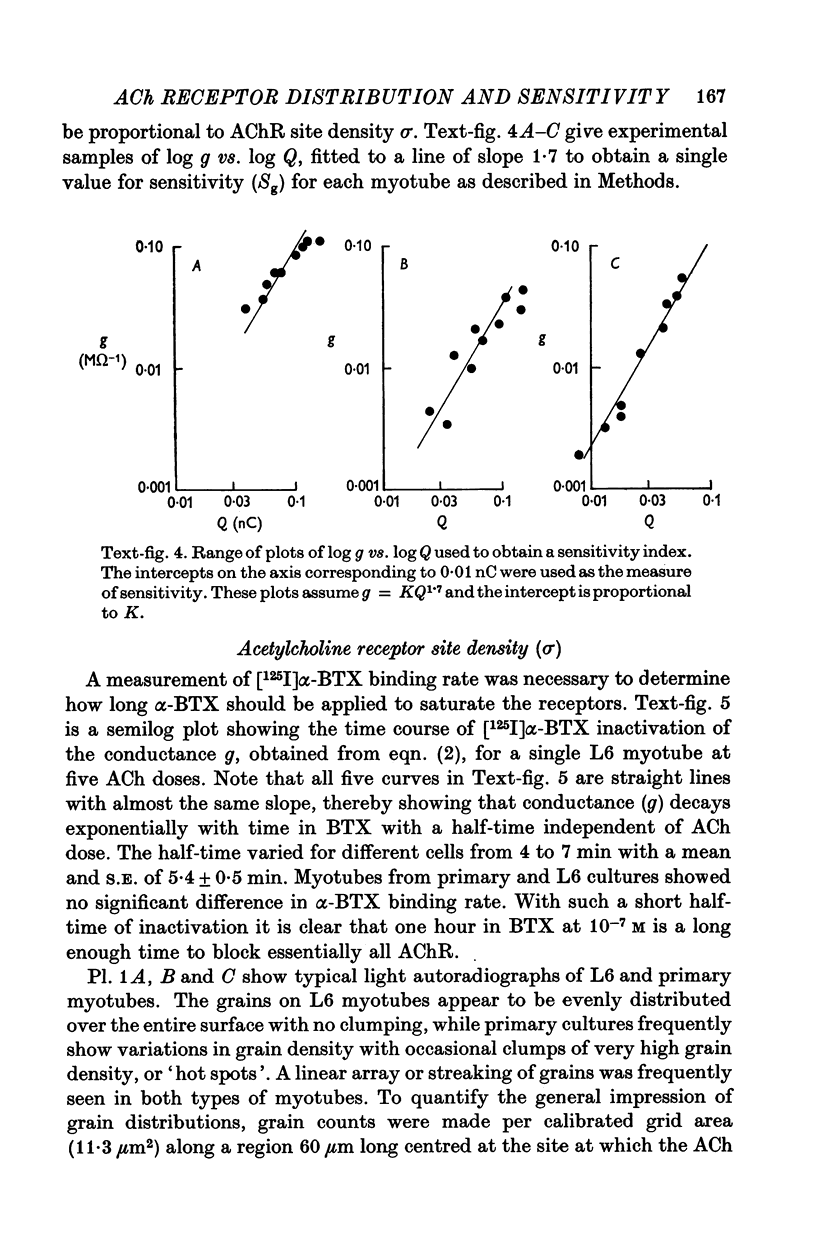
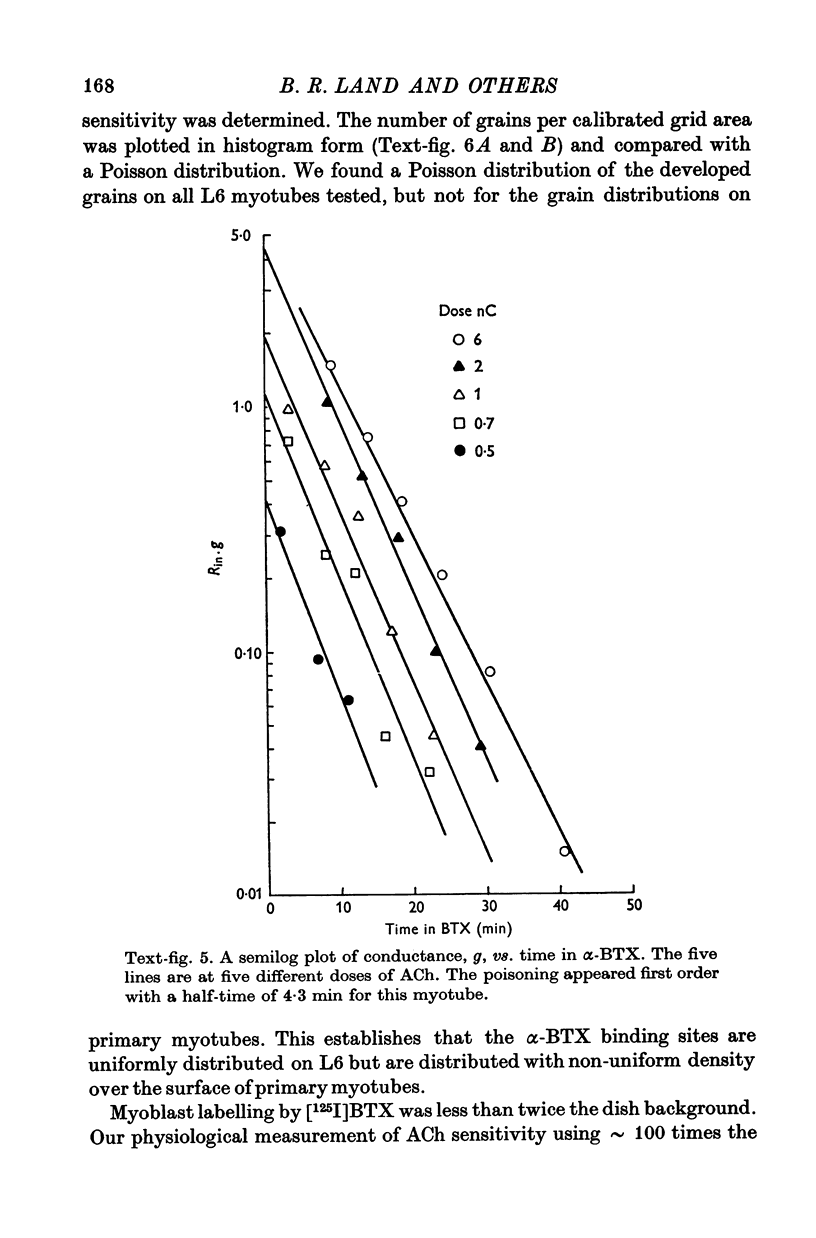
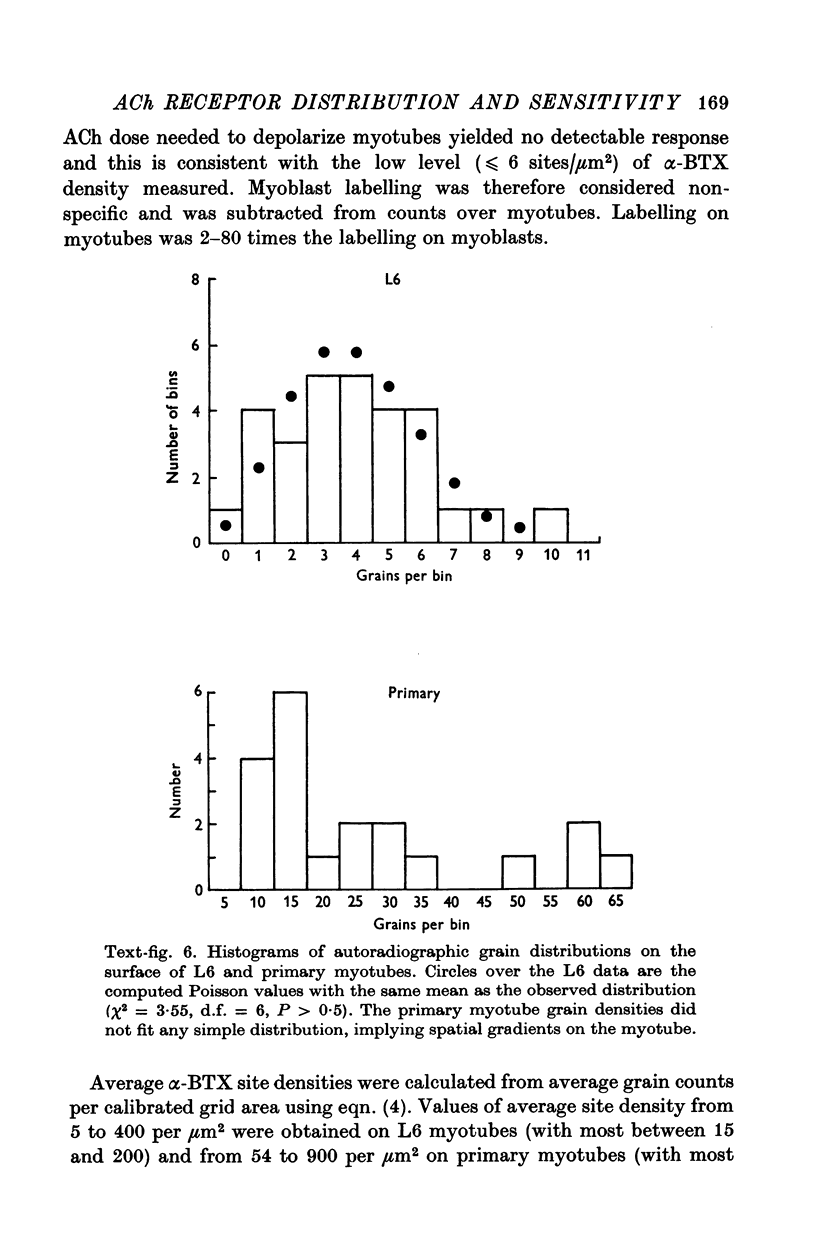
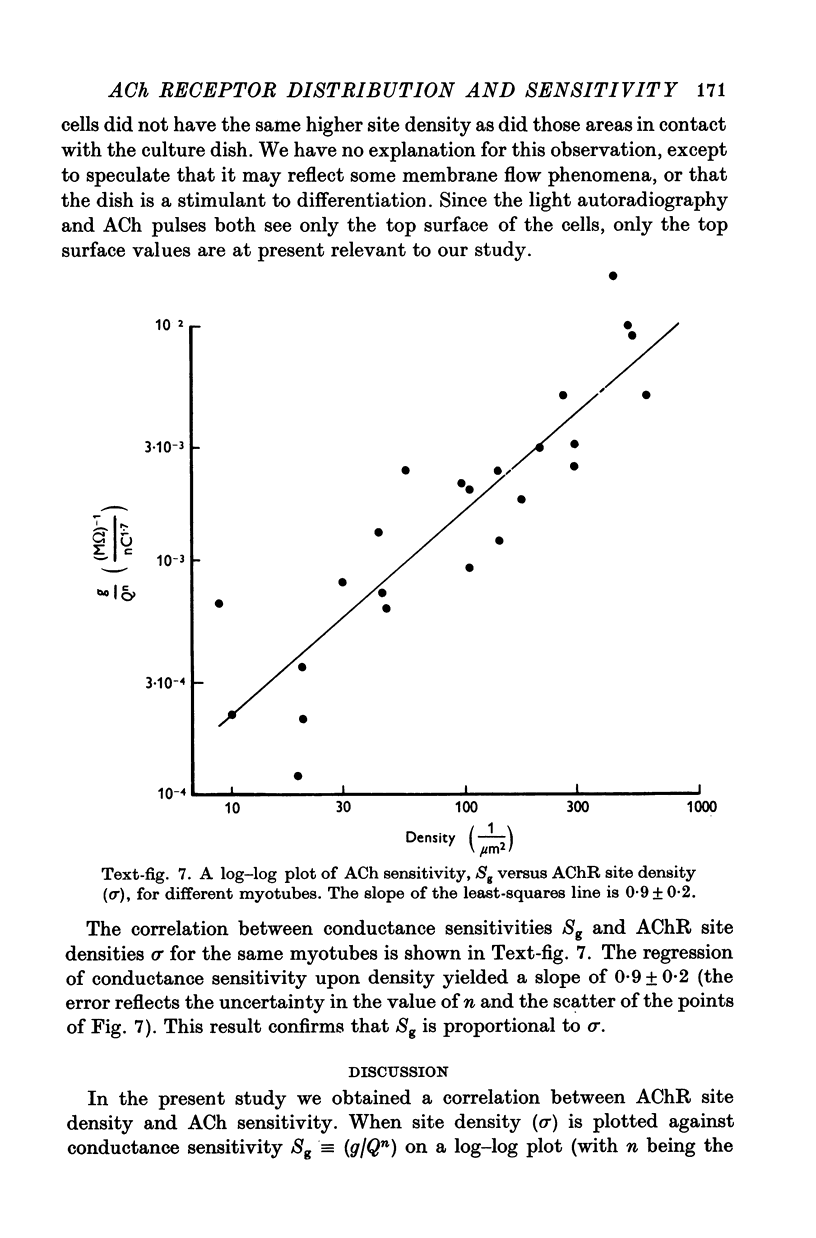
Abstract
1. A linear relation, with a slope of 0-9 +/- 0-2 on a log-log plot, was obtained between acetylcholine (ACh) sensitivity and alpha-bungarotoxin (alpha-BTX) binding site density in developing L6 and rat primary myotubes. ACh sensitivity was defined as g/Qn where g is conductance, Q is ACh charge and n is the Hill coefficient. Experimentally we found n approximately 1-7 for our myotubes, which is similar in value to that reported for adult systems. 2. The linear relationship is compatible with an organization whereby each ion channel is always complexed with a fixed number of ACh receptors such that the dose-response characteristics of each such complex are independent of average ACh receptor density. 3. Light microscope autoradiography showed that the alpha-bungarotoxin binding sites on L6 myotubes are uniformly distributed over the surface, while primary rat myotubes exhibit gradients and hot spots. Electron microscope autoradiography indicated that about 70% of the [125I]alpha-bungarotoxin label was on the surface of the myotubes. The alpha-bungarotoxin site density, after subtracting myoblast background, varied from 5 to 400 sites/micrometer2 on different L6 myotubes, and from 54 to 900 sites/micrometer2 on primary rat myotubes, with occasional hot spots of 3000-4000 sites/micrometer2. The conductance sensitivities varied from 10(-4) to 2 X 10(-2) Momega-1/nC1-7.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ada G. L., Humphrey J. H., Askonas B. A., McDevitt H. O., Nossal G. J. Correlation of grain counts with radioactivity (125I and tritium) in autoradiography. Exp Cell Res. 1966 Mar;41(3):557–572. doi: 10.1016/s0014-4827(66)80106-4. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Dolly J. O., Porter C. W., Albuquerque E. X. The acetylcholine receptor and the ionic conductance modulation system of skeletal muscle. Exp Neurol. 1975 Jul;48(1):1–28. doi: 10.1016/0014-4886(75)90219-8. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Wieckowski J., Chiu T. H. Cholinergic receptor molecules and cholinesterase molecules at mouse skeletal muscle junctions. Nature. 1971 Nov 26;234(5326):207–209. doi: 10.1038/234207a0. [DOI] [PubMed] [Google Scholar]
- CHANG C. C., LEE C. Y. ISOLATION OF NEUROTOXINS FROM THE VENOM OF BUNGARUS MULTICINCTUS AND THEIR MODES OF NEUROMUSCULAR BLOCKING ACTION. Arch Int Pharmacodyn Ther. 1963 Jul 1;144:241–257. [PubMed] [Google Scholar]
- Changeux J. P., Podleski T. R. On the excitability and cooperativity of the electroplax membrane. Proc Natl Acad Sci U S A. 1968 Mar;59(3):944–950. doi: 10.1073/pnas.59.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J., Tung Y., Kittel C. On the cooperativity of biological membranes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):335–341. doi: 10.1073/pnas.57.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G. S. Solid state lactoperoxidase: a highly stable enzyme for simple, gentle iodination of proteins. Biochem Biophys Res Commun. 1972 Jul 25;48(2):464–471. doi: 10.1016/s0006-291x(72)80074-3. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Density and dose-response curve of acetylcholine receptors in frog neuromuscular junction. Nature. 1975 Feb 20;253(5493):641–643. doi: 10.1038/253641a0. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Iontophoretic application of acetylcholine: advantages of high resistance micropipettes in connection with an electronic current pump. Pflugers Arch. 1974 Apr 22;348(3):263–272. doi: 10.1007/BF00587417. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Mallart A. An analysis of acetylcholine responses of junctional and extrajunctional receptors of frog muscle fibres. J Physiol. 1971 Oct;218(1):85–100. doi: 10.1113/jphysiol.1971.sp009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976 Apr;69(1):144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Sensitivity in electron microscope autoradiography for 125I. J Histochem Cytochem. 1974 Feb;22(2):80–87. doi: 10.1177/22.2.80. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Cohen S. A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev Biol. 1973 Mar;31(1):147–162. doi: 10.1016/0012-1606(73)90326-6. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetycholine receptor production and incorporation into membranes of developing muscle fibers. Dev Biol. 1973 Jan;30(1):153–165. doi: 10.1016/0012-1606(73)90054-7. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A. On the application of "a plausible model" of allosteric proteins to the receptor for acetylcholine. J Theor Biol. 1967 Aug;16(2):306–320. doi: 10.1016/0022-5193(67)90011-2. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Heinemann S. Synapse formation between clonal muscle cells and rat spinal cord explants. Nature. 1974 Dec 13;252(5484):593–594. doi: 10.1038/252593a0. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Sastre A., Podleski T. R. Tetrodotoxin-sensitive and -insensitive action potentials in myotubes. J Cell Physiol. 1973 Dec;82(3):497–510. doi: 10.1002/jcp.1040820318. [DOI] [PubMed] [Google Scholar]
- Miller M. M., Revel J. P. Scanning electron microscopy of epithelia prepared by blunt dissection. Anat Rec. 1975 Oct;183(2):339–357. doi: 10.1002/ar.1091830209. [DOI] [PubMed] [Google Scholar]
- Rang H. P. Drug receptors and their function. Nature. 1971 May 14;231(5298):91–96. doi: 10.1038/231091a0. [DOI] [PubMed] [Google Scholar]
- Ritchie A. K., Fambrough D. M. Electrophysiological properties of the membrane and acetylcholine receptor in developing rat and chick myotubes. J Gen Physiol. 1975 Sep;66(3):327–355. doi: 10.1085/jgp.66.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALPETER M. M., BACHMANN L. AUTORADIOGRAPHY WITH THE ELECTRON MICROSCOPE. A PROCEDURE FOR IMPROVING RESOLUTION, SENSITIVITY, AND CONTRAST. J Cell Biol. 1964 Aug;22:469–477. doi: 10.1083/jcb.22.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Fertuck H. C., Salpeter E. E. Resolution in electron microscope autoradiography. III. Iodine-125, the effect of heavy metal staining, and a reassessment of critical parameters. J Cell Biol. 1977 Jan;72(1):161–173. doi: 10.1083/jcb.72.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H. Acetylcholine responses on clonal myogenic cells in vitro. J Physiol. 1975 May;247(2):393–405. doi: 10.1113/jphysiol.1975.sp010937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H., Harris A. J., Patrick J., Schubert D., Heinemann S. Nerve-muscle interaction in vitro. Role of acetylcholine. J Gen Physiol. 1973 Sep;62(3):255–270. doi: 10.1085/jgp.62.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytkowski A. J., Vogel Z., Nirenberg M. W. Development of acetylcholine receptor clusters on cultured muscle cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):270–274. doi: 10.1073/pnas.70.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Developmental changes preceding cell fusion during muscle differentiation in vitro. Exp Cell Res. 1971 May;66(1):33–48. doi: 10.1016/s0014-4827(71)80008-3. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]