Abstract
Small colony variants (SCVs) of Staphylococcus aureus are slow-growing subpopulations that cause persistent and relapsing infections. The altered phenotype of SCV can arise from defects in menadione or hemin biosynthesis, which disrupt the electron transport chain and decrease ATP concentrations. With SCVs, virulence is altered by a decrease in exotoxin production and susceptibility to various antibiotics, allowing their intracellular survival. The expression of bacterial adhesins by SCVs is poorly documented. We tested fibrinogen- and fibronectin-mediated adhesion of a hemB mutant of S. aureus 8325-4 that is defective for hemin biosynthesis and exhibits a complete SCV phenotype. In this strain, adhesion to fibrinogen and fibronectin was significantly higher than that of its isogenic, normally growing parent and correlated with the increased surface display of these adhesins as assessed by flow cytometry. Real-time quantitative reverse transcription-PCR demonstrated increased expression of clfA and fnb genes by the hemB mutant compared to its isogenic parent. The influence of the hemB mutation on altered adhesin expression was confirmed by showing complete restoration of the wild-type adhesive phenotype in the hemB mutant, either by complementing with intact hemB or by supplementing the growth medium with hemin. Increased surface display of fibrinogen and fibronectin adhesins by the hemB mutation occurred independently from agr, a major regulatory locus of virulence factors in S. aureus. Both agr-positive and agr-lacking hemB mutants were also more efficiently internalized by human embryonic kidney cells than were their isogenic controls, presumably because of increased surface display of their fibronectin adhesins.
During persistent or relapsing staphylococcal infections, a bacterial subpopulation may emerge that can persist for months or even years in a dormant metabolic state (44). These bacteria with decreased metabolic activity are called small colony variants (SCVs) to reflect a phenotypic consequence of their low rate of growth and the formation of minute colonies on solid media. The physiological basis of the SCV phenotype in Staphylococcus aureus (46), Staphylococcus epidermidis, and other coagulase-negative staphylococcal species has been studied (2, 67). These SCVs frequently were auxotrophic for menadione or hemin, molecules that are required precursors of the components of a complete electron transport chain. Menadione is isoprenylated to form menaquinone, the first electron acceptor in the electron transport chain. The cytochromes that receive electrons from menaquinone and form the chain of higher-redox-potential complexes require a heme prosthetic group. Defects in either menadione or heme biosynthesis that disrupt the functional activity of the electron transport chain lead to an overall decrease in intracellular ATP (3, 44). This metabolic change has pleiotropic effects, such as altering pigmentation, carbohydrate utilization profiles, and exotoxin expression. In addition, SCVs display increased resistance to some cell wall-active antibiotics and decreased uptake of aminoglycoside antibiotics (45). These differences in susceptibility are presumably due to the decreased capacity for cell wall and teichoic acid biosynthesis and reduction in antibiotic uptake due to decreased membrane potential. One consequence of the observed SCV phenotype is the ability of SCVs to survive within endothelial cells (64) and potentially other cell lines. Intracellular survival of SCVs, perhaps the source of recurrent infections, may be a result of decreased alpha-toxin production of SCVs, which, at wild-type levels, normally lyses eukaryotic cells (57).
Recent studies brought molecular support to this physiological model by showing that a knockout hemB mutant, defective for hemin biosynthesis, developed a complete SCV phenotype (66). This was confirmed by restoring the SCV hemB mutant into a fully normal phenotype by complementation with an intact hemB gene or by growing the auxotrophic SCV mutant in a hemin-supplemented broth medium (66).
As far as we know, the impact of the SCV phenotype on S. aureus adhesion to extracellular or host cell components, an important step in the initiation of bacterial infection, has not been described. S. aureus and other bacterial species are known to express on their surface protein adhesins (8-11) collectively known as microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) (39). These molecules specifically interact with some major plasma or extracellular matrix protein components of normal tissues or those coating biomedical devices (58). The most important host proteins interacting with S. aureus are fibronectin (59-61), fibrinogen (18, 20, 32), collagen (39, 41), vitronectin (27), laminin (20, 29), thrombospondin (19), bone sialoprotein (70), elastin (69), and von Willebrand factor (17). Cloning and sequencing of the genes coding for fibronectin-binding proteins FnBPA (53) and FnBPB (23), fibrinogen-binding proteins ClfA (32) and ClfB (35), collagen- and elastin-binding proteins (38, 40, 42), and von Willebrand factor (16) of S. aureus have been reported. The two adjacent genes coding for FnBPA and FnBPB are partly redundant since both must be inactivated to eliminate S. aureus adhesion to fibronectin (13). The functional significance of each category of S. aureus adhesins was explored in vitro and in vivo by using specific mutants. The mutant strains either expressed defective attachment to their respective host proteins or restored wild-type phenotypes by complementation (12-14, 32, 41, 62).
The regulatory circuits governing the expression of MSCRAMMs remain to be fully defined. Available data suggest that transcription of fnbA and fnbB genes is downregulated by the activation of the agr locus (47). The agr locus encodes an autoinducing, quorum-sensing, signal transduction circuit that becomes active in the postexponential phase of growth in laboratory cultures (22, 28, 31, 37). The appearance of one agr transcript, RNAIII, correlates with downregulation of fnb, as well as the spa gene encoding protein A (25, 47, 50). However, other MSCRAMM genes like clfA appear to be independent of the effect of RNAIII (68). Other regulatory loci, sar and/or sar homologues (sarH1, sarR, sarT, and rot), also play a role in the regulation of MSCRAMMs (30, 34, 52, 56). For example, it is known that SarA, the major regulatory protein encoded within the sar locus, can activate fnb and clfA genes while repressing the transcription of spa (5-7, 68).
The aims of this study were to (i) characterize alterations in quantitative adhesion to fibrinogen- and fibronectin-coated surfaces of isogenic hemB mutants expressing SCV phenotypes (66) compared to their normally growing parents, (ii) quantify the number of fibrinogen- or fibronectin-binding sites expressed by hemin mutants and their parents, (iii) evaluate the potential impact of the global regulator agr activity on the regulation and functional expression of fibrinogen or fibronectin adhesins in wild-type and agr-defective mutants of S. aureus 8325-4, (iv) evaluate by real-time quantitative reverse transcription (RT)-PCR the expression of clfA and fnb genes by hemB mutants, and (v) correlate the altered expression of fibronectin-binding proteins by hemB mutants expressing SCV phenotypes with their endocytosis by human embryonic kidney cells.
MATERIALS AND METHODS
Bacterial strains.
Strain 8325-4G is a spontaneous agr RNAIII-defective nonhemolytic mutant of strain 8325-4. The defect in RNAIII production of strain 8325-4G was assessed by Northern blot analysis and confirmed by real-time RT-PCR (TaqMan 7770, Perkin-Elmer Applied Biosystems, Foster City, Calif.). The origin of the molecular defect in RNAIII production of strain 8325-4G is unknown, but we could exclude any mutation in either the sarA locus (nucleotides 37 to 1317) or agr P2-P3 interregion and RNAIII coding region (nucleotides 1480 to 1781) whose DNA sequences were found to be identical to those of strain 8325-4.
Construction of hemB-deficient mutants.
hemB mutants of S. aureus strains 8325-4G, 6850, Cowan I, and COL were constructed by allelic replacement with an ermB cassette-inactivated hemB gene, as previously described with wild-type strain 8325-4 and its hemB mutant strain I10 (66) (Table 1). In brief, the PCR-amplified hemB gene from S. aureus 8325-4 genomic DNA was cloned into pUC19, yielding plasmid pCE3 propagated in Escherichia coli DH5α. A 1.4-kb ermB cassette was inserted into the hemB gene and cloned into plasmid pBT9, a shuttle vector conferring chloramphenicol resistance with a temperature-sensitive replicon for staphylococci, yielding plasmid pCE8. After propagation in the restriction-negative strain SA113 of S. aureus, pCE8 was transferred to the different strains of S. aureus by electroporation and selection of transformants with chloramphenicol (10 μg/ml); the transformation efficiency was 104 transformants/μg of plasmid DNA. Disruption of the intact hemB gene in the recipient strains was produced by homologous recombination as described previously (66), using growth at a nonpermissive temperature and selection for erythromycin resistance. Successful chromosomal insertion of the ermB cassette was indicated by the appearance of chloramphenicol-sensitive and erythromycin-resistant SCV colonies. The successful recombination was verified by PCR; the PCR products of all hemB mutants were 1.4 kb larger than the products of their respective isogenic parents.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype, phenotype, and/or property | Source or reference |
|---|---|---|
| Strains | ||
| S. aureus | ||
| 8325-4 | NCTC 8325 cured of prophages and plasmids, rsbU | www.genome.ou.edu |
| I10 | 8325-4 hemB::ermB, SCV | 66 |
| A2 | 8325-4 hemB::ermB, pCE12 | 66 |
| 8325-4G | 8325-4 defective in agr RNAIII production | This study |
| I15 | 8325-4G hemB::ermB, SCV | This study |
| K1 | 8325-4 hemB::ermB, pCE12 | This study |
| Cowan I | NCTC8530, septic arthritis isolate | ATCC 1298 |
| IIb41 | Cowan I, hemB::ermB, SCV | This study |
| 6850 | Clinical isolate | ATCC 53657 |
| IIb13 | 6850, hemB::ermB, SCV | This study |
| COL | MRSA strain | www.tigr.org |
| Ia48 | COL, hemB::ermB, SCV | This study |
| SA113 | 8325, restriction deficient | ATCC 35556 (21) |
| DU5883 | 8325-4, fnbA::tetM, fnbB::ermB | 13 |
| DU5880 | 8325-4, clfA::Tn917 | 13 |
| S. epidermidis KH11 | 43 | |
| Plasmids | ||
| pUC19 | Cloning vector | 71 |
| pCE3 | pUC19::hemB | 66 |
| pBT9 | E. coli-S. aureus shuttle vector | 72 |
| pCE8 | pBT9::hemB::ermB | 66 |
| pCE12 | pCX19::hemB | 66 |
Complementation of the hemB mutants.
The PCR-amplified hemB gene was cloned in Staphylococcus carnosus with the vector pCX19, which has a xylose-inducible promoter, yielding plasmid pCE12 (66). The hemB mutants of strains 8325-4 and 8325-4G were transformed with pCE12 as described previously (66). Transformation resulted in normal growth of each hemB mutant on both liquid and solid media.
Bacterial adhesion assay.
In vitro attachment of S. aureus to protein-coated surfaces was measured by the method of Bisognano et al. (4), using polymethylmethacrylate (PMMA) coverslips coated in vitro with purified fibrinogen (32) or fibronectin (13) as described elsewhere. To optimize adsorption of fibronectin from concentrations below 1 μg/ml, PMMA coverslips were precoated with gelatin (1 mg/ml) as described previously (62). Each native or gelatin-surfaced coverslip was coated in duplicate with increasing amounts of immobilized fibrinogen or fibronectin, respectively, by incubation for 60 min at 37°C with three different concentrations (0.25, 0.5, and 1 μg/ml) of either protein in phosphate-buffered saline (PBS), followed by rinsing in PBS as described previously (13, 14). This resulted in a linear coating of PMMA surfaces with fibrinogen and fibronectin ranging from 41 to 145 ng (28 to 101 ng/cm2) and 46 to 190 ng (31 to 128 ng/cm2) per coverslip, respectively.
The adhesion characteristics of parental strains and their hemB mutants were evaluated by incubating the protein-coated coverslips with 107 CFU of washed cultures of late-logarithmic-phase cells, metabolically radiolabeled with [3H]thymidine during growth without shaking for 5 h at 37°C in Mueller-Hinton broth (MHB) as previously described (4). In some experiments, hemB mutants were restored to normally growing cells by supplementing MHB with hemin (1 μg/ml) (66).
Precultures of hemB mutants complemented with intact hemB+ were prepared by an overnight incubation in MHB supplemented with chloramphenicol (5 μg/ml) to avoid loss of plasmid pCE12. Complemented strains were then incubated for 5 h in antibiotic-free MHB containing both [3H]thymidine as described above and 0.5% xylose to promote hemB+ expression. The presence of plasmid pCE12 in the 5-h-radiolabeled cultures was verified by CFU counts on chloramphenicol-containing Mueller-Hinton agar (MHA).
At the end of the attachment period, the fluids containing unbound bacteria were removed, the coverslips were rinsed, and radioactivity on the coverslips was determined as described previously (61). Albumin-coated and gelatin-coated PMMA coverslips were used as controls of nonspecific adhesion to fibrinogen- and fibronectin-coated surfaces, respectively.
Validation of comparative bacterial adhesion data.
To validate and normalize bacterial adhesion data of hemB mutants compared to their isogenic normally growing parents, whose biomass and cell-associated radioactivity may significantly differ from each other, the following control experiments were performed. First, both CFU and [3H]thymidine cell-associated counts of each SCV or normally growing culture were systematically determined. Average CFU counts of the hemB mutant strain I10 represented 38% of those of its isogenic parent strain 8325-4. Similar data were recorded with hemB mutants of strains 8325-4G, Cowan, 6850, and COL (data not shown). Complementation of all hemB mutants with an intact hemB gene led to CFU counts identical to those of their respective wild type parents (data not shown). Average [3H]thymidine cell-associated counts performed in parallel indicated that hemB mutants were optimally labeled. Average counts of the hemB mutant strain I10 (counts per minute) represented 82% of those of its isogenic parent. Similar data were recorded with hemB mutants of strains 8325-4G, Cowan, 6850, and COL (data not shown).
To normalize adhesion data of parental strains and their hemB mutants, the data were first scored as the percentage of attached radiolabeled bacteria and then normalized to a fixed inoculum of 107 CFU/ml, as previously described (4, 63). Each experiment was performed at least three times, and the results were expressed as means ± standard errors of the means (SEM). The significance of differences in bacterial adhesion of hemB mutants compared to their isogenic wild-type or hemB+-complemented parents was evaluated by paired t tests (49). Statistical significance was evaluated for each individual concentration of fibrinogen or fibronectin immobilized on PMMA coverslips, and data were considered significant when P was <0.05 by using two-tailed significance levels.
Quantification of fibrinogen-binding ClfA antigens and FnBPs by flow cytometry.
The number of ClfA-mediated fibrinogen-binding sites and FnBP-mediated fibronectin-binding sites displayed by hemB mutants of S. aureus compared to their isogenic wild-type or hemB-complemented parents was monitored by flow cytometry as previously described (15), except when indicated. The specificity of flow cytometry data for ClfA and FnBPs, respectively, was evaluated by running in parallel ClfA and FnBP knockout mutants of strain 8325-4, respectively.
Late-log-phase cells to be assayed for ClfA after growth (without shaking) for 5 h in MHB were fixed using 0.5% formaldehyde (vol/vol) in PBS (pH 7.2), rinsed in PBS. The bacteria were incubated first for 1 h with purified Fab fragments (1:100) of rabbit anti-ClfA immunoglobulin G antibodies, prepared from whole sera following manufacturer instruction (Immunopure Fab kit; Pierce) and then with (Fab′)2 fragments of goat anti-rabbit immunoglobulin G (1:100) conjugated with fluorescein isothiocyanate (FITC)(Chemicon, Temecula, Calif.). Samples were rinsed twice in FACS buffer (2.5% bovine serum albumin in PBS). The threshold settings for nonspecific fluorescence were obtained by using bacterial cells incubated without primary or secondary antibodies.
Comparative surface display of FnBPs by hemB mutants and parental strains was monitored as previously described (12) by incubating formaldehyde-fixed bacteria for 2 h at 4°C with FITC (2 μg/ml)-conjugated fibronectin or FITC-conjugated albumin used as control. Background fluorescence recorded with bacteria incubated with FITC-albumin was subtracted from fluorescence recorded in the presence of FITC-fibronectin (12). Each experiment was performed at least three times, and the results were expressed as means ± SEM. Differences in anti-ClfA or FITC-fibronectin binding by hemB mutants compared to their hemB+-complemented derivatives or respective parents were evaluated by paired t tests (49) and considered significant when P was <0.05 by using two-tailed significance levels.
Endocytic uptake assay by human embryonic kidney cells.
All media components were from Gibco-BRL. 293 cells (adenovirus type 5 DNA-transformed primary human embryonic kidney) were obtained from ATCC (CRL-1573); maintained in Dulbecco's modified Eagle medium (DMEM)-Nut mix F-12 (Glutamax I) supplemented with 10% fetal calf serum, penicillin (50 IU/ml) and streptomycin (50 μg/ml); and split twice weekly 1:5 by trypsinization. Cells were maintained in humidified air with 5% CO2 at 37°C. The lysostaphin protection assay was performed as previously described (54). Bacterial suspensions were washed with saline, suspended in PBS containing 0.5 mg of bovine serum albumin per ml, and adjusted to 107 CFU/ml using the optical density at 540 nm. MHA plates were also inoculated with serial dilutions of these suspensions to control the inoculum. 293 cells (0.2 × 106 to 0.3 × 106 cells per plate) were transferred to plates at 2 days before the assay and washed with DMEM devoid of antibiotics. Then, cell culture medium was replaced with 0.1 ml of bacterial suspensions, resulting in ∼1 × 106 CFU/well in invasion medium (DMEM-Nut mix F-12, 1% human serum albumin, 10 mM HEPES), yielding an estimated bacterium/cell ratio (multiplicity of infection) of ∼4:1. Cells were incubated for 0, 1, or 2 h at 37°C; bacterial suspensions were replaced by lysostaphin medium (DMEM-Nut mix F-12, 10% fetal calf serum, lysostaphin [20 μg/ml]) and further incubated for 30 min. Cells were subsequently lysed in 1 ml of sterile distilled H2O containing 0.2% Triton X-100. The presence of the detergent does not affect bacterial viability (not shown). Appropriate serial dilutions of the cell lysate in PBS were transferred to plates of MHA, and CFU were counted manually.
Each experiment was performed at least three times, and the results were expressed as means ± SEM. Differences in endocytic uptake of hemB mutants compared to their isogenic wild type or hemB+-complemented parents were evaluated by paired t tests (49) and considered significant when P was <0.05 by using two-tailed significance levels.
Real-time RT-PCR.
Total RNA was extracted from S. aureus strains by using the RNeasy mini Kit (Qiagen). RNA yields were analyzed spectroscopically and by 16S rRNA measurements. mRNA levels were determined using the Platinum quantitative RT-PCR kit (Gibco-BRL). PrimerExpress software (version 1.5; Applied Biosystems) was used to design primers and probes, which were used at 0.2 and 0.1 μM, respectively. All the primers, except the sarA probe, a minor groove binder (Applied Biosystems), and probes harboring a 5′FAM (6-carboxy-fluorescein) and a 3′TAMRA (6-carboxy-tetramethyl-rhodamine) were synthesized by Eurogentec (Seraing, Belgium) and are listed in Table 2. Fluorescence emission was detected by the sequence detector ABI Prism 7700 and analyzed with Sequence Detector software (version 1.7; Applied Biosystems). The mRNA levels of target genes extracted from hemB mutants and their isogenic parents were normalized on the basis of their 16S rRNA levels, which were assayed in each round of real-time RT-PCR as internal controls.
TABLE 2.
TaqMan primers and probes used in this study
| Gene | Forward primer | Reverse primer | Probe (5′→3′) |
|---|---|---|---|
| fnbB | 803F-CACCGAAAACTGTGCAAGCA | 889R-TTCCTGTAGTTTCCTTATCAGCAACTT | 830T-TAGAAACTTCGCGAGTTGATTTGCCATCGa |
| fnbA | 268F-ACAAGTTGAAGTGGCACAGCC | 341R-CCGCTACATCTGCTGATCTTGTC | 290T-AGAACGGCATCAGAAAGTAAGCCACGTGa |
| clfA | 1014F-ATGTGACAGTTGGTATTGACTCTGG | 1100R-TAGGCACTGAAAAACCATAATTCAGT | 1040T-CGACTGTGTATCCGCACCAAGCAGGb |
| RNAIII | 367F-TTCACTGTGTCGATAATCCA | 436R-TGATTTCAATGGCACAAGAT | 388T-TTTACTAAGTCACCGATTGTTGAAATGAa |
| agrA | 34F-CAAAGAGAAAACATGGTTACCATTATTAA | 135R-CTCAAGCACCTCATAAGGATTATCAG | 83T-AAAAGCCTATGGAAATTGCCCTCGCAa |
| 16S | 551F-GGCAAGCGTTATCCGGAATT | 651R-GTTTCCAATGACCCTCCACG | 573T-CCTACGCGCGCTTTACGCCCAa |
| sarA | 17F-ACATGGCAATTACAAAAATCAATGAT | 167R-TCTTTCTCTTTGTTTTCGCTGATG | 45T-CTTTGAGTTGTTATCAATGGTc |
5′FAM and 3′TAMRA labeled.
5′JOE (6-carboxy-4,5-dichloro-2,7-dimethoxyfluorescein) and 3′TAMRA labeled.
5′FAM Minor Groove Binder and dark quencher labeled.
RESULTS
Increased adhesion of the hemB mutant of S. aureus 8325-4 on fibrinogen- and fibronectin-coated surfaces.
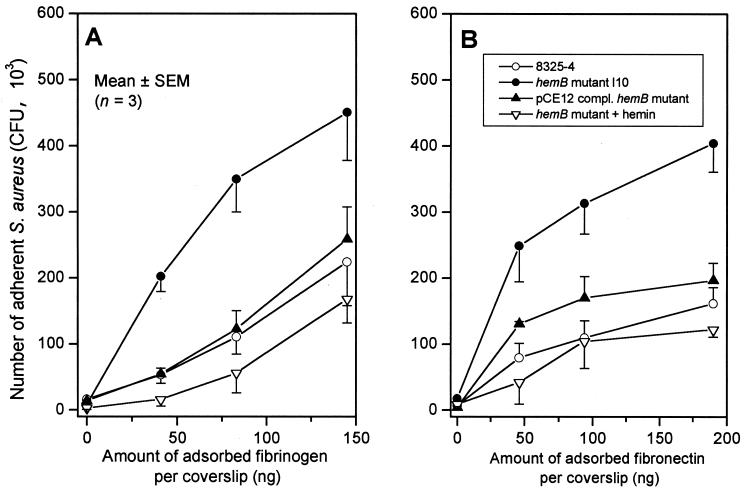
The bacterial attachment profiles of the parental strain 8325-4 to either fibrinogen- or fibronectin-coated coverslips compared to the hemB mutant strain I10, expressed as a function of the amount of adsorbed protein per coverslip, are shown in Fig. 1. The hemB mutant displaying an SCV phenotype exhibited a significantly higher attachment to each concentration of immobilized fibrinogen (Fig. 1A) or fibronectin (Fig. 1B), respectively, compared to its normally growing parental strain 8325-4. Average attachment increases of SCV strain I10 over the three coating concentrations of fibrinogen and fibronectin were 3.0- and 2.8-fold, respectively, compared to 8325-4.
FIG. 1.
Adhesion to fibrinogen-coated (A) or fibronectin-coated (B) coverslips of the parental strain (8325-4), its hemB mutant (strain I10) with (1 μg/ml) or without supplementation with hemin, and the pCE12-complemented mutant (strain A2) with 0.5% xylose in MHB.
Complementation of the hemB mutant I10 with plasmid pCE12 expressing the intact hemB gene (strain A2), or growth in hemin-supplemented medium, which both led to a normally growing phenotype, reduced bacterial attachment to immobilized fibrinogen or fibronectin to the parental levels (Fig. 1). These data confirmed that the increased attachment of the SCV derivative of 8325-4 to both fibrinogen- and fibronectin-coated surfaces was indeed linked to the mutation in hemB.
The hemB mutant displayed increased attachment in the absence of agr RNAIII production.
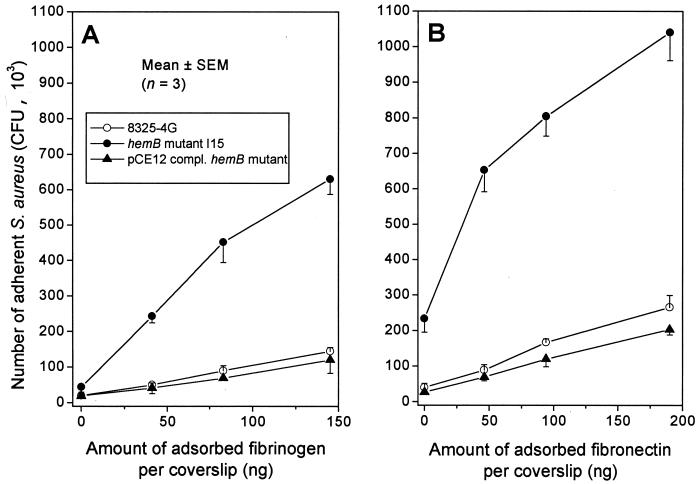
To exclude the possibility that the increased bacterial attachment of strain I10 over its parent 8325-4 might simply result from different growth-phase-dependent activities of the global regulator agr in the SCV hemB mutant compared to its parent, we tested the attachment properties of an isogenic pair of strain 8325-4G, a spontaneous RNAIII-defective mutant of strain 8325-4, and its hemB derivative mutant I15. Compared to its hemB+ parent 8325-4G, the RNAIII-defective hemB mutant I15 exhibited a significant increase in bacterial attachment, averaging 4.7- and 5.4-fold on the three concentrations of immobilized fibrinogen- (Fig. 2A) or fibronectin-coated coverslips (Fig. 2B), respectively. An unexpected finding was the higher attachment of strain I15 occurring on control coverslips coated with gelatin without any fibronectin (zero point). The higher attachment of strain I15 to gelatin cannot be related to any MSCRAMM thus far described, the 8325-4 family being devoid of the collagen adhesin cna gene (39), and may therefore reflect a nonspecific binding mechanism.
FIG. 2.
Adhesion to fibrinogen-coated (A) or fibronectin-coated (B) coverslips of the parental RNAIII-defective strain (8325-4G), its hemB mutant (strain I15), and the pCE12-complemented mutant (strain K1) with 0.5% xylose in MHB.
Complementation of strain I15 by hemB+ returned it to the parental levels of bacterial attachment to immobilized fibrinogen or fibronectin, as seen in the normally growing strain K1. Taken together, these data ruled out the possibility that upregulation of fibrinogen and fibronectin adhesins in hemB mutants displaying an SCV phenotype required the contribution of RNAIII, a major effector of the global regulator agr.
Quantification of fibrinogen-binding ClfA antigens and FnBPs by flow cytometry.
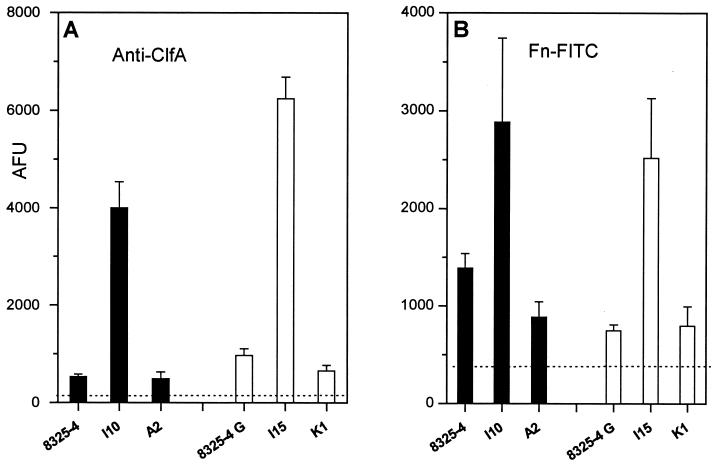
To confirm that increased bacterial adhesion resulted from increased surface display of fibronectin and fibrinogen adhesin molecules by the hemB mutants compared to their wild-type or hemB+-complemented parents, ClfA-mediated fibrinogen-binding sites and FnBP-mediated fibronectin-binding sites were monitored by flow cytometry (15). Figure 3 shows that the hemB mutant I10 bound 7.6-fold more anti-ClfA antibodies and 2.1-fold more FITC-labeled fibronectin than its parent 8325-4 (P < 0.01). In comparison, the hemB mutant I15 bound 6.5-fold more anti-ClfA antibodies and 3.4-fold more FITC-labeled fibronectin than its parent 8325-4G (P < 0.01). These differences were indeed linked to the defective hemB gene, as they were abolished in the hemB+-complemented strains A2 and K1. Overall, these flow cytometry data brought further evidence of an increased surface display of fibrinogen-binding ClfA and FnBPs adhesins by hemB mutants of both strains 8325-4 and 8325-4G, which also explained the higher SCV attachment to surface-bound fibrinogen and fibronectin.
FIG. 3.
Binding of soluble anti-ClfA antibodies (A) or FITC-labeled fibronectin (Fn-FITC) (B) by strain 8325-4 or its hemB (strain I10) or pCE12-complemented (strain A2) hemB mutants was compared to that of the RNAIII-defective strain 8325-4G and its respective hemB (strain I15) or pCE12-complemented (strain K1) hemB mutants. Dashed lines represent the baseline binding of anti-ClfA and Fn-FITC by clfA mutant DU5880 (A) and fnbA fnbB mutant DU5883 (B) strains, respectively. Results are means + SEM (error bars) of three experiments performed in duplicate and scored in arbitrary fluorescence units (AFU).
Increased uptake of SCV hemB mutants by human embryonic kidney cells.
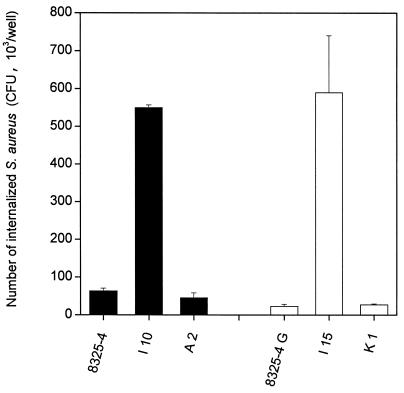
The hemB mutant strains I10 and I15 were internalized at an 8.7- and 25.6-fold-higher rate by HEK cells compared to their parents 8325-4 and 8325-4G, respectively, whose uptake was marginal, in a lysostaphin protection assay (Fig. 4). Since recent studies provided evidence that S. aureus invasion of 293 cells critically depends on fibronectin bridging between S. aureus FnBPs and the host receptor integrin α5β1, the present data strongly suggest that increased surface display of FnBPs by strains I10 and I15 is responsible for increased internalization of these SCV hemB mutants. Restoration of low levels of internalization observed in the hemB+-complemented strains A2 and K1 by HEK cells, compared to parental strains I10 and I15, respectively, is likely explained by their aforementioned decreased surface display of FnBPs (Fig. 4).
FIG. 4.
Increased uptake of SCV hemB mutants by human embryonic kidney cells, scored as the number of internalized (lysostaphin-protected) bacteria. Internalization of strain 8325-4 or its hemB (strain I10) or pCE12-complemented (strain A2) hemB mutants was compared to that of the RNAIII-defective strain 8325-4G and its respective hemB (strain I15) or pCE12-complemented (strain K1) hemB mutants. Results are presented as means + SEM (error bars)
Evaluation of steady-state mRNA of clfA, fnbA, and fnbB genes in hemB mutants and parental strains of S. aureus.
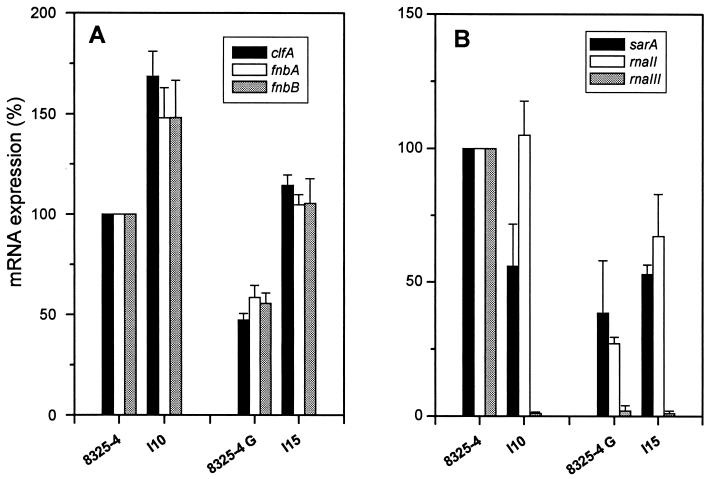
To explore the mechanisms leading to increased expression of fibrinogen and fibronectin adhesins in SCV hemB mutants of 8325-4 and 8325-4G compared to their respective parents, their contents in steady-state mRNAs encoded by clfA, fnbA, and fnbB genes were assayed by real-time RT-PCR (TaqMan). To normalize the transcript levels of clfA, fnbA, and fnbB assayed in these differently growing strains, 16S rRNA of both hemB mutants and parents were assayed in parallel by real-time RT-PCR. Figure 5A shows significantly increased contents of clfA, fnbA, and fnbB mRNAs in the hemB mutant I10, averaging 169% ± 13%, 148% ± 15%, and 148% ± 18%, respectively (means ± SEM), of those assayed in the parent 8325-4.
FIG. 5.
mRNA expression of clfA, fnbA, and fnbB (A) or sarA, rnaII, and rnaIII (B) genes determined by real-time RT-PCR of 16S rRNA adjusted extracts. Data are presented as means + SEM (error bars) of three experiments performed in triplicate.
Despite the fact that the RNAIII-defective strain 8325-4G yielded contents of clfA, fnbA, and fnbB mRNAs which were reduced by approximately one-half compared to those of the agr+ parent 8325-4, the hemB mutant I15 also showed significantly increased levels in mRNAs for each of these adhesins (Fig. 5A). Namely, clfA, fnbA, and fnbB mRNAs assayed in strain I15 in the absence of any significant RNAIII production represented 241% ± 18%, 179% ± 15%, and 189% ± 34%, respectively (means ± SEM), of those assayed in strain 8325-4G.
To evaluate whether the different levels of clfA, fnbA, and fnbB mRNAs assayed in the 8325-4 and 8325-4G parental strains and their respective hemB derivatives were due to functional changes in their major global regulators sar and agr, we assayed in parallel the quantitative levels of mRNAs coding for SarA, RNAII, and RNAIII, respectively. Figure 5B shows that both hemB mutants I10 and I15 and the RNAIII-defective parent 8325-4G yielded similar decreased levels of sarA transcripts compared to the agr+ parent 8325-4. Thus, these data revealed that the levels of clfA, fnbA, and fnbB messages are not correlated with sarA transcript levels in these different strains.
More contrasted results were found when assaying RNA transcript levels originating from P2 compared to P3 agr promoters in normally growing versus hemB mutant strains (Fig. 5B). In strain 8325-4 and its hemB mutant I10, identical levels of AgrA-encoding mRNAs reflecting the P2 promoter activity were recorded. In contrast, RNAII levels in strain 8325-4G and the hemB mutant I15 represented only 27 and 67%, respectively, of the levels found in strain 8325-4. Thus, there was no consistent link between RNAII levels of the P2 agr promoter and the level of clfA, fnbA, and fnbB messages found in these different strains.
The most impressive differences were observed in the P3-derived RNAIII transcript levels of 8325-4 compared to the three other strains. While the sharp reduction in RNAIII transcript levels compared to the agr+ parent 8325-4 essentially confirmed that both strain 8325-4G and its hemB derivative I15 displayed stable RNAIII-defective phenotypes, the similar >99% decrease in RNAIII transcript in the agr+ hemB strain I10 compared to 8325-4 was unexpected. More-extensive studies are required to understand the molecular basis of RNAIII-defective production by the agr+ hemB mutant strain I10 compared to its isogenic parent 8325-4.
Taken together, neither differences in sarA nor agr RNAII and RNAIII transcript levels in normally growing parents and their hemB mutants could consistently explain the overexpression of clfA, fnbA, and fnbB adhesins by SCVs compared to normally growing organisms. In other words, increased expression of these adhesin genes by hemB mutants appear to be mediated by agr- and sar-independent pathways.
Impact of the hemB mutation on adhesin expression by other strains of S. aureus.
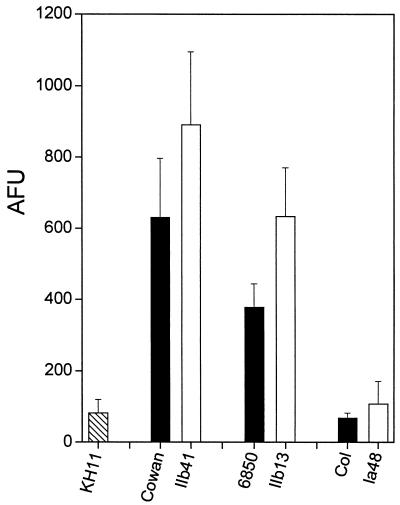
Figure 6 shows flow cytometry data of fibronectin binding by hemB mutant strains IIb41, IIb13, and Ia48, compared to their respective normally growing parents Cowan, 6850, and COL. SCV strains IIb41 and IIb13 displayed a significantly higher fibronectin binding of 65% ± 7% and 44% ± 10% (means ± SEM) compared to their isogenic parents Cowan and 6850, respectively. In contrast, the hemB mutant strain Ia48 of methicillin-resistant S. aureus (MRSA) strain COL exhibited no significant difference in fibronectin binding compared to its normally growing parent. Very low levels of fibronectin binding were exhibited by both strains Ia48 and COL, which were not significantly higher than those of S. epidermidis strain KH11, used as a negative control of FnBP display in our assay. The low adhesin content of strain COL and its hemB derivative may be due to a previously described mechanism of defective surface display occurring in some clinical and laboratory MRSA isolates (51, 63).
FIG. 6.
Binding of soluble FITC-labeled fibronectin (Fn-FITC) by strain Cowan and its hemB mutant IIb41, 6850 and its hemB mutant IIb13, and MRSA COL and its hemB mutant Ia48, compared to S. epidermidis strain KH11 taken as a negative control of FnBP display. Results are presented as means + SEM (error bars) of four experiments performed in duplicate and scored in arbitrary fluorescence units (AFU).
DISCUSSION
Several experimental and microbiological observations provided evidence that a majority of clinical SCV isolates of S. aureus are auxotrophic for either hemin or menadione (24, 65). Under natural conditions, any mutation affecting one of the multiple genes of the hem operon, namely, hemAXCDBL, hemN, and hemYHE (26), may potentially lead to similar hemin-auxotrophic SCV phenotypes. These considerations also apply to the menadione (3, 65) and thymidine synthetic pathways (24). Extensive genotypic and phenotypic studies of clinical SCVs are hardly feasible because of the difficulty to maintain pure cultures of this phenotype, which generally require addition of antibiotics to protect the slow-growing forms to be overgrown by a minority of normally growing parental organisms (24, 65). The knockout mutation in the hemB gene in S. aureus represents a very useful experimental system for obtaining a stable SCV phenotype that can be easily reversed by exogenous hemin supplementation or endogenous complementation with an intact hemB gene. In three additional unrelated strains, namely, Cowan, 6850, and COL, the inactivation of the hemB gene led to pleiotropic changes in bacterial physiology, which essentially mimicked all major features of clinically encountered SCV phenotypes (66). Our data showing increased surface display of both fibrinogen and fibronectin adhesins in hemB mutants of strains 8325-4, Cowan, and 6850 stand in contrast with the general notion that SCVs exhibit decreased expression of virulence factors compared to the normally growing parent population of S. aureus. While the decreased expression of cytolytic toxins by SCVs may contribute to their prolonged residence and survival in an intracellular milieu without harming the host cells (44), the increased expression of MSCRAMMs by S. aureus SCVs might be viewed as an important selective advantage for these slow-growing bacterial cells. In particular, the higher surface display of FnBPs involved in fibronectin-mediated bridging with the host receptor integrin α5β1 is known to promote endocytic uptake by some epithelial and endothelial cell lines (54, 55).
Comparison of slow-growing hemB mutants with their normally growing parents for quantitative expression of various virulence factors represents a difficult challenge, since major metabolic parameters, such as rates of DNA, RNA, protein, and cell wall synthesis, are significantly decreased in SCVs compared to their normally growing parents. To overcome these potential difficulties, we decided to compare surface display of fibrinogen and fibronectin adhesins by two different procedures, namely, the bacterial adhesion and flow cytometric assays. Both types of binding assays allowed normalization of adhesin expression by equivalent cell numbers of SCVs and wild-type parents and provided convergent evidence for increased surface display of fibrinogen and fibronectin adhesins by slow-growing compared to normally growing populations. While the increased expression of fibronectin adhesins was less important with SCV derivatives of S. aureus strains Cowan I and 6850 than with hemB mutants of strain 8325-4 and 8325-4G, at least a similar trend was observed with these genetically unrelated strains of S. aureus. The defective adhesin expression of MRSA strain COL and its hemB derivative Ia48 is compatible with previous observations showing a defective surface display of major S. aureus adhesins in either natural or genetically engineered MRSA strains (51, 63).
An additional difficulty in comparing the regulatory mechanisms of virulence factors of SCVs compared to their normally growing isogenic parents is the unknown influence of growth phase and environmental control on the activities of global regulators of S. aureus virulence in the slow-growing populations. In particular, the limiting ATP supply of hemB mutants might significantly alter the time course of activation of the global regulators agr and sar compared to those of normally growing populations (47). To avoid a potentially significant bias in comparing slow-growing with normally growing populations, we also compared the adhesin surface display of one hemB mutant with its isogenic RNAIII-defective parent 8325-4G. This spontaneous nonhemolytic mutant whose exact molecular defect has not yet been mapped was preferred to other well-characterized agr mutants that already carry erythromycin resistance determinants and also because strain 8325-4G exhibits an extensive defect in RNAIII production. The comparison between strains 8325-4G and I15 provided evidence that the increased display of fibrinogen and fibronectin adhesins could also occur in a hemB mutant constructed in RNAIII-defective background and thus ruled out any significant involvement of the growth cycle-dependent global regulator agr in the upregulation of these adhesins. Additional support for agr- and sar-independent mechanisms leading to increased adhesin gene expression and surface display was provided by real-time RT-PCR comparisons, which failed to reveal any consistent link between steady-state transcript levels of each adhesin gene with those of their putative global regulators. While increased transcript levels of clfA and both fnb genes seem to contribute in part to increased surface display of these important adhesins, the potential contribution of further posttranscriptional and posttranslational regulatory mechanisms should also be considered. This contribution is likely when comparing the mRNA transcript levels with surface expression of ClfA and FnBP adhesins in the hemB agr mutant strain I15 and wild-type 8325-4. While adhesion and flow cytometry data showed a markedly enhanced surface expression of ClfA and FnBPs in strain I15 compared to 8325-4, their respective levels of clfA, fnbA, and fnbB mRNA were equivalent. These apparently conflicting data might be explained by a different processing of surface expressed adhesins, as a global consequence of their widely different agr activities. A number of studies (see references 1 and 36 for reviews) demonstrated that upregulation of RNAIII promotes the production of serine proteases and their secretion into the extracellular medium. Recent studies documented the contribution of extracellular serine proteases in the transition of an adhesive to a nonadhesive phenotype through rapid degradation of FnBPs and potentially other cell surface proteins (1, 33, 48).
More-elaborate functional genomic approaches evaluating global transcriptomic and proteomic alterations of SCVs compared to their normally growing parents should contribute to identifying the most important regulatory circuits involved in the metabolic adjustments of the slow-growing bacterial populations. The identification and subsequent understanding of the role of these regulatory pathways in vitro and in vivo might be helpful for the development of new drug categories targeting the persistent slow-growing populations of S. aureus.
Acknowledgments
This work was supported by grants 3200-063710.00 (to P.V.), 632-57950.99 (to J.S.), and 31-55344.98 (to D.L.) from the Swiss National Foundation; American Heart Association Beginning Grant-in-Aid 9960328Z to P.J.M.; NIH grant AI42072 (to R.A.P.); and Innovative Medical Research, Germany, grant EI 119912 (to C.V.E.).
We thank M. Bento, S. Weber, and S. Hubrich for excellent technical assistance.
Editor: V. J. DiRita
REFERENCES
- 1.Arvidson, S. 2000. Extracellular enzymes, p. 379-391. In V. A. Fischetti, R. P. Novick, J. J. Ferreti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- 2.Baddour, L. M., W. A. Simpson, J. J. Weems, M. M. Hill, Jr., and G. D. Christensen. 1988. Phenotypic selection of small-colony variant forms of Staphylococcus epidermidis in the rat model of endocarditis. J. Infect. Dis. 157:757-763. [DOI] [PubMed] [Google Scholar]
- 3.Balwit, J. M., P. Van Langevelde, J. M. Vann, and R. A. Proctor. 1994. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 170:1033-1037. [DOI] [PubMed] [Google Scholar]
- 4.Bisognano, C., P. E. Vaudaux, D. P. Lew, E. Y. W. Ng, and D. C. Hooper. 1997. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob. Agents Chemother. 41:906-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung, A. L., K. Eberhardt, and J. H. Heinrichs. 1997. Regulation of protein A synthesis by the sar and agr loci of Staphylococcus aureus. Infect. Immun. 65:2243-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung, A. L., K. J. Eberhardt, E. Chung, M. R. Yeaman, P. M. Sullam, M. Ramos, and A. S. Bayer. 1994. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J. Clin. Investig. 94:1815-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien, Y., A. C. Manna, S. J. Projan, and A. L. Cheung. 1999. SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J. Biol. Chem. 274:37169-37176. [DOI] [PubMed] [Google Scholar]
- 8.Foster, T. J., O. Hartford, and D. O'Connel. 1997. Host-pathogen protein-protein interactions in Staphylococcus, p. 67-94. In M. A. McCrae, J. R. Saunders, C. J. Smyth, and N. D. Stow (ed.), Molecular aspects of host-pathogen interaction. Cambridge University Press, Cambridge, United Kingdom.
- 9.Foster, T. J., and M. Höök. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 10.Foster, T. J., and M. Höök. 2000. Molecular basis of adherence of Staphylococcus aureus to biomaterials, p. 27-39. In F. A. Waldvogel and A. L. Bisno (ed.), Infections associated with indwelling medical devices. ASM Press, Washington, D.C.
- 11.Foster, T. J., and D. McDevitt. 1994. Surface-associated proteins of Staphylococcus aureus: their possible roles in virulence. FEMS Microbiol. Lett. 118:199-206. [DOI] [PubMed] [Google Scholar]
- 12.Francois, P., J. Schrenzel, C. Stoerman-Chopard, H. Favre, M. Herrmann, T. J. Foster, D. P. Lew, and P. Vaudaux. 2000. Identification of plasma proteins adsorbed on hemodialysis tubing that promote Staphylococcus aureus adhesion. J. Lab. Clin. Med. 135:32-42. [DOI] [PubMed] [Google Scholar]
- 13.Greene, C., D. McDevitt, P. François, P. Vaudaux, D. P. Lew, and T. J. Foster. 1995. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin binding proteins and studies on the expression of fnb genes. Mol. Microbiol. 17:1143-1152. [DOI] [PubMed] [Google Scholar]
- 14.Greene, C., P. E. Vaudaux, P. François, R. A. Proctor, D. McDevitt, and T. J. Foster. 1996. A low-fibronectin-binding mutant of Staphylococcus aureus 879R4S has Tn918 inserted into its single fnb gene. Microbiology 142:2153-2160. [DOI] [PubMed] [Google Scholar]
- 15.Hartford, O., P. Francois, P. Vaudaux, and T. J. Foster. 1997. The dipeptide repeat region of the fibrinogen-binding protein (clumping factor) is required for functional expression of the fibrinogen-binding domain on the Staphylococcus aureus cell surface. Mol. Microbiol. 25:1065-1076. [DOI] [PubMed] [Google Scholar]
- 16.Hartleib, J., N. Köhler, R. B. Dickinson, G. S. Chhatwal, J. J. Sixma, O. M. Hartford, T. J. Foster, G. Peters, B. E. Kehrel, and M. Herrmann. 2000. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood 96:2149-2156. [PubMed] [Google Scholar]
- 17.Herrmann, M., J. Hartleib, B. Kehrel, R. R. Montgomery, J. J. Sixma, and G. Peters. 1997. Interaction of von Willebrand factor with Staphylococcus aureus. J. Infect. Dis. 176:984-991. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann, M., Q. J. Lai, R. M. Albrecht, D. F. Mosher, and R. A. Proctor. 1993. Adhesion of Staphylococcus aureus to surface-bound platelets: role of fibrinogen/fibrin and platelet integrins. J. Infect. Dis. 167:312-322. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann, M., S. J. Suchard, L. A. Boxer, F. A. Waldvogel, and D. P. Lew. 1991. Thrombospondin binds to Staphylococcus aureus and promotes staphylococcal adherence to surfaces. Infect. Immun. 59:279-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann, M., P. Vaudaux, D. Pittet, R. Auckenthaler, D. P. Lew, F. Schumacher-Perdreau, G. Peters, and F. A. Waldvogel. 1988. Fibronectin, fibrinogen and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 158:693-701. [DOI] [PubMed] [Google Scholar]
- 21.Iordanescu, S., and M. Surdeanu. 1976. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J. Gen. Microbiol. 96:277-281. [DOI] [PubMed] [Google Scholar]
- 22.Ji, G. Y., R. C. Beavis, and R. P. Novick. 2059. 1995. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. USA 92:12055-12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonsson, K., C. Signäs, H. P. Muller, and M. Lindberg. 1991. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur. J. Biochem. 202:1041-1048. [DOI] [PubMed] [Google Scholar]
- 24.Kahl, B., M. Herrmann, A. S. Everding, H. G. Koch, K. Becker, E. Harms, R. A. Proctor, and G. Peters. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023-1029. [DOI] [PubMed] [Google Scholar]
- 25.Kornblum, J., B. N. Kreiswirth, S. J. Projan, H. Ross, and R. P. Novick. 1990. Agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus, p. 373-402. In R. P. Novick (ed.), Molecular biology of the staphylococci. VCH Publishers, New York, N.Y.
- 26.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Z. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Q. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, et al. 2001. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 27.Liang, O. D., M. Maccarana, J.-I. Flock, M. Paulsson, K. T. Preissner, and T. Wadström. 1993. Multiple interactions between human vitronectin and Staphylococcus aureus. Biochim. Biophys. Acta 1225:57-63. [DOI] [PubMed] [Google Scholar]
- 28.Lina, G., S. Jarraud, G. Y. Ji, T. Greenland, A. Pedraza, J. Etienne, R. P. Novick, and F. Vandenesch. 1998. Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol. Microbiol. 28:655-662. [DOI] [PubMed] [Google Scholar]
- 29.Lopez, J. D., M. Dos Reis, and R. R. Bretani. 1985. Presence of laminin receptors in Staphylococcus aureus. Science 229:275-277. [DOI] [PubMed] [Google Scholar]
- 30.Manna, A., and A. L. Cheung. 2001. Characterization of sarR, a modulator of sar expression in Staphylococcus aureus. Infect. Immun. 69:885-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayville, P., G. Y. Ji, R. Beavis, H. M. Yang, M. Goger, R. P. Novick, and T. W. Muir. 1999. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc. Natl. Acad. Sci. USA 96:1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDevitt, D., P. François, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 33.McGavin, M. J., C. Zahradka, K. Rice, and J. E. Scott. 1997. Modification of the Staphylococcus aureus fibronectin binding phenotype by V8 protease. Infect. Immun. 65:2621-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNamara, P. J., K. C. Milligan-Monroe, S. Khalili, and R. A. Proctor. 2000. Identification, cloning, and initial characterization of rot, a locus encoding a regulator of virulence factor expression in Staphylococcus aureus. J. Bacteriol. 182:3197-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni, E. D., S. Perkins, P. Francois, P. Vaudaux, M. Hook, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 36.Novick, R. P. 2000. Pathogenicity factors and their regulation, p. 393-407. In V. A. Fischetti, R. P. Novick, J. J. Ferreti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- 37.Novick, R. P., S. J. Projan, J. Kornblum, H. F. Ross, G. Ji, B. Kreiswirth, F. Vandenesch, and S. Moghazeh. 1995. The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol. Gen. Genet. 248:446-458. [DOI] [PubMed] [Google Scholar]
- 38.Park, P. W., J. Rosenbloom, W. R. Abrams, and R. P. Mecham. 1996. Molecular cloning and expression of the gene for elastin-binding protein (ebpS) in Staphylococcus aureus. J. Biol. Chem. 271:15803-15809. [DOI] [PubMed] [Google Scholar]
- 39.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Höök. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 40.Patti, J. M., J. O. Boles, and M. Höök. 1993. Identification and biochemical characterization of the ligand binding domain of the collagen adhesin from Staphylococcus aureus. Biochemistry 32:11428-11435. [DOI] [PubMed] [Google Scholar]
- 41.Patti, J. M., T. Bremell, D. Krajewska-Pietrasik, A. Abdelnour, A. Tarkowski, C. Rydén, and M. Höök. 1994. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect. Immun. 62:152-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patti, J. M., H. Jonsson, B. Guss, L. M. Switalski, K. Wiberg, M. Lindberg, and M. Höök. 1992. Molecular characterization and expression of a gene encoding Staphylococcus aureus collagen adhesin. J. Biol. Chem. 267:4766-4772. [PubMed] [Google Scholar]
- 43.Peters, G., R. Locci, and G. Pulverer. 1982. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J. Infect. Dis. 146:479-482. [DOI] [PubMed] [Google Scholar]
- 44.Proctor, R. A. 2000. Microbial pathogenic factors: small-colony variants, p. 41-54. In F. A. Waldvogel and A. L. Bisno (ed.), Infections associated with medical devices. ASM Press, Washington, D.C.
- 45.Proctor, R. A., B. Kahl, C. Von Eiff, P. E. Vaudaux, D. P. Lew, and G. Peters. 1998. Staphylococcal small colony variants have novel mechanisms for antibiotic resistance. Clin. Infect. Dis. 27(Suppl. 1):S68-S74 [DOI] [PubMed] [Google Scholar]
- 46.Proctor, R. A., P. Van Langevelde, M. Kristjansson, J. N. Maslow, and R. D. Arbeit. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 20:95-102. [DOI] [PubMed] [Google Scholar]
- 47.Projan, S. J., and R. P. Novick. 1997. The molecular basis of pathogenicity, p. 55-81. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill Livingstone, New York, N.Y.
- 48.Rice, K., M. Huesca, D. Vaz, and M. J. McGavin. 2001. Variance in fibronectin binding and fnb locus polymorphisms in Staphylococcus aureus: identification of antigenic variation in a fibronectin binding protein adhesin of the epidemic CMRSA-1 strain of methicillin-resistant S. aureus. Infect. Immun. 69:3791-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosner, B. 1990. Analysis of variance, p. 474-526. In M. R. Payne, S. Hankinson, and S. London (ed.), Fundamentals of biostatistics. PWS-KENT Publishing Company, Belmont, Calif.
- 50.Saravia-Otten, P., H. P. Müller, and S. Arvidson. 1997. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J. Bacteriol. 179:5259-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savolainen, K., L. Paulin, B. Westerlund-Wikström, T. J. Foster, T. K. Korhonen, and P. Kuusela. 2001. Expression of pls, a gene closely associated with the mecA gene of methicillin-resistant Staphylococcus aureus, prevents bacterial adhesion in vitro. Infect. Immun. 69:3013-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt, K. A., A. C. Manna, S. Gill, and A. L. Cheung. 2001. SarT, a repressor of α-hemolysin in Staphylococcus aureus. Infect. Immun. 69:4749-4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Signäs, C., K. Raucci, K. Jönsson, P.-E. Lindgren, G. M. Anantharamaiah, M. Höök, and M. Lindberg. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc. Natl. Acad. Sci. USA 86:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinha, B., P. Francois, Y. A. Que, M. Hussain, C. Heilmann, P. Moreillon, D. Lew, K. H. Krause, G. Peters, and M. Herrmann. 2000. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect. Immun. 68:6871-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha, B., P. P. Francois, O. Nüsse, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1:101-118. [DOI] [PubMed] [Google Scholar]
- 56.Tegmark, K., A. Karlsson, and S. Arvidson. 2000. Identification and characterization of SarH1, a new global regulator of virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 37:398-409. [DOI] [PubMed] [Google Scholar]
- 57.Vann, J. M., and R. A. Proctor. 1988. Cytotoxic effects of ingested Staphylococcus aureus on bovine endothelial cells: role of S. aureus alpha-hemolysin. Microb. Pathog. 4:443-453. [DOI] [PubMed] [Google Scholar]
- 58.Vaudaux, P., P. Francois, D. P. Lew, and F. A. Waldvogel. 2000. Host factors predisposing to and influencing therapy of foreign body infections, p. 1-26. In F. A. Waldvogel and A. L. Bisno (ed.), Infections associated with indwelling medical devices. ASM Press, Washington, D.C.
- 59.Vaudaux, P., D. Pittet, A. Haeberli, P. G. Lerch, J. J. Morgenthaler, R. A. Proctor, F. A. Waldvogel, and D. P. Lew. 1993. Fibronectin is more active than fibrin or fibrinogen in promoting Staphylococcus aureus adherence to inserted intravascular catheters. J. Infect. Dis. 167:633-641. [DOI] [PubMed] [Google Scholar]
- 60.Vaudaux, P., R. Suzuki, F. A. Waldvogel, J. J. Morgenthaler, and U. E. Nydegger. 1984. Foreign body infection: role of fibronectin as a ligand for the adherence of Staphylococcus aureus. J. Infect. Dis. 150:546-553. [DOI] [PubMed] [Google Scholar]
- 61.Vaudaux, P., F. A. Waldvogel, J. J. Morgenthaler, and U. E. Nydegger. 1984. Adsorption of fibronectin onto polymethylmethacrylate and promotion of Staphylococcus aureus adherence. Infect. Immun. 45:768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaudaux, P. E., P. François, R. A. Proctor, D. McDevitt, T. J. Foster, R. M. Albrecht, D. P. Lew, H. Wabers, and S. L. Cooper. 1995. Use of adhesion-defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting bacterial adhesion to canine arteriovenous shunts. Infect. Immun. 63:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaudaux, P. E., V. Monzillo, P. Francois, D. P. Lew, T. J. Foster, and B. Berger-Bächi. 1998. Introduction of the mec element (methicillin resistance) into Staphylococcus aureus alters in vitro functional activities of fibrinogen and fibronectin adhesins. Antimicrob. Agents Chemother. 42:564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vesga, O., M. C. Groeschel, M. F. Otten, D. W. Brar, J. M. Vann, and R. A. Proctor. 1996. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J. Infect. Dis. 173:739-742. [DOI] [PubMed] [Google Scholar]
- 65.Von Eiff, C., D. Bettin, R. A. Proctor, B. Rolauffs, N. Lindner, W. Winkelmann, and G. Peters. 1997. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin. Infect. Dis. 25:1250-1251. [DOI] [PubMed] [Google Scholar]
- 66.Von Eiff, C., C. Heilmann, R. A. Proctor, C. Woltz, G. Peters, and F. Götz. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179:4706-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Von Eiff, C., P. Vaudaux, B. C. Kahl, D. Lew, S. Emler, A. Schmidt, G. Peters, and R. A. Proctor. 1999. Bloodstream infections caused by small-colony variants of coagulase-negative staphylococci following pacemaker implantation. Clin. Infect. Dis. 29:932-934. [DOI] [PubMed] [Google Scholar]
- 68.Wolz, C., P. Pöhlmann-Dietze, A. Steinhuber, Y. T. Chien, A. Manna, W. Van Wamel, and A. Cheung. 2000. Agr-independent regulation of fibronectin-binding protein(s) by the regulatory locus sar in Staphylococcus aureus. Mol. Microbiol. 36:230-243. [DOI] [PubMed] [Google Scholar]
- 69.Woo Park, P., D. D. Roberts, L. E. Grosso, W. C. Parks, J. Rosenbloom, W. R. Abrams, and R. P. Mecham. 1991. Binding of elastin to Staphylococcus aureus. J. Biol. Chem. 266:23399-23406. [PubMed] [Google Scholar]
- 70.Yacoub, A., P. Lindahl, K. Rubin, M. Wendel, D. Heinegård, and C. Rydén. 1994. Purification of a bone sialoprotein-binding protein from Staphylococcus aureus. Eur. J. Biochem. 222:919-925. [DOI] [PubMed] [Google Scholar]
- 71.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 72.Youngman, P., H. Poth, B. Green, K. York, G. Olmedo, and K. Smith. 1989. Methods for genetic manipulation, cloning and functional analysis of sporulation genes in Bacillus subtilis, p. 65-87. In I. Smith, R. A. Slepecky, and P. Setlow (ed.), Regulation of procaryotic development. American Society for Microbiology, Washington, D.C.