Abstract
1. The discharges of ganglion cells in the cat's retina were recorded under conditions intended to isolate the cone system.
2. Stiles' two-colour threshold technique permitted the photopic system to be studied when at its highest sensitivity. The absolute sensitivity of a ganglion cell, expressed in equivalent photons of λmax at the cornea per impulse discharged, was about 2500 times less when driven by cones than when driven by rods. This ratio improves to around 200 when allowance is made for the much smaller fraction absorbed by cones of photons incident on the cornea.
3. The number of extra impulses discharged in response to a brief flash was approximately proportional to the number of photons in the flash, up to a limit.
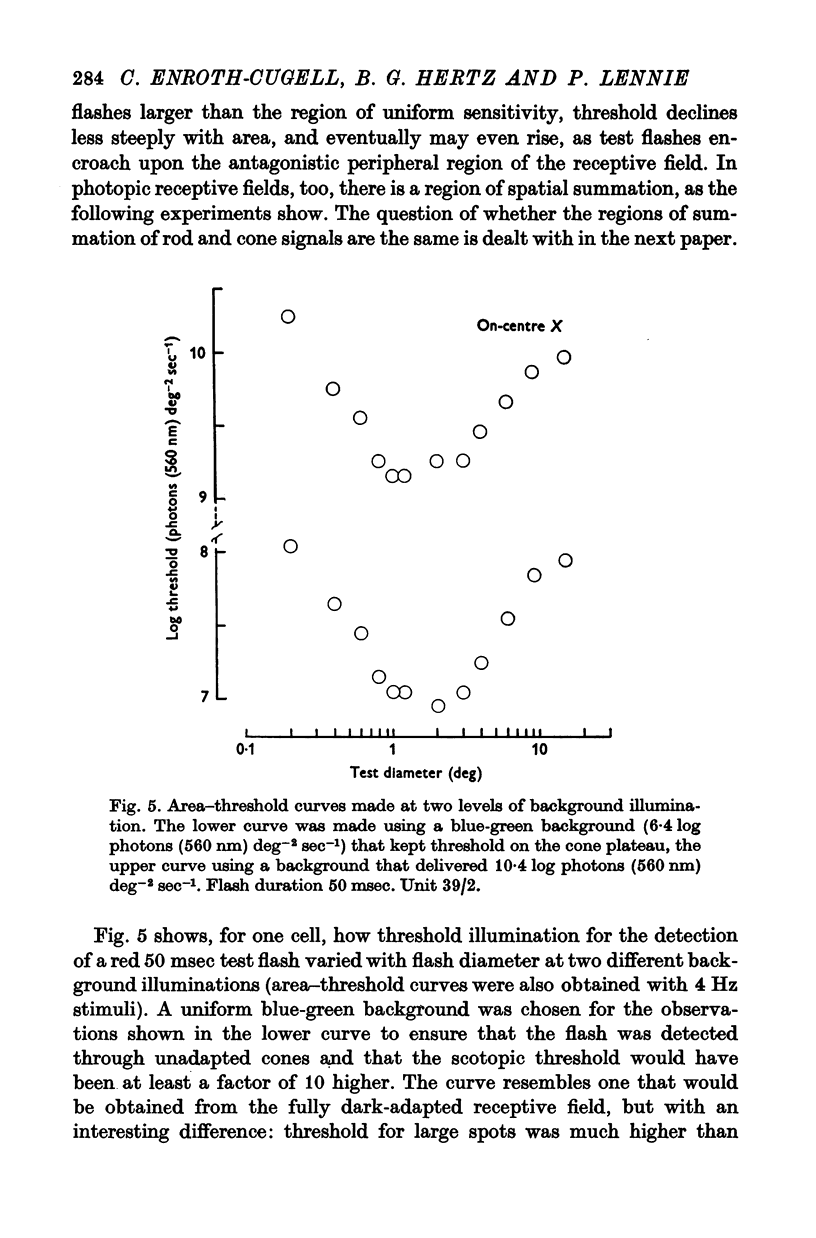
4. There was a region in the middle of the receptive field within which the area of a test spot and its illumination for threshold varied inversely. A flash extending over the peripheral part of the receptive field raised threshold above its minimum, presumably as a result of surround antagonism. Assessed from area—threshold curves, the balance of centre-surround antagonism in the photopic receptive field did not seem to depend upon background illumination.
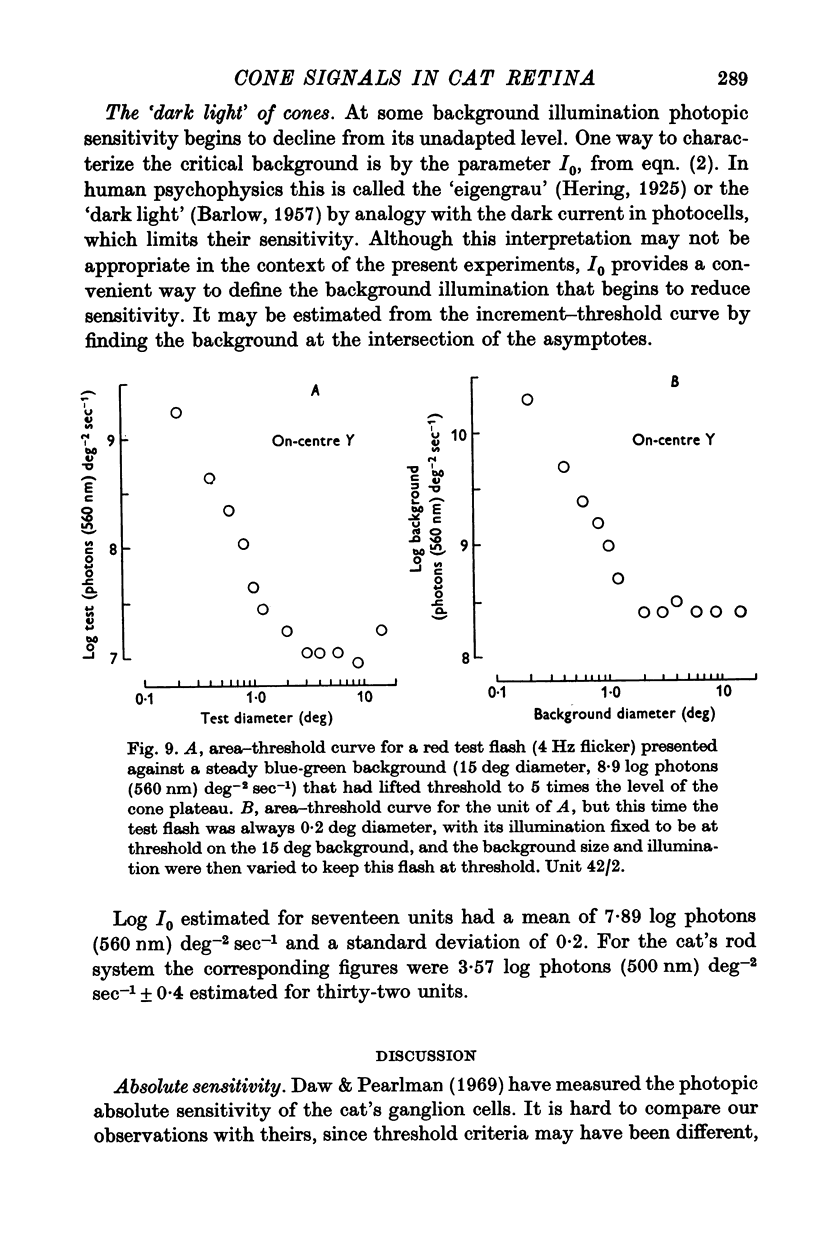
5. The threshold for a small (0·2°) flash confined to the middle of the receptive field was independent of background illumination until the background exceeded a particular level, the `dark light' (Io). In different units this ranged about a mean of 7·89 log photons (560 nm equivalent) deg-2 sec-1. For backgrounds that exceeded Io, threshold followed approximately Weber's law up to the highest illuminations that could be produced.
6. With test flashes that filled the centre of the receptive field, the Weber fraction (test flash illumination/background illumination) in some units fell below 1%.
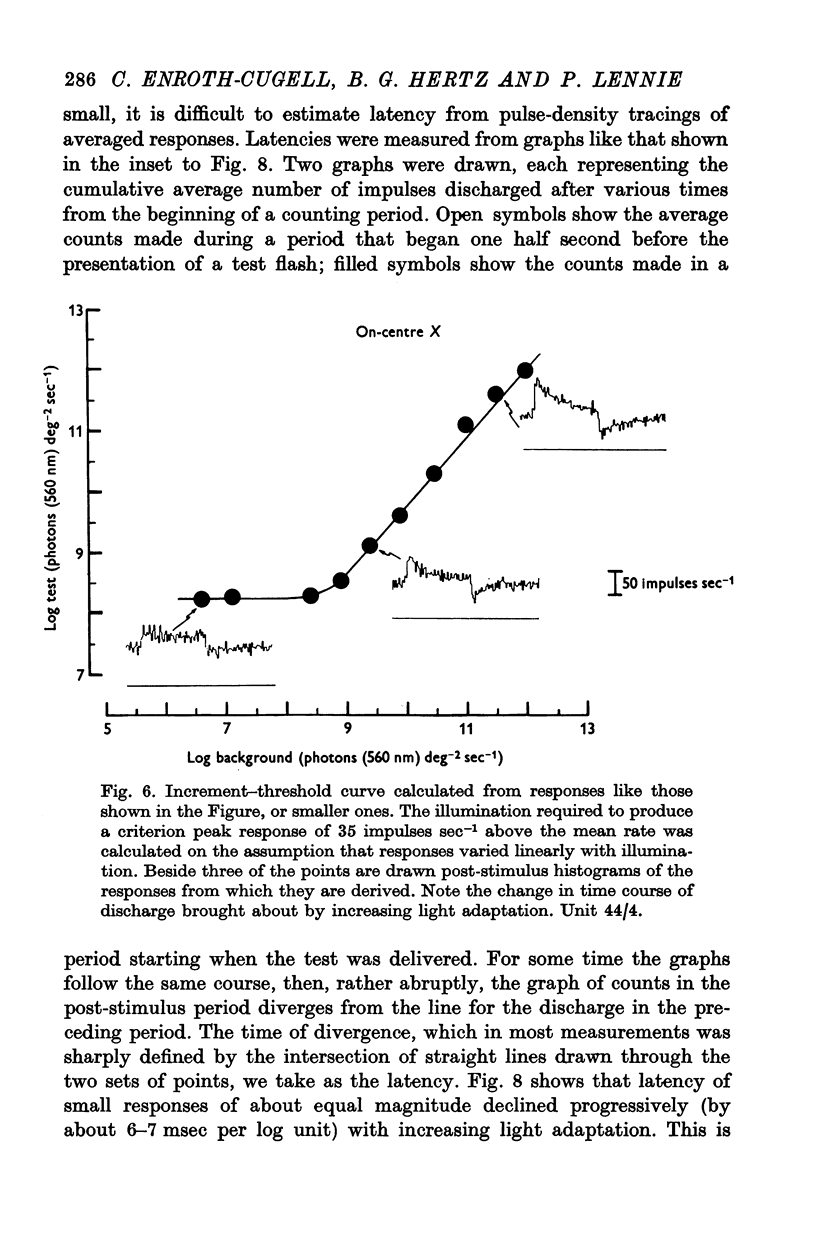
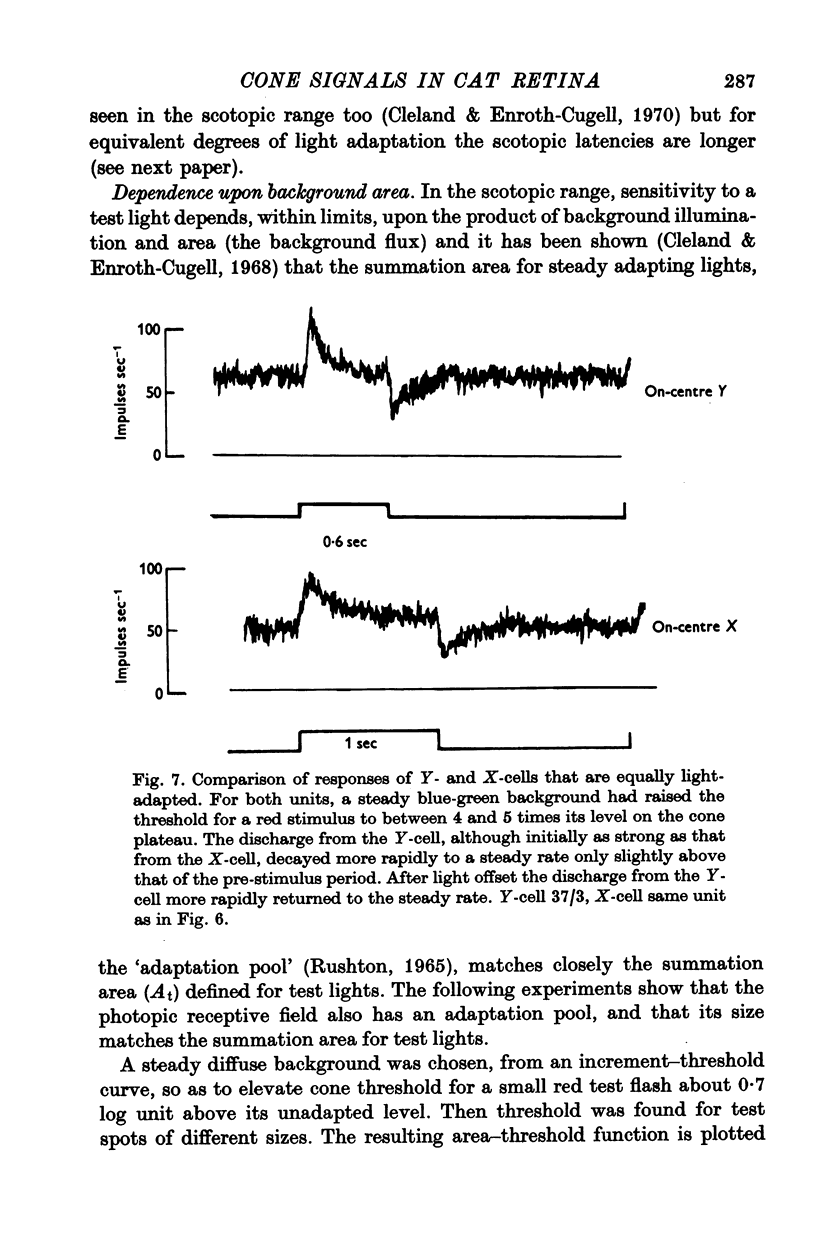
7. Changes in the time course and latency of response accompanied the changes in sensitivity caused by alterations in background illumination. Responses of both X- and Y-cells became more transient and faster.
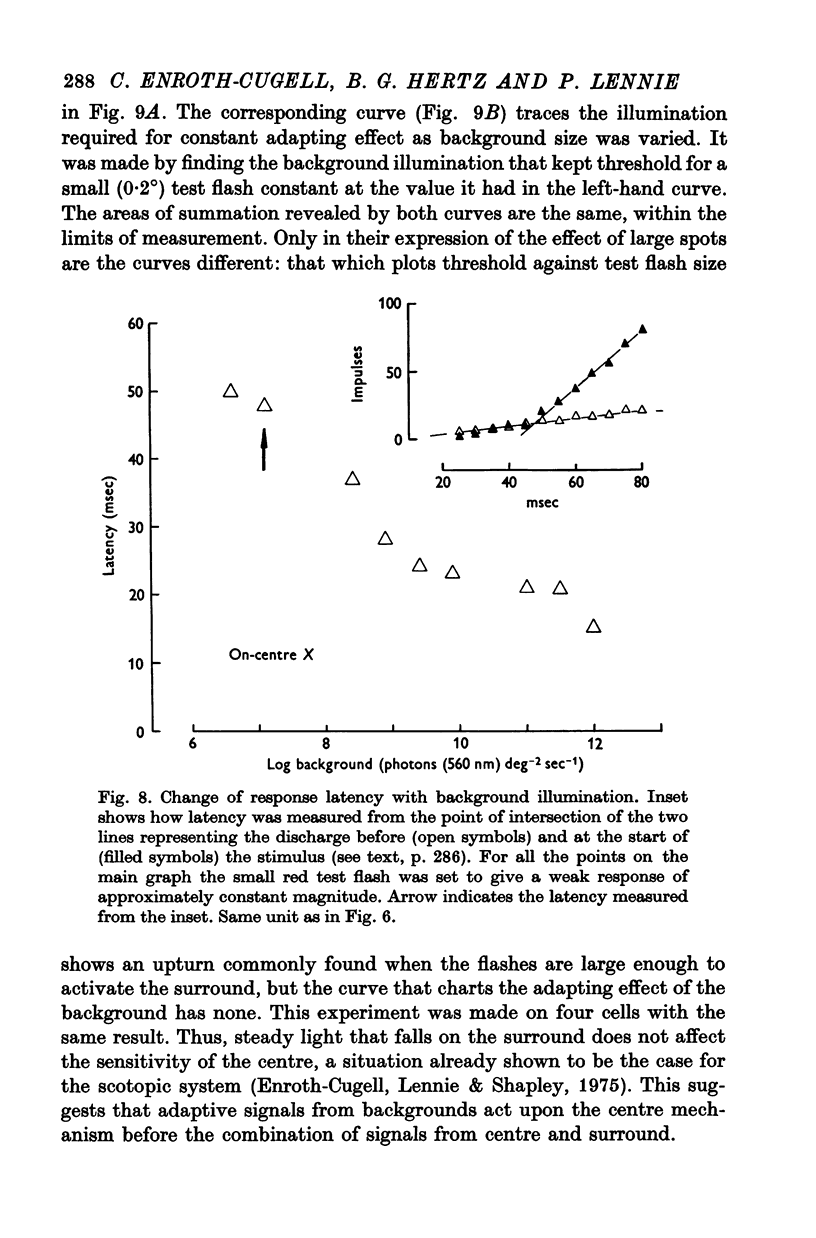
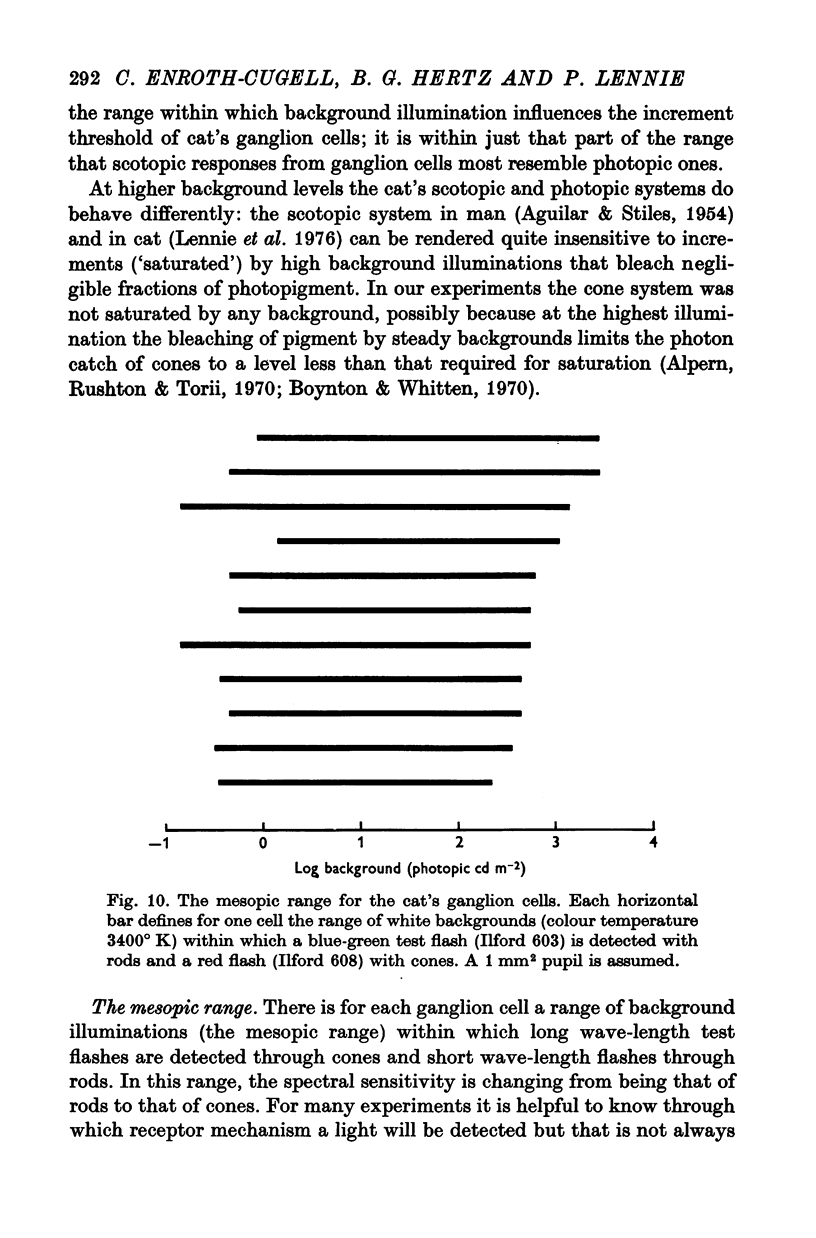
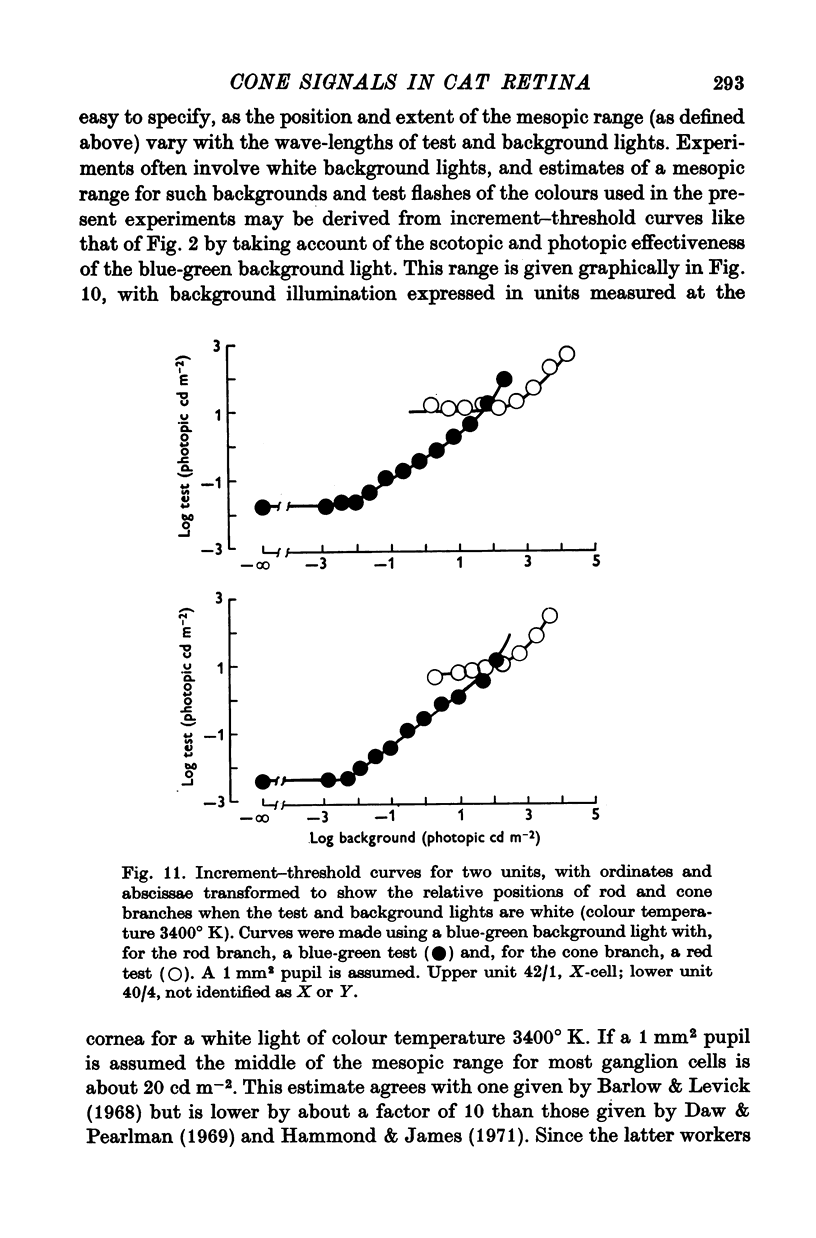
8. The loss of sensitivity to a test flash brought about by a steady background light depended upon the size of that light. Sensitivity varied inversely with background area within a central region that matched closely the summing area for test flashes.
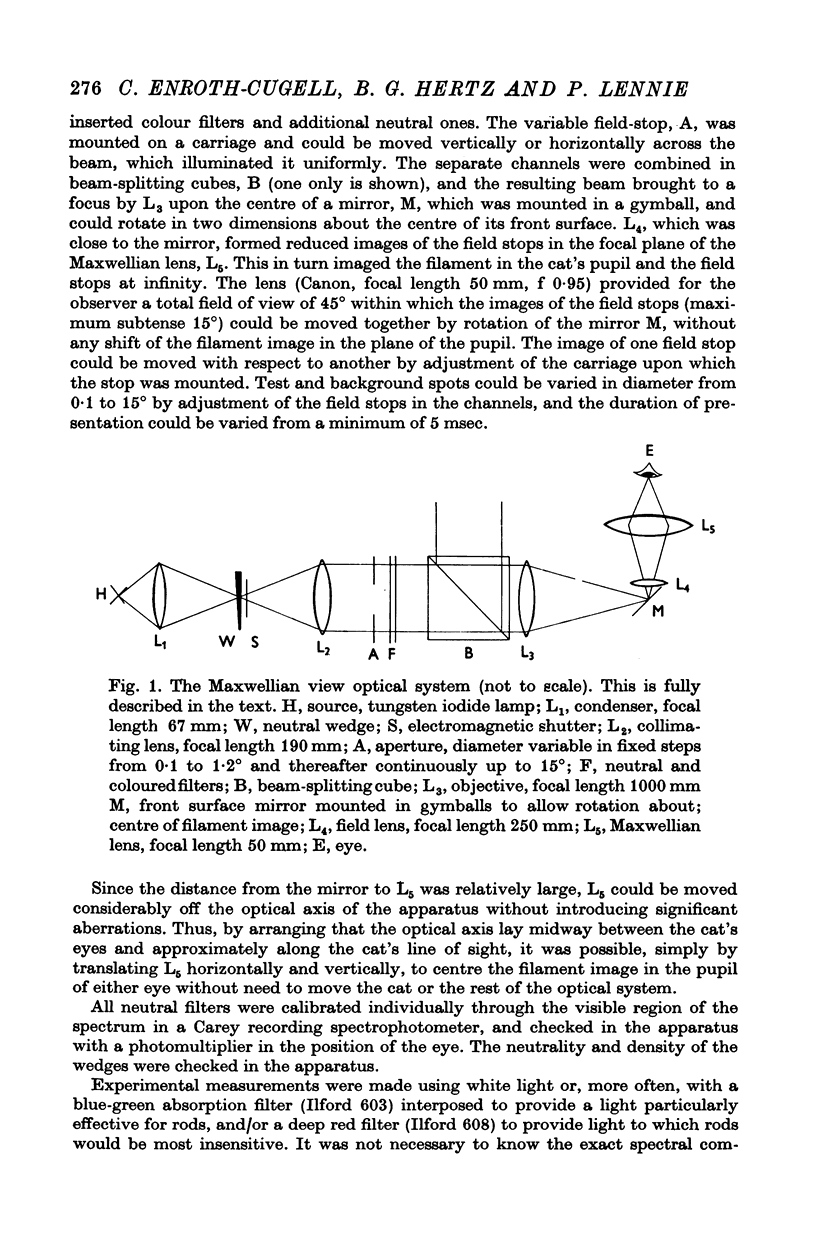
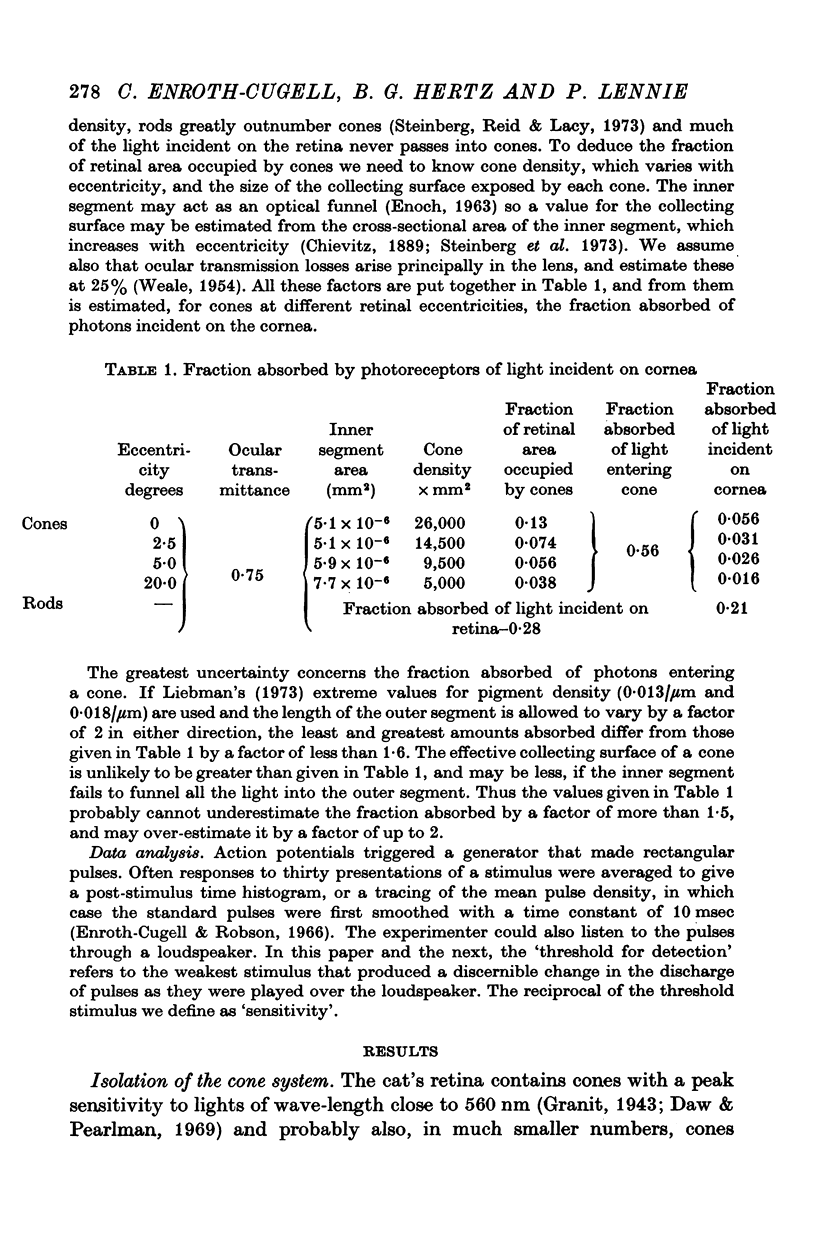
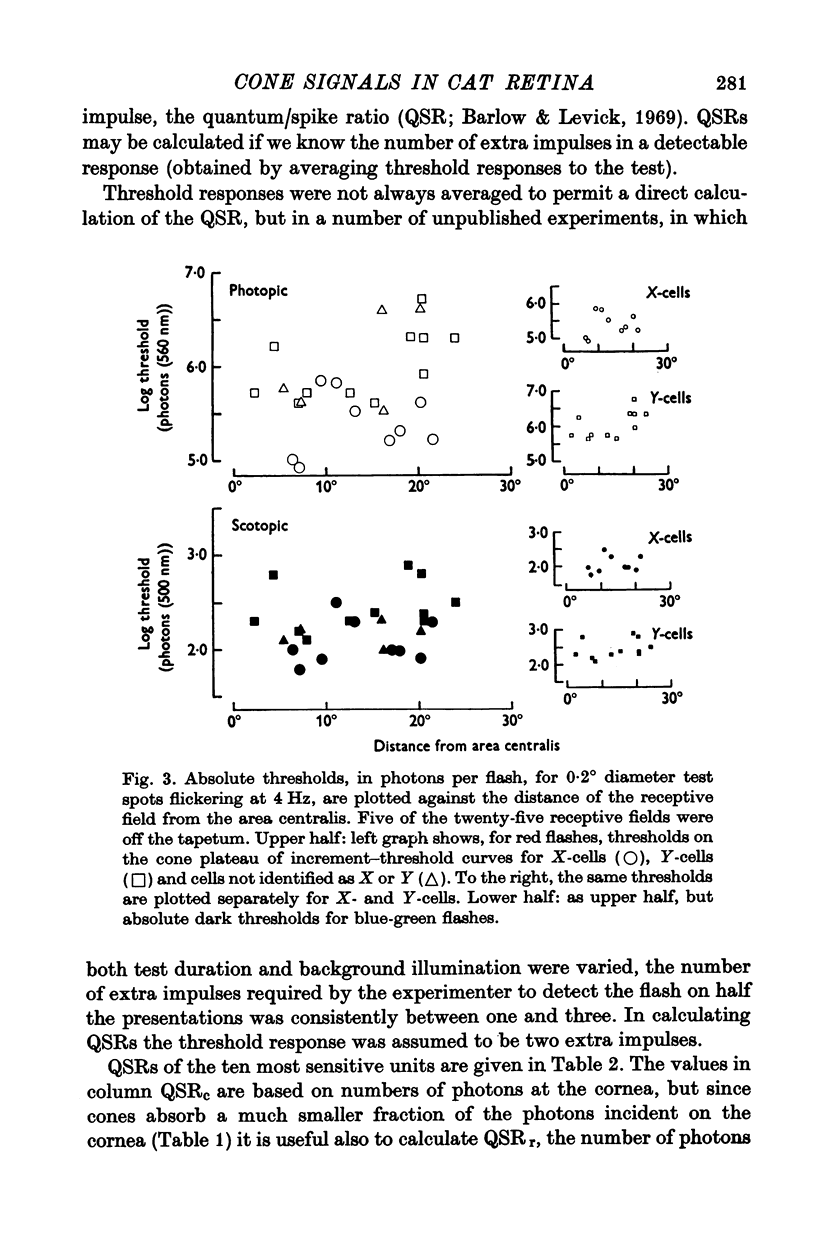
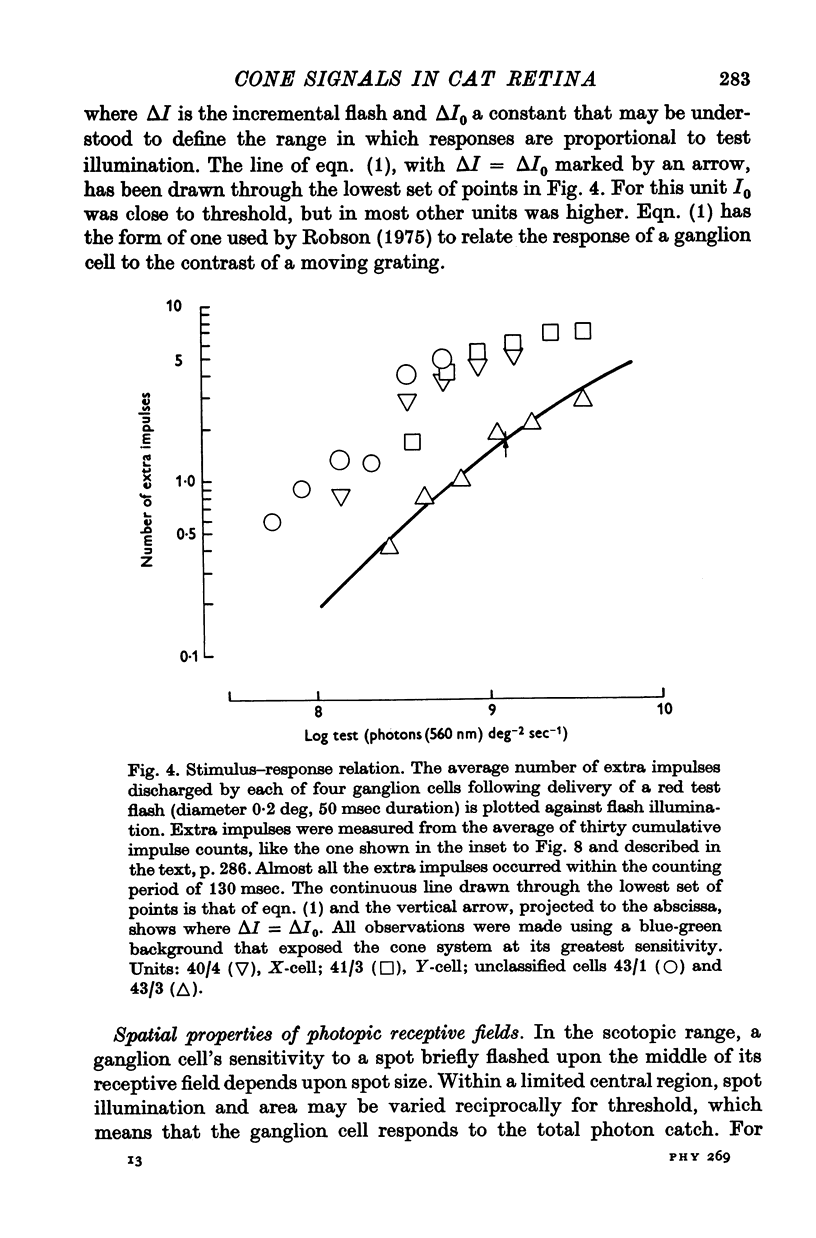
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern M., Rushton W. A., Torii S. Signals from cones. J Physiol. 1970 Apr;207(2):463–475. doi: 10.1113/jphysiol.1970.sp009073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Increment thresholds at low intensities considered as signal/noise discriminations. J Physiol. 1957 May 23;136(3):469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R., Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res. 1971;Suppl 3:87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds A. B., MacLeod D. I. The bleaching and regeneration of rhodopsin in the cat. J Physiol. 1974 Oct;242(1):237–253. doi: 10.1113/jphysiol.1974.sp010704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-Cugell C. Quantitative aspects of gain and latency in the cat retina. J Physiol. 1970 Jan;206(1):73–91. doi: 10.1113/jphysiol.1970.sp008998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: evidence for more than one cone process. J Physiol. 1970 Nov;211(1):125–137. doi: 10.1113/jphysiol.1970.sp009270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: one cone process or several? J Physiol. 1969 May;201(3):745–764. doi: 10.1113/jphysiol.1969.sp008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Croz J. J., Rushton W. A. The separation of cone mechanisms in dark adaptation. J Physiol. 1966 Mar;183(2):481–496. doi: 10.1113/jphysiol.1966.sp007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz B. G., Lennie P. Convergence of rod and cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):297–318. doi: 10.1113/jphysiol.1977.sp011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Lennie P., Shapley R. M. Surround contribution to light adaptation in cat retinal ganglion cells. J Physiol. 1975 Jun;247(3):579–588. doi: 10.1113/jphysiol.1975.sp010948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Adaptation and dynamics of cat retinal ganglion cells. J Physiol. 1973 Sep;233(2):271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Dowling J. E. Intracellular recordings from single rods and cones in the mudpuppy retina. Science. 1973 Jun 15;180(4091):1178–1181. doi: 10.1126/science.180.4091.1178. [DOI] [PubMed] [Google Scholar]
- Hammond P., James C. R. The Purkinje shift in cat: extent of the mesopic range. J Physiol. 1971 Jul;216(1):99–109. doi: 10.1113/jphysiol.1971.sp009511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- Jakiela H. G., Enroth-Cugell C. Adaptation and dynamics in X-cells and Y-cells of the cat retina. Exp Brain Res. 1976 Feb 26;24(4):335–342. doi: 10.1007/BF00235001. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H., Brown J. L. Dark adaptation and spectral sensitivity in the cat. Vision Res. 1970 Aug;10(8):703–716. doi: 10.1016/0042-6989(70)90017-9. [DOI] [PubMed] [Google Scholar]
- Lennie P., Hertz B. G., Enroth-Cugell C. Saturation of rod pools in cat. Vision Res. 1976;16(9):935–940. doi: 10.1016/0042-6989(76)90223-6. [DOI] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. THE SENSITIVITY OF RODS UNDER ILLUMINATION. J Physiol. 1965 May;178:141–160. doi: 10.1113/jphysiol.1965.sp007620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H., Reid M., Lacy P. L. The distribution of rods and cones in the retina of the cat (Felis domesticus). J Comp Neurol. 1973 Mar 15;148(2):229–248. doi: 10.1002/cne.901480209. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H. The rod after-effect in S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1345–1355. doi: 10.1016/0042-6989(69)90071-6. [DOI] [PubMed] [Google Scholar]
- VAKKUR G. J., BISHOP P. O., KOZAK W. VISUAL OPTICS IN THE CAT, INCLUDING POSTERIOR NODAL DISTANCE AND RETINAL LANDMARKS. Vision Res. 1963 Nov;61:289–314. doi: 10.1016/0042-6989(63)90004-x. [DOI] [PubMed] [Google Scholar]
- VAKKUR G. J., BISHOP P. O. THE SCHEMATIC EYE IN THE CAT. Vision Res. 1963 Nov;61:357–381. doi: 10.1016/0042-6989(63)90009-9. [DOI] [PubMed] [Google Scholar]
- WEALE R. A. Light absorption in the crystalline lens of the cat. Nature. 1954 May 29;173(4413):1049–1050. doi: 10.1038/1731049a0. [DOI] [PubMed] [Google Scholar]
- WEALE R. A. Photochemical reactions in the living cat's retina. J Physiol. 1953 Nov 28;122(2):322–331. doi: 10.1113/jphysiol.1953.sp005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEALE R. A. The spectral reflectivity of the cat's tapetum measured in situ. J Physiol. 1953 Jan;119(1):30–42. doi: 10.1113/jphysiol.1953.sp004826. [DOI] [PMC free article] [PubMed] [Google Scholar]


