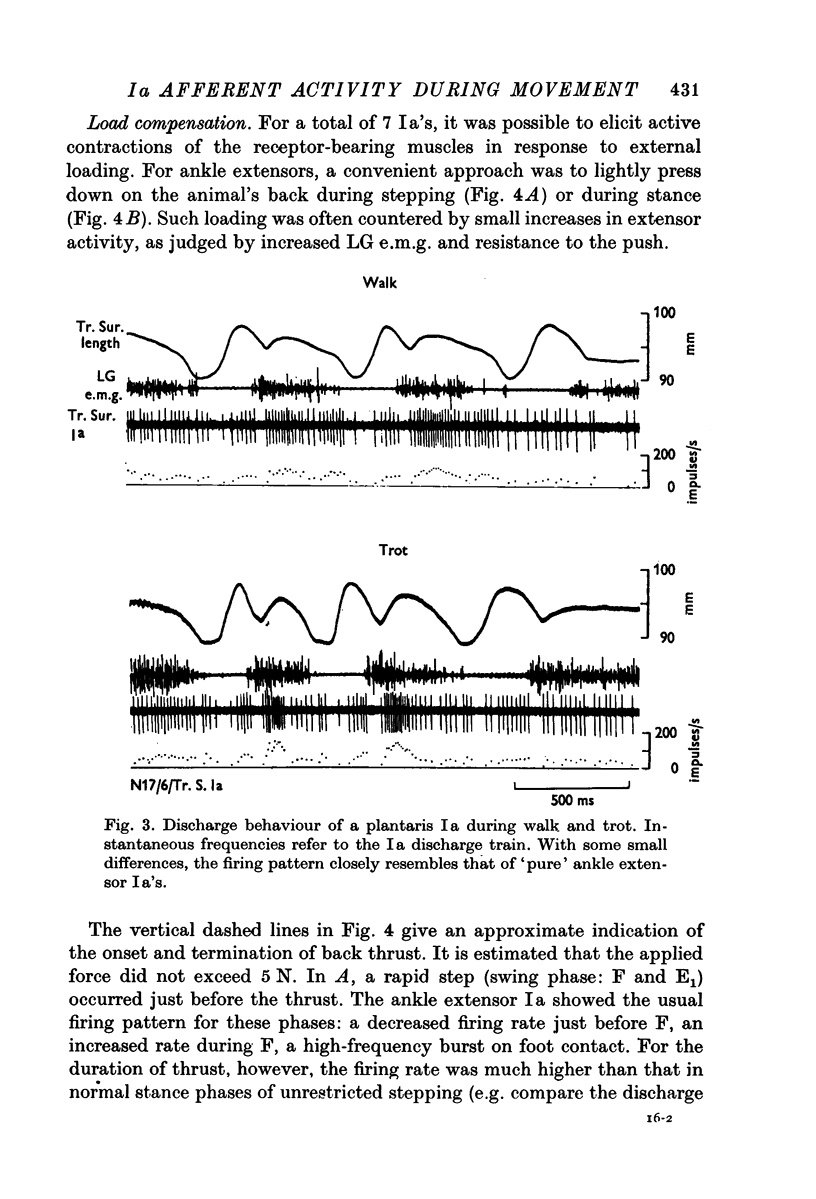
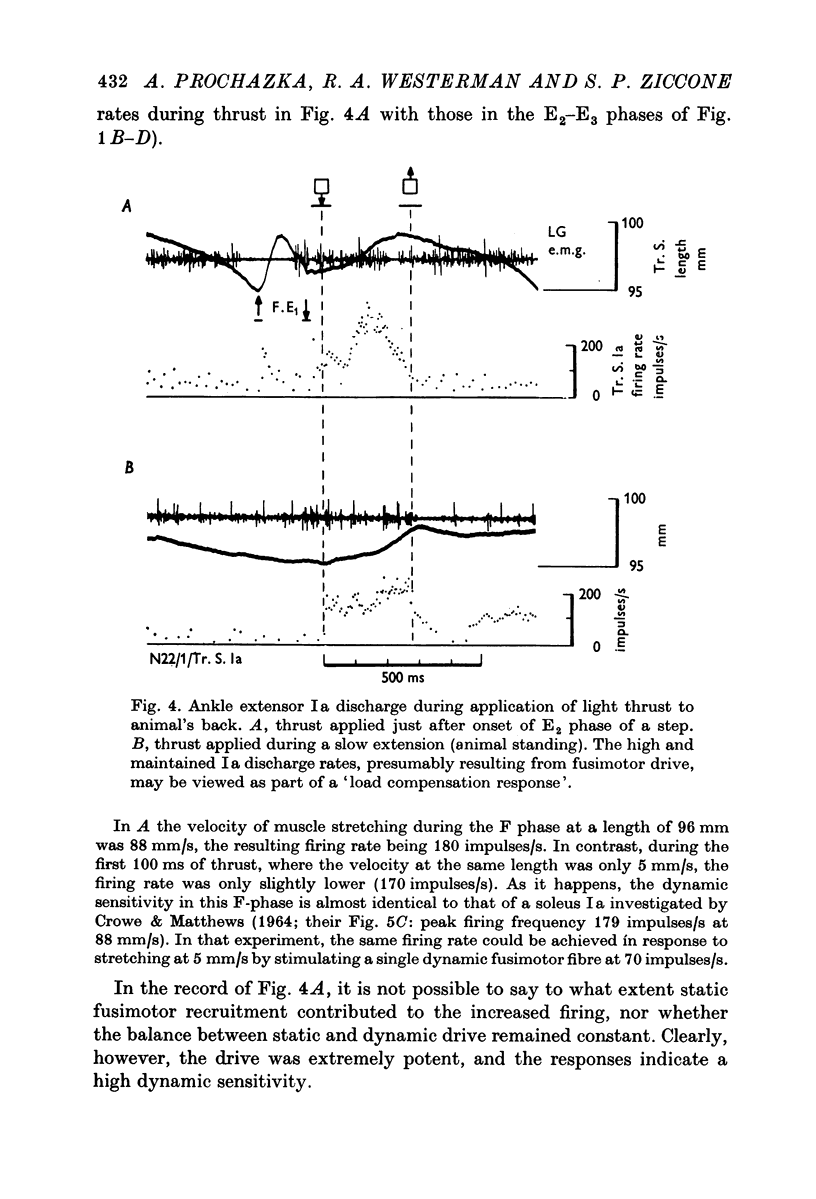
5. Light thrust applied to the animal's back evoked a potent fusimotor response. This load compensation effect may provide an explanation for the apparently higher degree of α-γ co-activation seen in the mesencephalic locomotor preparation.
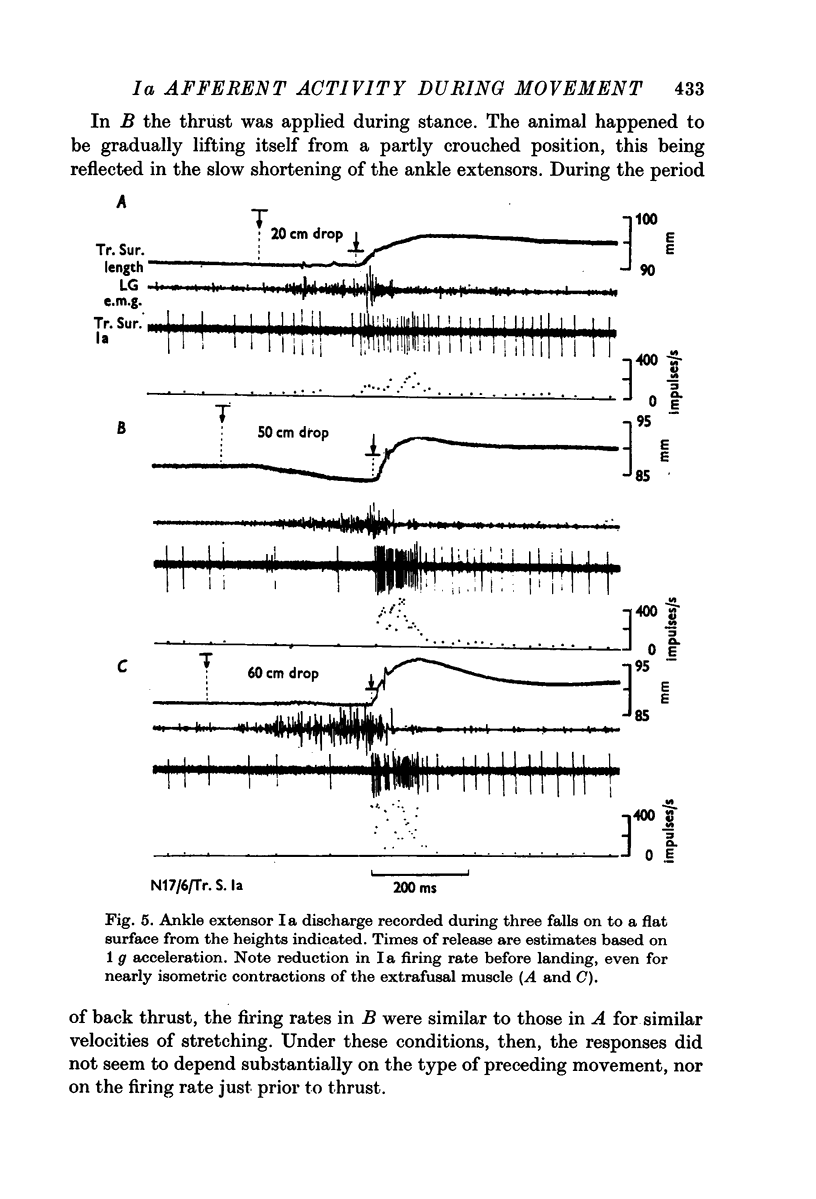
6. Ankle extensor Ia discharge decreased during falls, despite an increase in extensor e.m.g. This is seen as a clear example of independent α and γ control.
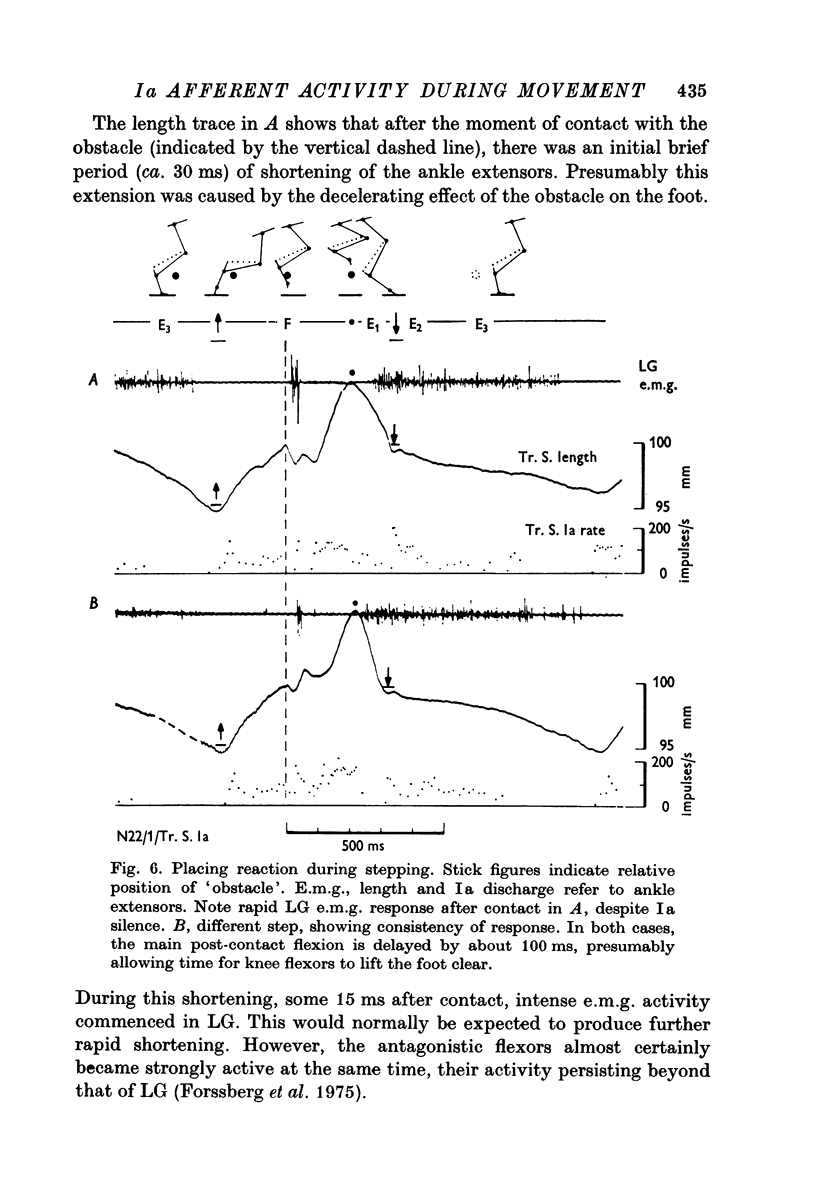
7. Placing reactions during walking were consistent with the notion that cutaneous inputs dominate over proprioceptive inputs in these movements.
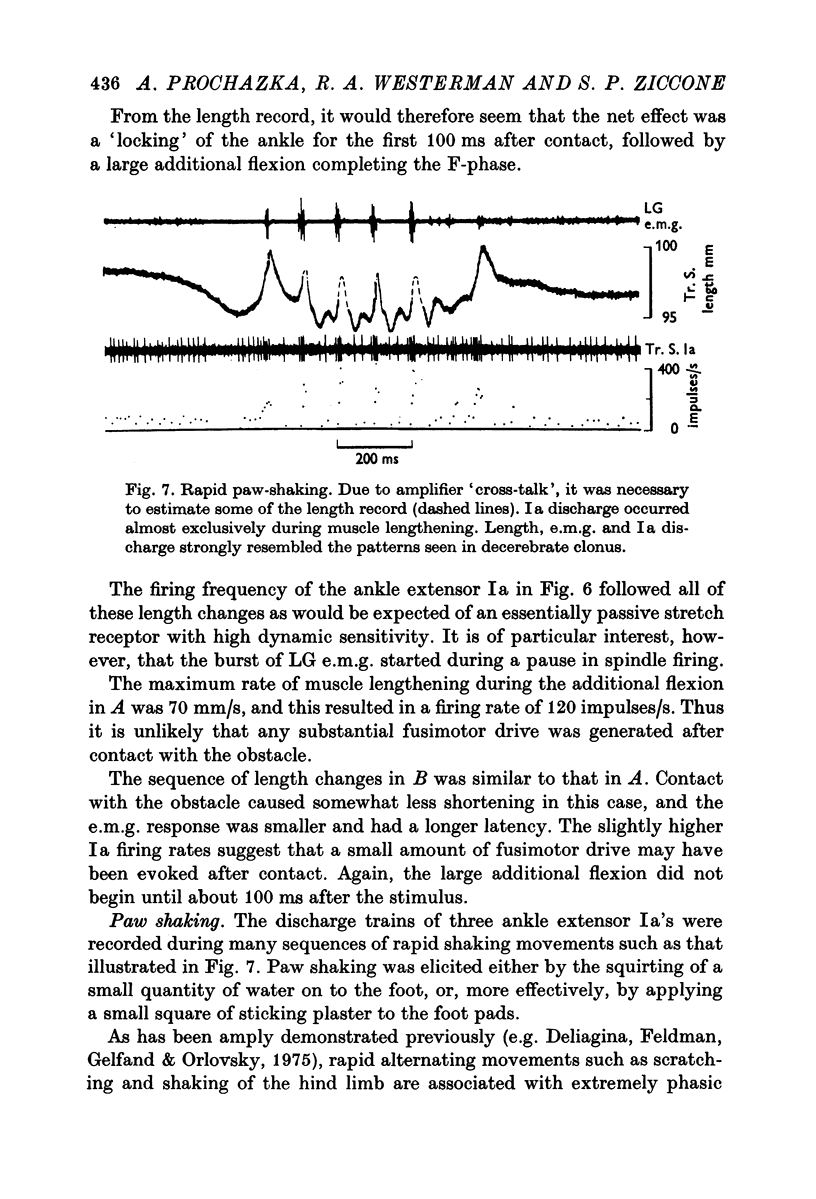
8. α and Ia discharge during paw-shaking showed many of the characteristics of that in decerebrate and spastic clonus. The present results suggest that movements resembling clonus may be part of the animal's normal repertoire.
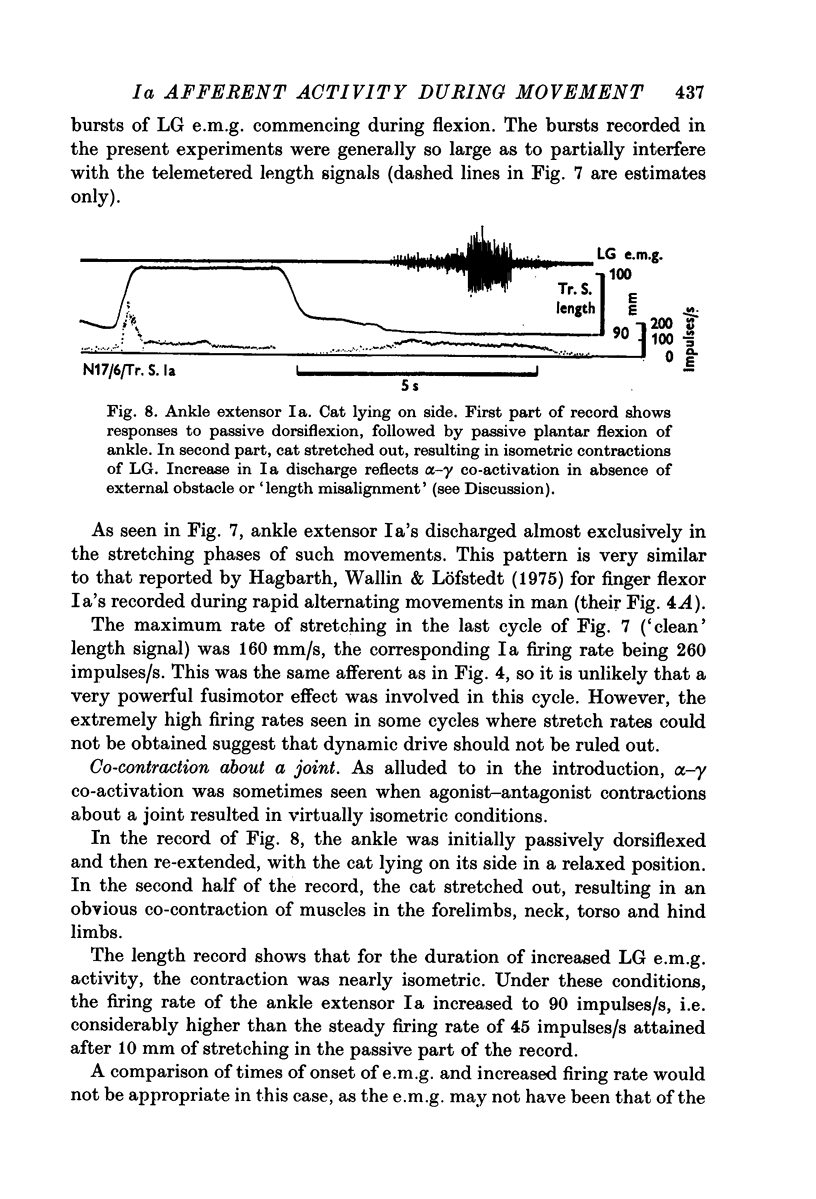
9. Isometric co-contraction of agonists and antagonists was found to involve α-γ co-activation.
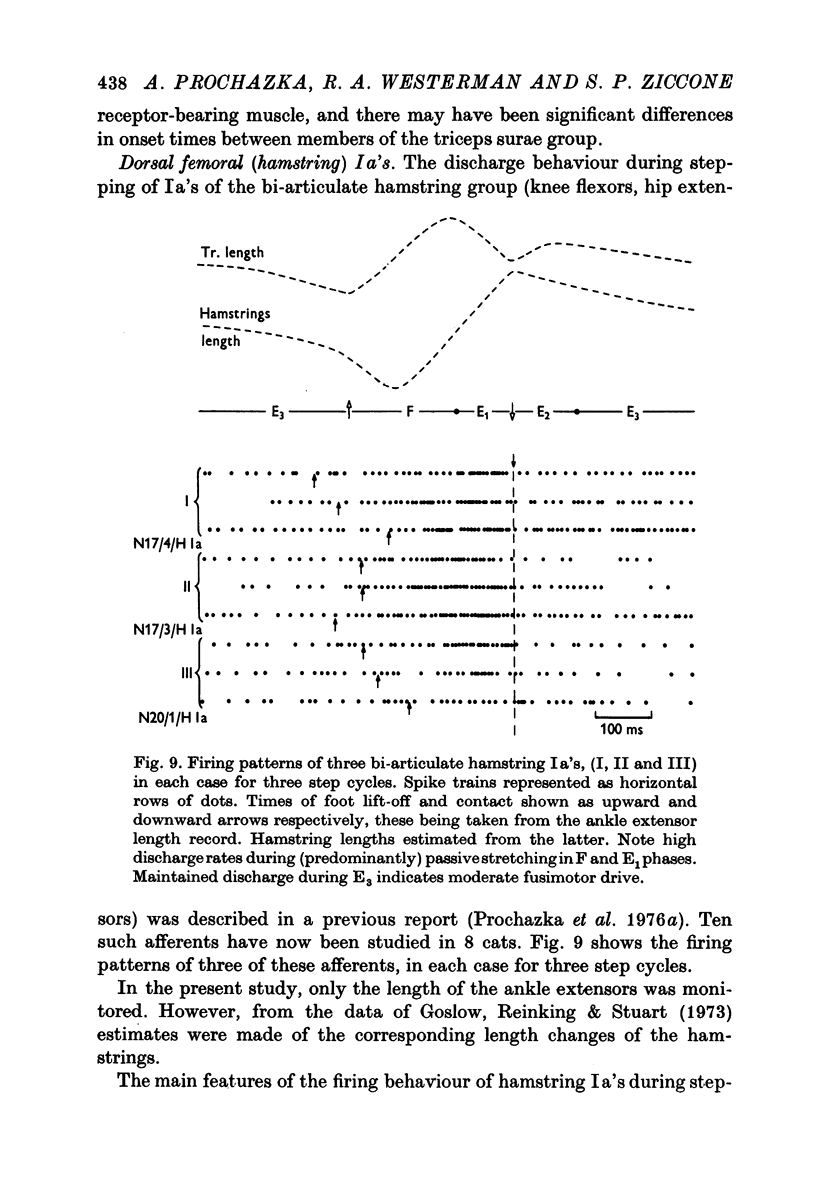
10. Hamstring Ia discharge behaviour during stepping further highlighted the increases in firing rate which normally occur during passive muscle stretching in `pre-programmed' movements.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki M., McIntyre A. K. Cortical and long spinal actions on lumbosacral motoneurones in the cat. J Physiol. 1975 Oct;251(3):569–587. doi: 10.1113/jphysiol.1975.sp011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Laporte Y., Pagès B. Frequencygrams of spindle primary endings elicited by stimulation of static and dynamic fusimotor fibres. J Physiol. 1968 May;196(1):47–63. doi: 10.1113/jphysiol.1968.sp008493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Pagés B. Cinematographic analysis of contractile events produced in intrafusal muscle fibres by stimulation of static and dynamic fusimotor axons. J Physiol. 1975 Nov;252(2):397–427. doi: 10.1113/jphysiol.1975.sp011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D., Kroin J. S., Moore G. P., Stauffer E. K., Stuart D. G. Correlation analysis of muscle spindle responses to single motor unit contractions. J Physiol. 1976 May;257(2):325–336. doi: 10.1113/jphysiol.1976.sp011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Goodwin G. M., Matthews P. B. After-effects of fusimotor stimulation on the response of muscle spindle primary afferent endings. J Physiol. 1969 Dec;205(3):677–694. doi: 10.1113/jphysiol.1969.sp008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. THE EFFECTS OF STIMULATION OF STATIC AND DYNAMIC FUSIMOTOR FIBRES ON THE RESPONSE TO STRETCHING OF THE PRIMARY ENDINGS OF MUSCLE SPINDLES. J Physiol. 1964 Oct;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody F. W., Harrison L. M., Taylor A. Analysis of activity of muscle spindles of the jaw-closing muscles during normal movements in the cat. J Physiol. 1975 Dec;253(2):565–582. doi: 10.1113/jphysiol.1975.sp011207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. N., Sears T. A. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol. 1970 Aug;209(3):711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagnina T. G., Feldman A. G., Gelfand I. M., Orlovsky G. N. On the role of central program and afferent inflow in the control of scratching movements in the cat. Brain Res. 1975 Dec 19;100(2):297–313. doi: 10.1016/0006-8993(75)90484-9. [DOI] [PubMed] [Google Scholar]
- Duysens J., Pearson K. G. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res. 1976 Jan 26;24:245–255. doi: 10.1007/BF00235013. [DOI] [PubMed] [Google Scholar]
- ELDRED E., GRANIT R., MERTON P. A. Supraspinal control of the muscle spindles and its significance. J Physiol. 1953 Dec 29;122(3):498–523. doi: 10.1113/jphysiol.1953.sp005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H., Grillner S., Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975 Feb 21;85(1):103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- Fromm C., Noth J. Reflex responses of gamma motoneurones to vibration of the muscle they innervate. J Physiol. 1976 Mar;256(1):117–136. doi: 10.1113/jphysiol.1976.sp011315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERNANDT B. E., GILMAN S. Descending vestibular activity and its modulation by proprioceptive, cerebellar, and reticular influences. Exp Neurol. 1959 Aug;1:274–304. doi: 10.1016/0014-4886(59)90005-6. [DOI] [PubMed] [Google Scholar]
- GRANIT R., HOLMGREN B., MERTON P. A. The two routes for excitation of muscle and their subservience to the cerebellum. J Physiol. 1955 Oct 28;130(1):213–224. doi: 10.1113/jphysiol.1955.sp005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. M., Hulliger M., Matthews P. B. The effects of fusimotor stimulation during small amplitude stretching on the frequency-response of the primary ending of the mammalian muscle spindle. J Physiol. 1975 Dec;253(1):175–206. doi: 10.1113/jphysiol.1975.sp011186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. M., Luschei E. S. Discharge of spindle afferents from jaw-closing muscles during chewing in alert monkeys. J Neurophysiol. 1975 May;38(3):560–571. doi: 10.1152/jn.1975.38.3.560. [DOI] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Reinking R. M., Stuart D. G. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973 Sep;141(1):1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Stauffer E. K., Nemeth W. C., Stuart D. G. Digit flexor muscles in the cat: their action and motor units. J Morphol. 1972 Jul;137(3):335–342. doi: 10.1002/jmor.1051370305. [DOI] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Stauffer E. K., Nemeth W. C., Stuart D. G. The cat step cycle; responses of muscle spindles and tendon organs to passive stretch within the locomotor range. Brain Res. 1973 Sep 28;60(1):35–54. doi: 10.1016/0006-8993(73)90849-4. [DOI] [PubMed] [Google Scholar]
- Granit R. The functional role of the muscle spindles--facts and hypotheses. Brain. 1975 Dec;98(4):531–556. doi: 10.1093/brain/98.4.531. [DOI] [PubMed] [Google Scholar]
- Greenwood R., Hopkins A. Muscle responses during sudden falls in man. J Physiol. 1976 Jan;254(2):507–518. doi: 10.1113/jphysiol.1976.sp011242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S., Hongo T., Lund S. Descending monosynaptic and reflex control of gamma-motoneurones. Acta Physiol Scand. 1969 Apr;75(4):592–613. doi: 10.1111/j.1748-1716.1969.tb04414.x. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Wallen G., Löfstedt L. Muscle spindle activity in man during voluntary fast alternating movements. J Neurol Neurosurg Psychiatry. 1975 Jul;38(7):625–635. doi: 10.1136/jnnp.38.7.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk J. C., Singer J. J., Henneman E. Adequate stimulus for tendon organs with observations on mechanics of ankle joint. J Neurophysiol. 1971 Nov;34(6):1051–1065. doi: 10.1152/jn.1971.34.6.1051. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. J Physiol. 1971 Jul;215(3):637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. M., Watt D. G. Muscular control of landing from unexpected falls in man. J Physiol. 1971 Dec;219(3):729–737. doi: 10.1113/jphysiol.1971.sp009685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. M., Watt D. G. Observations on the control of stepping and hopping movements in man. J Physiol. 1971 Dec;219(3):709–727. doi: 10.1113/jphysiol.1971.sp009684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerstrand G., Thoden U. Muscle spindle responses to concomitant variations in lenght and in fusimotor activation. Acta Physiol Scand. 1968 Sep-Oct;74(1):153–165. doi: 10.1111/j.1748-1716.1968.tb04224.x. [DOI] [PubMed] [Google Scholar]
- Lewis D. M., Proske U. The effect of muscle length and rate of fusimotor stimulation on the frequency of discharge in primary endings from muscle spindles in the cat. J Physiol. 1972 May;222(3):511–535. doi: 10.1113/jphysiol.1972.sp009812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERTON P. A. The silent period in a muscle of the human hand. J Physiol. 1951 Jun;114(1-2):183–198. doi: 10.1113/jphysiol.1951.sp004610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T. R., Houk J. C. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol. 1976 Jan;39(1):119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Perret C., Berthoz A. Evidence of static and dynamic fusimotor actions on the spindle response to sinusoidal stretch during locomotor activity in the cat. Exp Brain Res. 1973 Sep 29;18(2):178–188. doi: 10.1007/BF00234722. [DOI] [PubMed] [Google Scholar]
- Phillips C. G. The Ferrier lecture, 1968. Motor apparatus of the baboon's hand. Proc R Soc Lond B Biol Sci. 1969 May 20;173(1031):141–174. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol. 1976 Sep;39(5):1090–1104. doi: 10.1152/jn.1976.39.5.1090. [DOI] [PubMed] [Google Scholar]
- Proske U., Lewis D. M. The effects of muscle stretch and vibration on fusimotor activity in the lightly anaesthetised cat. Brain Res. 1972 Nov 13;46:55–69. doi: 10.1016/0006-8993(72)90005-4. [DOI] [PubMed] [Google Scholar]
- Sjöström A., Zangger P. ALPHA-GAMMA-Linkage in the spinal generator for locomotion in the cat. Acta Physiol Scand. 1975 May;94(1):130–132. doi: 10.1111/j.1748-1716.1975.tb05869.x. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Reinking R. M., Stuart D. G. Tendon organs of cat medial gastrocnemius: responses to active and passive forces as a function of muscle length. J Neurophysiol. 1975 Sep;38(5):1217–1231. doi: 10.1152/jn.1975.38.5.1217. [DOI] [PubMed] [Google Scholar]
- Szumski A. J., Burg D., Struppler A., Velho F. Activity of muscle spindles during muscle twitch and clonus in normal and spastic human subjects. Electroencephalogr Clin Neurophysiol. 1974 Dec;37(6):589–597. doi: 10.1016/0013-4694(74)90072-8. [DOI] [PubMed] [Google Scholar]
- Taylor A., Cody F. W. Jaw muscle spindle activity in the cat during normal movements of eating and drinking. Brain Res. 1974 May 17;71(2-3):523–530. doi: 10.1016/0006-8993(74)90996-2. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B. Human muscle spindle discharge during isometric voluntary contractions. Amplitude relations between spindle frequency and torque. Acta Physiol Scand. 1974 Feb;90(2):319–336. doi: 10.1111/j.1748-1716.1974.tb05594.x. [DOI] [PubMed] [Google Scholar]
- Watt D. G. Responses of cats to sudden falls: an otolith-originating reflex assisting landing. J Neurophysiol. 1976 Mar;39(2):257–265. doi: 10.1152/jn.1976.39.2.257. [DOI] [PubMed] [Google Scholar]
- Wetzel M. C., Gerlach R. L., Stern L. Z., Hannapel L. K. Behavior and histochemistry of functionally isolated cat ankle extensors. Exp Neurol. 1973 Mar-Apr;39(2):223–233. doi: 10.1016/0014-4886(73)90225-2. [DOI] [PubMed] [Google Scholar]
- Wetzel M. C., Stuart D. G. Ensemble characteristics of cat locomotion and its neural control. Prog Neurobiol. 1976;7(1):1–98. doi: 10.1016/0301-0082(76)90002-2. [DOI] [PubMed] [Google Scholar]



