Abstract
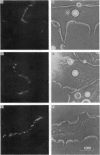
1. Theree-day-old cultures of myotomal muscle, obtained from embryos of Xenopus laevis, were stained with fluorescent conjugates of alpha-bungarotoxin and maintained in native toxin in order to ensure that ACh receptors subsequently inserted into the sarcolemma would not be stained. Neural tube cells were then added to the cultures. 2. When cultures were exmained 1-3 days later fluorescent stain was found to be associated with sites of nerve-muscle contact. In some cases the stain along the path of contact extended for greater distances than the patches of stain seen on non-contacted muscle cells. 3. The development of new areas of fluorescent stain at sites of nerve-muscle contact was confirmed by making successive observations on the same muscle cells over a period of a day. 4. Similar experiments on muscle cells not contacted by nerve revealed the formation of new receptor patches, usually in areas of cell growth. 5. The majority of fluorescent pathes on non-contacted muscle cells did not undergo changes in size or shape over the course of 1-2 days. However some examples of enlargement, shrinkage and disappearance were observed. 6. On the basis of these findings it is concluded that ACh receptors aggregate within the sarcolemma, spontaneously as well as in response to innervation. In the latter case extrajunctional receptors accumulate at the site of nerve contact thereby contributing to the development of high receptor density in the subneural muscle membrane. This process of receptors redistribution occurs in the absence of synaptic or contractile activity. 7. Possible mechanisms involved in the redistribution of ACh receptors are discussed in relation to those which appear to modulate ligand-induced changes in the distribution of lectin and immunoglobulin receptors.
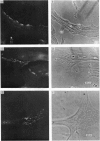
Full text
PDF
























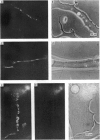
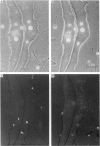
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Cohen M. W. Fluorescent staining of acetylcholine receptors in vertebrate skeletal muscle. J Physiol. 1974 Mar;237(2):385–400. doi: 10.1113/jphysiol.1974.sp010487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. J., Cohen M. W., Zorychta E. Effects of innervation on the distribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):731–756. doi: 10.1113/jphysiol.1977.sp011879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G., Taylor R. S. The formation of synapses in reinnervated and cross-reinnervated adult avian muscle. J Physiol. 1973 Apr;230(2):331–357. doi: 10.1113/jphysiol.1973.sp010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. K., Hall Z. W. Fate of alpha-bungarotoxin bound to acetylcholine receptors of normal and denervated muscle. Science. 1974 Apr 26;184(4135):473–475. doi: 10.1126/science.184.4135.473. [DOI] [PubMed] [Google Scholar]
- Berg D. K., Hall Z. W. Increased extrajunctional acetylcholine sensitivity produced by chronic acetylcholine sensitivity produced by chronic post-synaptic neuromuscular blockade. J Physiol. 1975 Jan;244(3):659–676. doi: 10.1113/jphysiol.1975.sp010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. K., Hall Z. W. Loss of alpha-bungarotoxin from junctional and extrajunctional acetylcholine receptors in rat diaphragm muscle in vivo and in organ culture. J Physiol. 1975 Nov;252(3):771–789. doi: 10.1113/jphysiol.1975.sp011169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray J. J., Harris A. J. Dissociation between nerve-muscle transmission and nerve trophic effects on rat diaphragm using type D botulinum toxin. J Physiol. 1975 Dec;253(1):53–77. doi: 10.1113/jphysiol.1975.sp011179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. P., Hall Z. W. Synthesis of acetylcholine receptor by denervated rat diaphragm muscle. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1368–1372. doi: 10.1073/pnas.72.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Chuang S. T., Huang M. C. Effects of chronic treatment with various neuromuscular blocking agents on the number and distribution of acetylcholine receptors in the rat diaphragm. J Physiol. 1975 Aug;250(1):161–173. doi: 10.1113/jphysiol.1975.sp011047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Huang M. C. Turnover of junctional and extrajunctional acetylcholine receptors of the rat diaphragm. Nature. 1975 Feb 20;253(5493):643–644. doi: 10.1038/253643a0. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Synthesis of acetylcholine receptors by cultured chick myotubes and denervated mouse extensor digitorum longus muscles. Proc Natl Acad Sci U S A. 1976 Jan;73(1):161–164. doi: 10.1073/pnas.73.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B., Witzke F. Trophic regulation of acetylcholine sensitivity of muscle: effect of electrical stimulation. Science. 1972 May 5;176(4034):514–516. doi: 10.1126/science.176.4034.514. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Fambrough D. Fluidity of the surface of cultured muscle fibers. Rapid lateral diffusion of marked surface antigens. J Cell Biol. 1973 Apr;57(1):27–37. doi: 10.1083/jcb.57.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Fex S., Sonesson B., Thesleff S., Zelená J. Nerve implants in botulinum poisoned mammalian muscle. J Physiol. 1966 Jun;184(4):872–882. doi: 10.1113/jphysiol.1966.sp007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Gautvik K., Sommerschild H. Cholinergic receptors at denervated mammalian motor end-plates. Acta Physiol Scand. 1975 Sep;95(1):66–76. doi: 10.1111/j.1748-1716.1975.tb10026.x. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Hogan P. G., Marshall J. M., Hall Z. W. Muscle activity decreases rate of degradation of alpha-bungarotoxin bound to extrajunctional acetylcholine receptors. Nature. 1976 May 27;261(5558):328–330. doi: 10.1038/261328a0. [DOI] [PubMed] [Google Scholar]
- Jones R., Vrbová G. Effect of muscle activity on denervation hypersensitivity. J Physiol. 1970 Sep;210(2):144P–145P. [PubMed] [Google Scholar]
- Jones R., Vrbová G. Two factors responsible for the development of denervation hypersensitivity. J Physiol. 1974 Feb;236(3):517–538. doi: 10.1113/jphysiol.1974.sp010450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE DEVELOPMENT OF ACETYLCHOLINE SENSITIVITY IN NERVE-FREE SEGMENTS OF SKELETAL MUSCLE. J Physiol. 1964 Mar;170:389–396. doi: 10.1113/jphysiol.1964.sp007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie P. A., Collier B., Tenehouse A. Comparison of alpha-bungarotoxin binding to skeletal muscles after inactivity or denervation. Nature. 1976 Mar 25;260(5549):349–350. doi: 10.1038/260349a0. [DOI] [PubMed] [Google Scholar]
- Letinsky M. S. Acetylcholine sensitivity changes in tadpole tail muscle fibers innervated by developing motor neurons. J Neurobiol. 1975 Nov;6(6):609–617. doi: 10.1002/neu.480060607. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974 Aug 2;185(4149):457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol. 1975 Nov;252(3):603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Cis- and trans-membrane control of cell surface topography. J Supramol Struct. 1973;1(4):410–416. doi: 10.1002/jss.400010419. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Painter R. G. Anionic sites of human erythrocyte membranes. II. Antispectrin-induced transmembrane aggregation of the binding sites for positively charged colloidal particles. J Cell Biol. 1973 Nov;59(2 Pt 1):395–406. doi: 10.1083/jcb.59.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. The interactions of lectins with animal cell surfaces. Int Rev Cytol. 1974;39:89–190. doi: 10.1016/s0074-7696(08)60939-0. [DOI] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B., Griffin J. W. Effect of muscle disuse on acetylcholine receptors. Nature. 1976 Mar 25;260(5549):352–353. doi: 10.1038/260352a0. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Purves D., Sakmann B. The effect of contractile activity on fibrillation and extrajunctional acetylcholine-sensitivity in rat muscle maintained in organ culture. J Physiol. 1974 Feb;237(1):157–182. doi: 10.1113/jphysiol.1974.sp010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Yahara I., Edelman G. M. Morphology, motility, and surface behavior of lymphocytes bound to nylon fibers. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1149–1153. doi: 10.1073/pnas.71.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Modulation of lymphocyte receptor mobility by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1579–1583. doi: 10.1073/pnas.72.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]