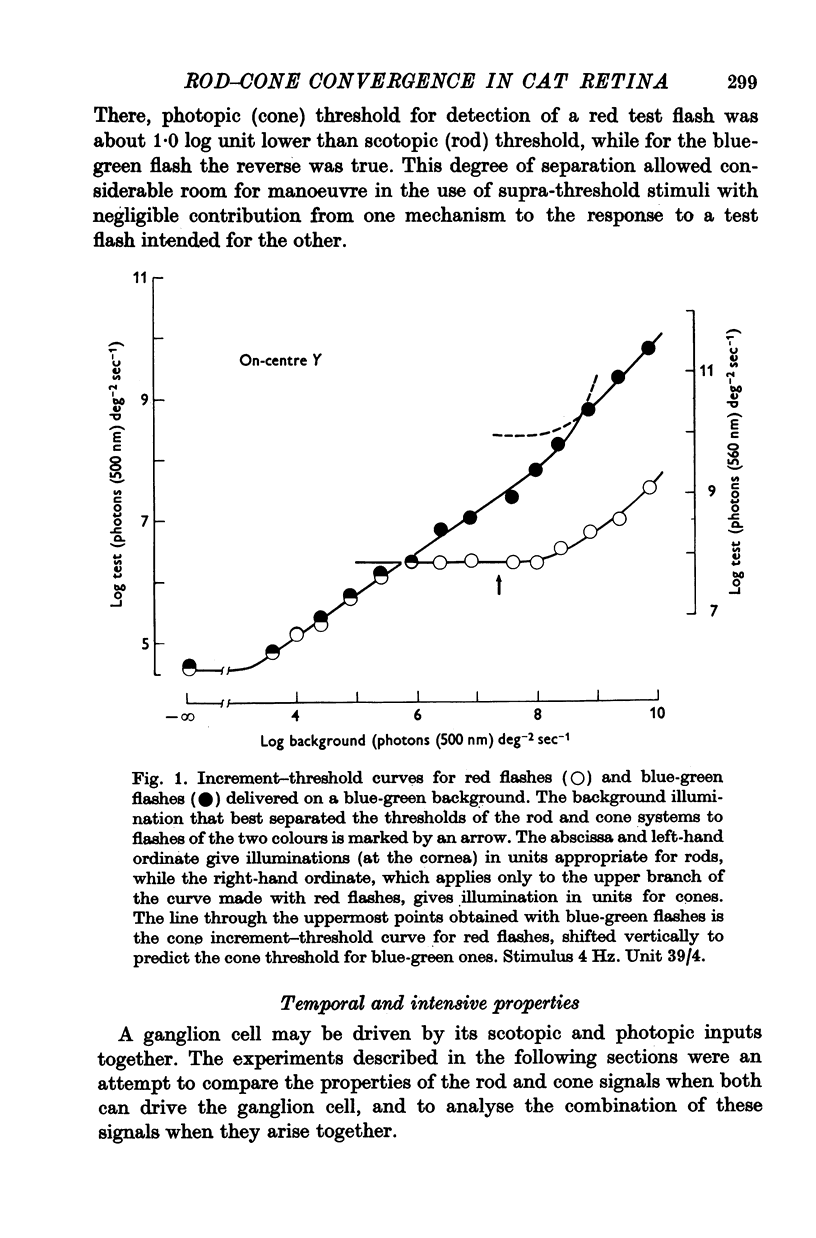
Abstract
1. In an attempt to understand the convergence of rod and cone signals in the cat's retina, ganglion cells that received inputs from both rods and cones were stimulated using lights chosen to excite one or other receptor system or both together.
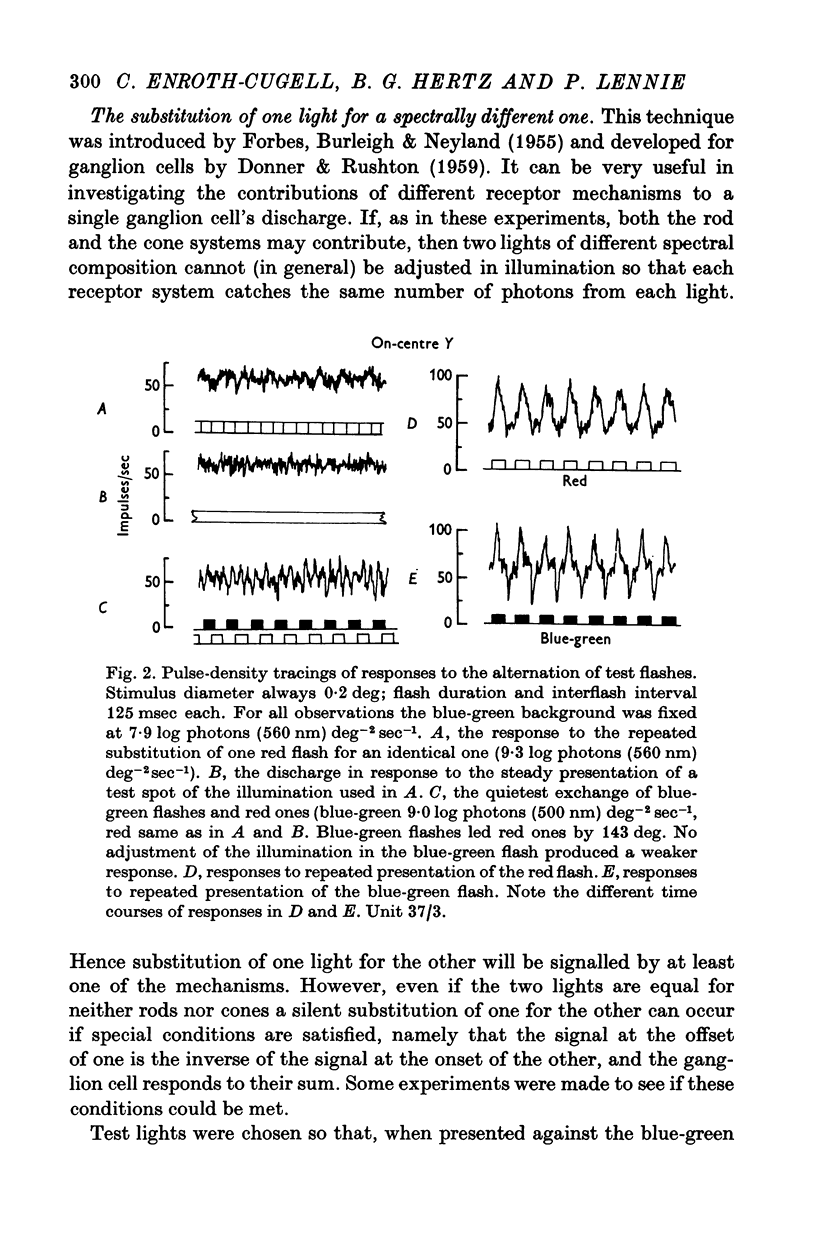
2. If a mesopic background was chosen to allow the ganglion cell to be excited by a blue-green test flash primarily through rods and a deep red flash primarily through cones, one light could not be alternated with the other without eliciting a response from the cell.
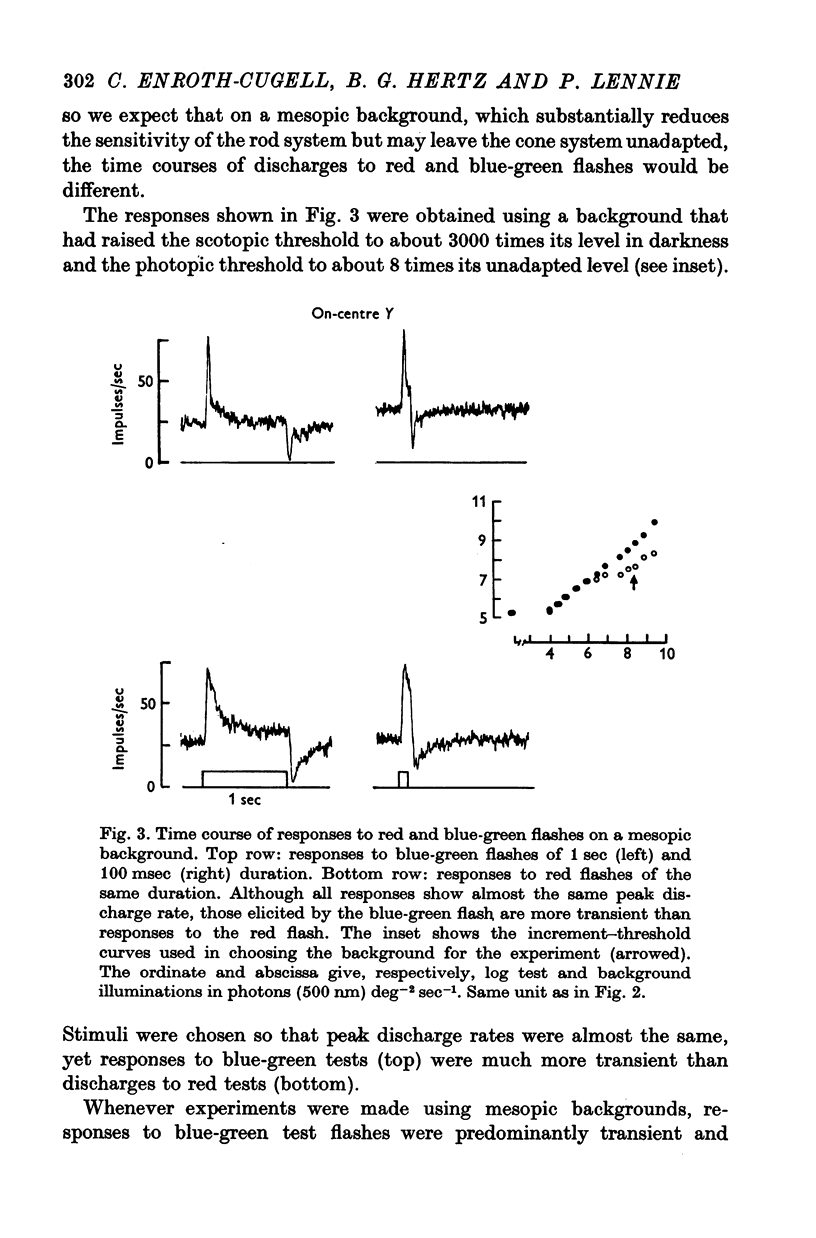
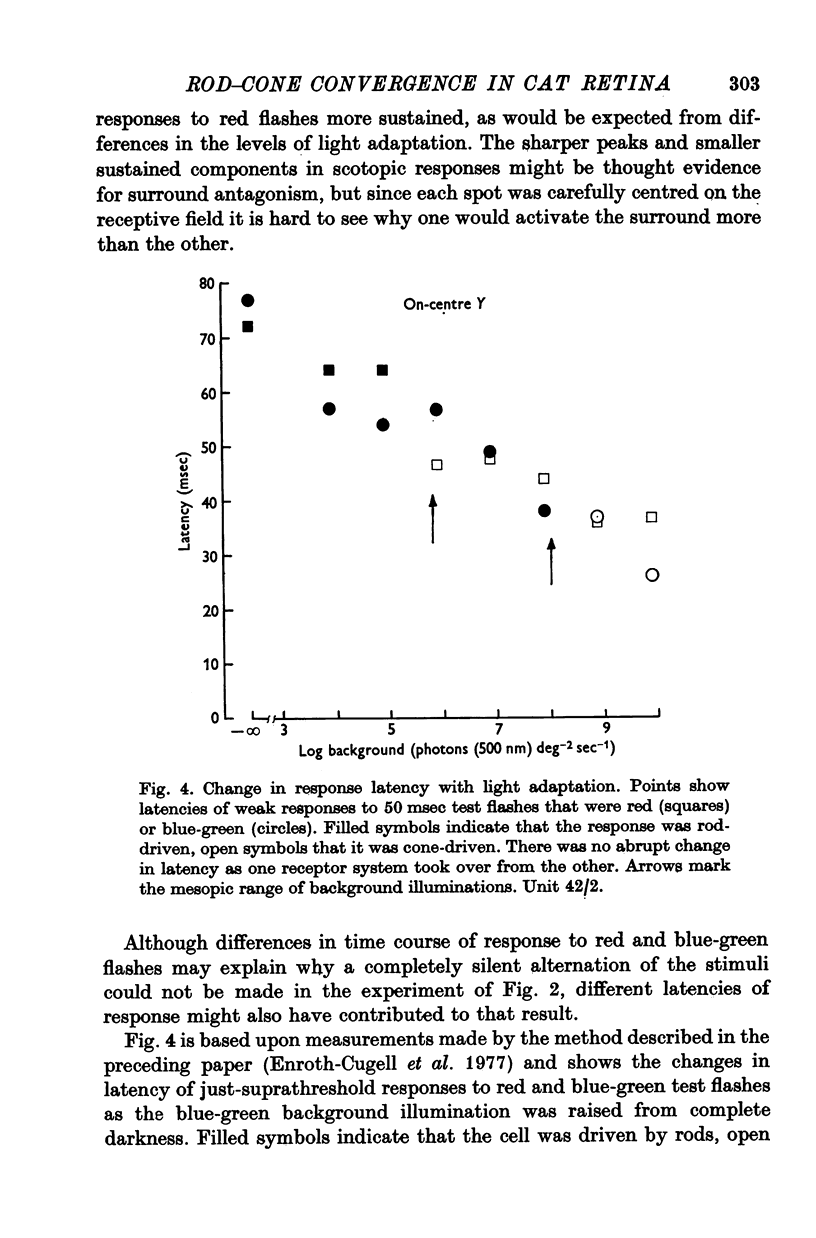
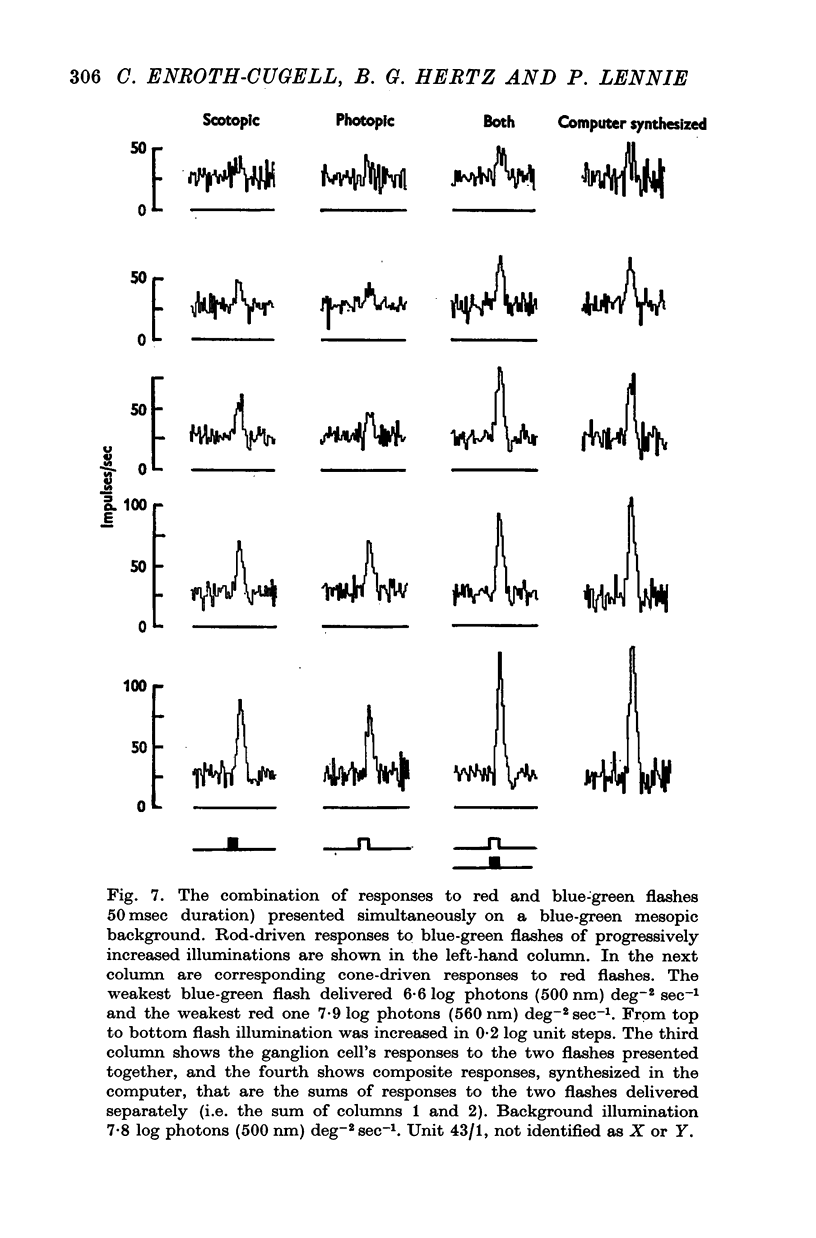
3. This appears to be a result of the different temporal properties of the scotopic and photopic systems. On the mesopic background responses to blue-green test flashes were transient. Responses to red test flashes arose with similar latency, but were more sustained.
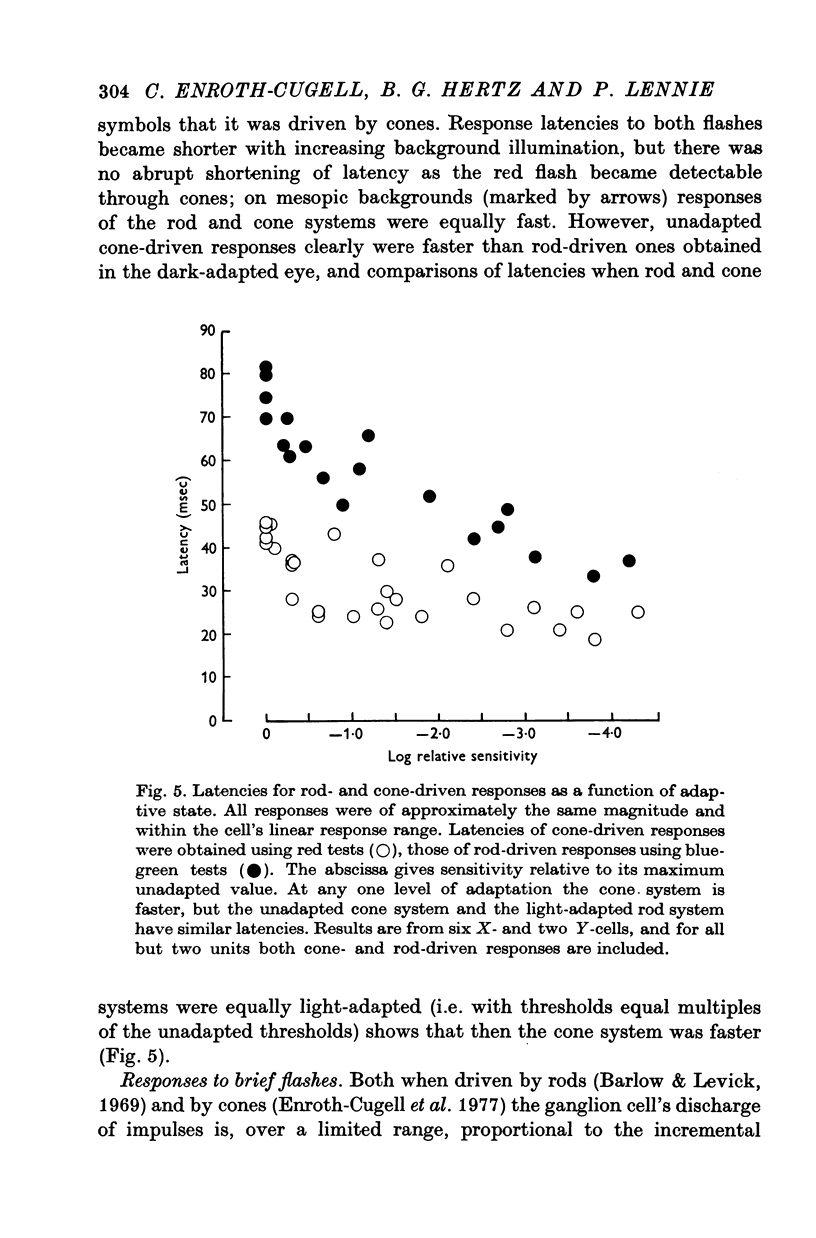
4. Rod and cone systems responded with similar latencies in the presence of the mesopic background that substantially light-adapted the rod system but left the full sensitivity of the cone system undiminished. When equivalently light-adapted, the cone system was faster.
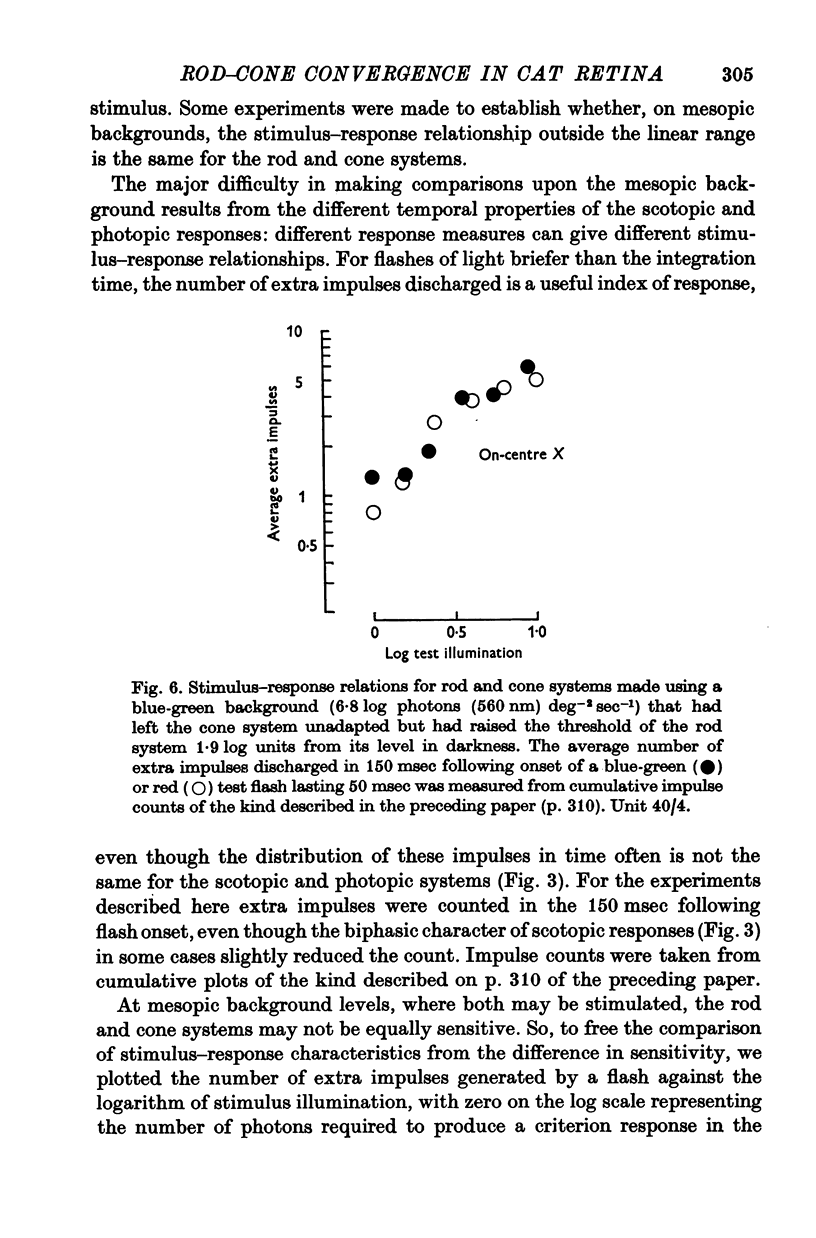
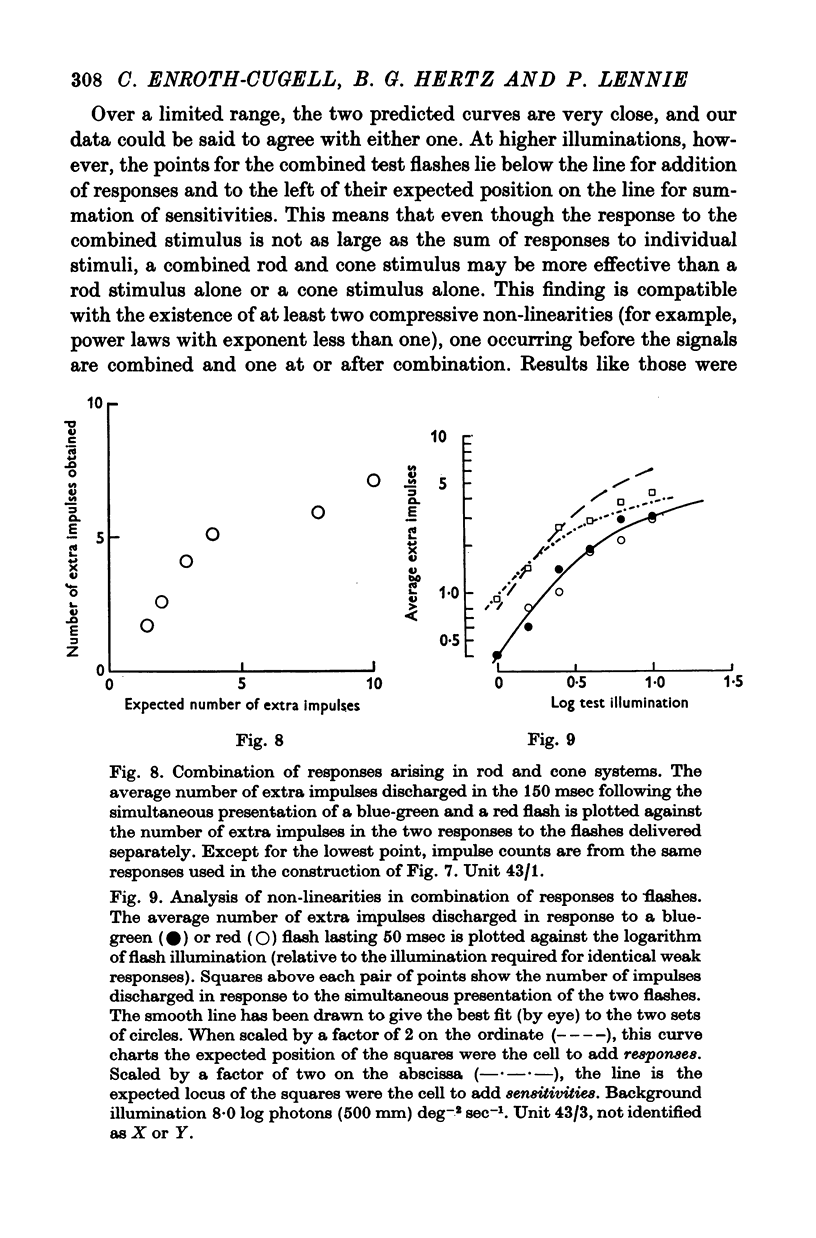
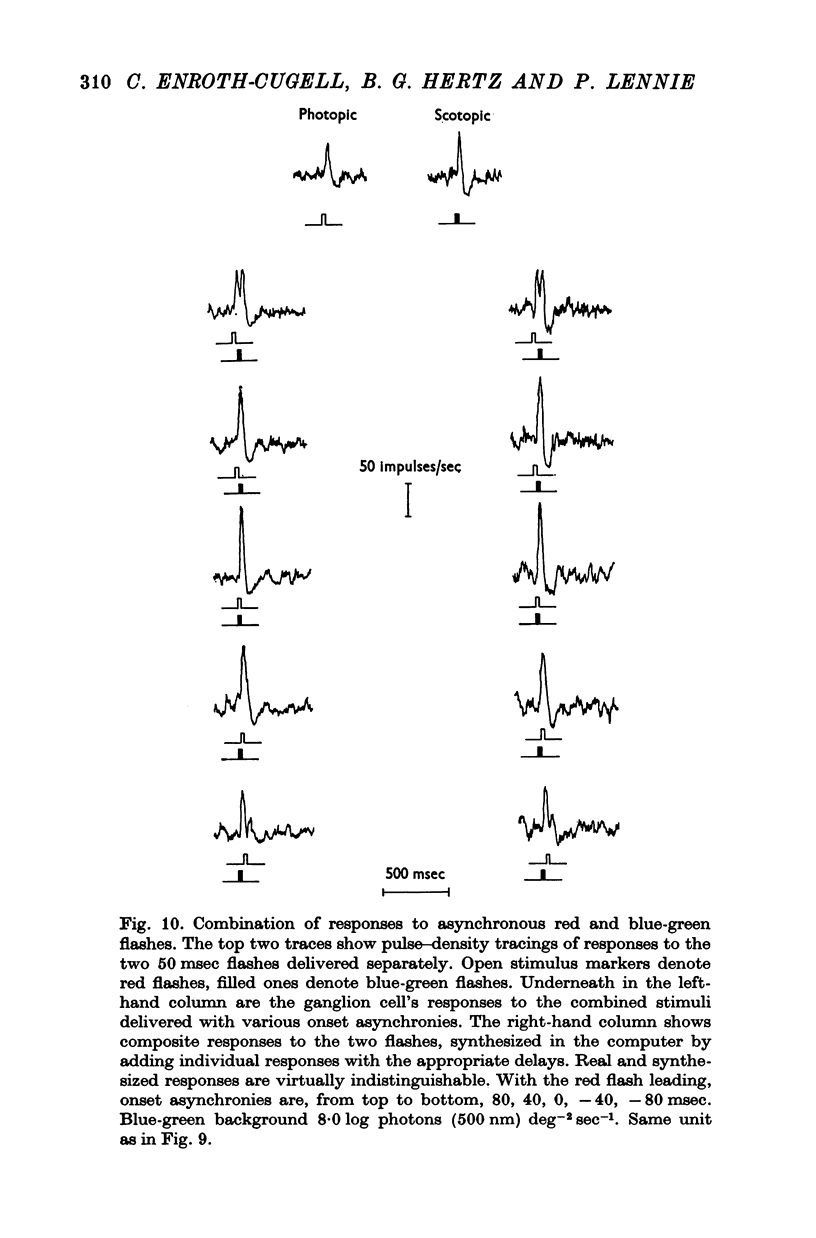
5. When brief flashes that acted through rods were presented with flashes that acted through cones the ganglion cell's response was the sum of the responses to the two flashes presented separately, as long as the flashes were weak. This linear relation ceased to hold when flashes were strong, but the breakdown appears not to be the result of mutual inhibition between rod and cone signals.
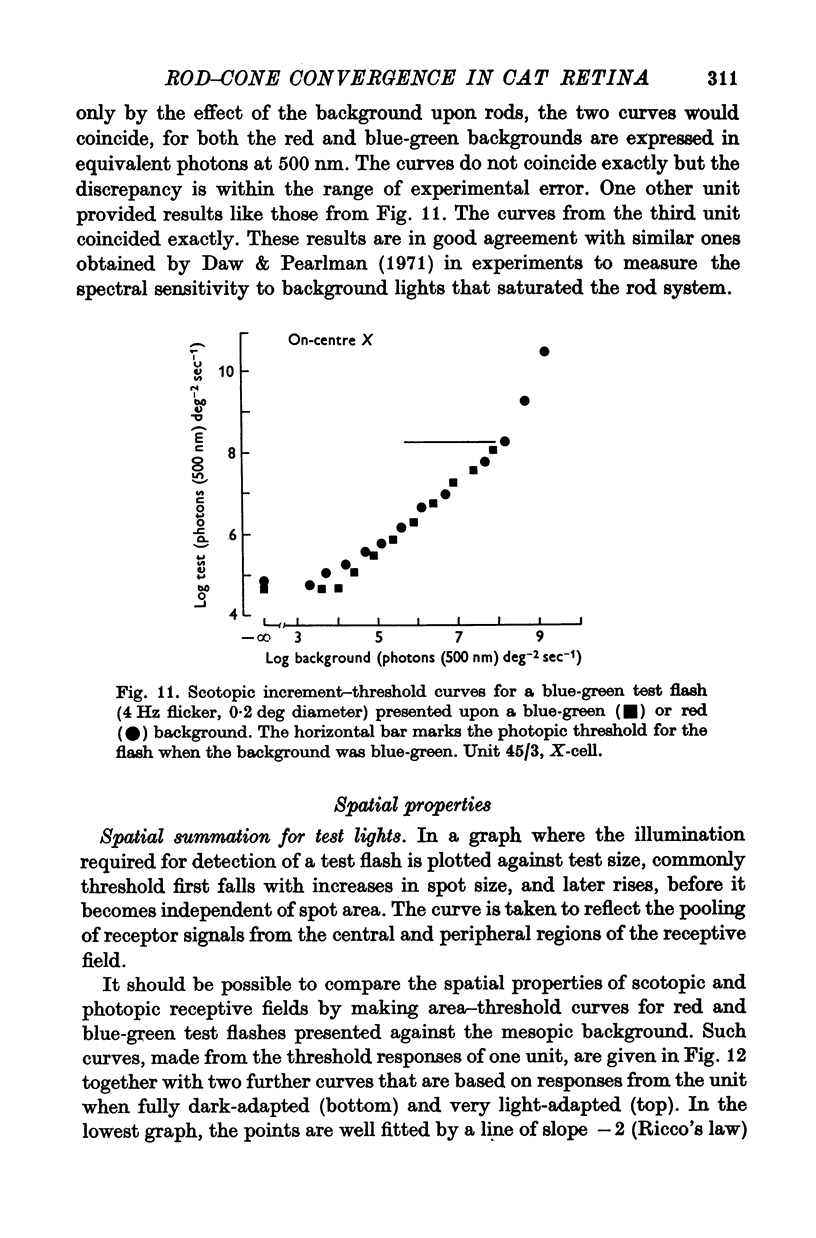
6. When a background light excited both rod and cone systems it appeared to reduce sensitivity independently in each.
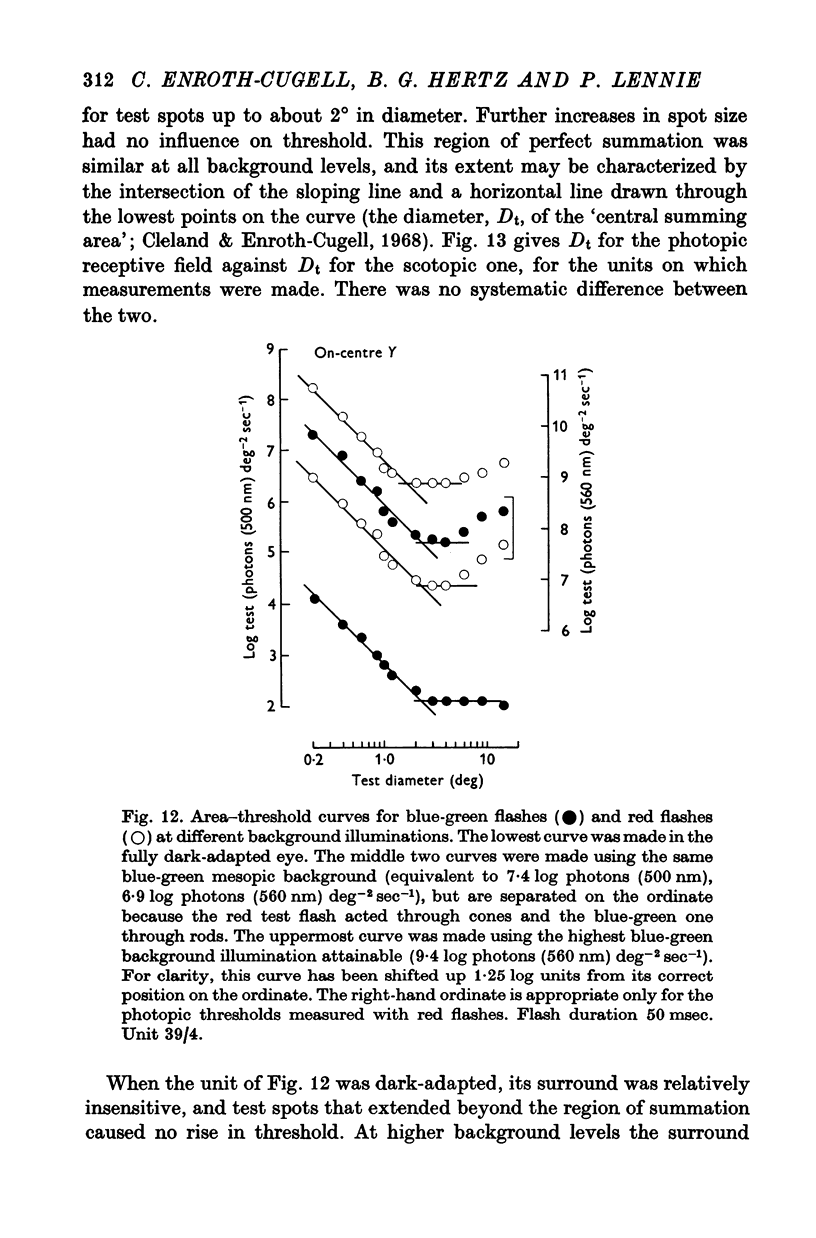
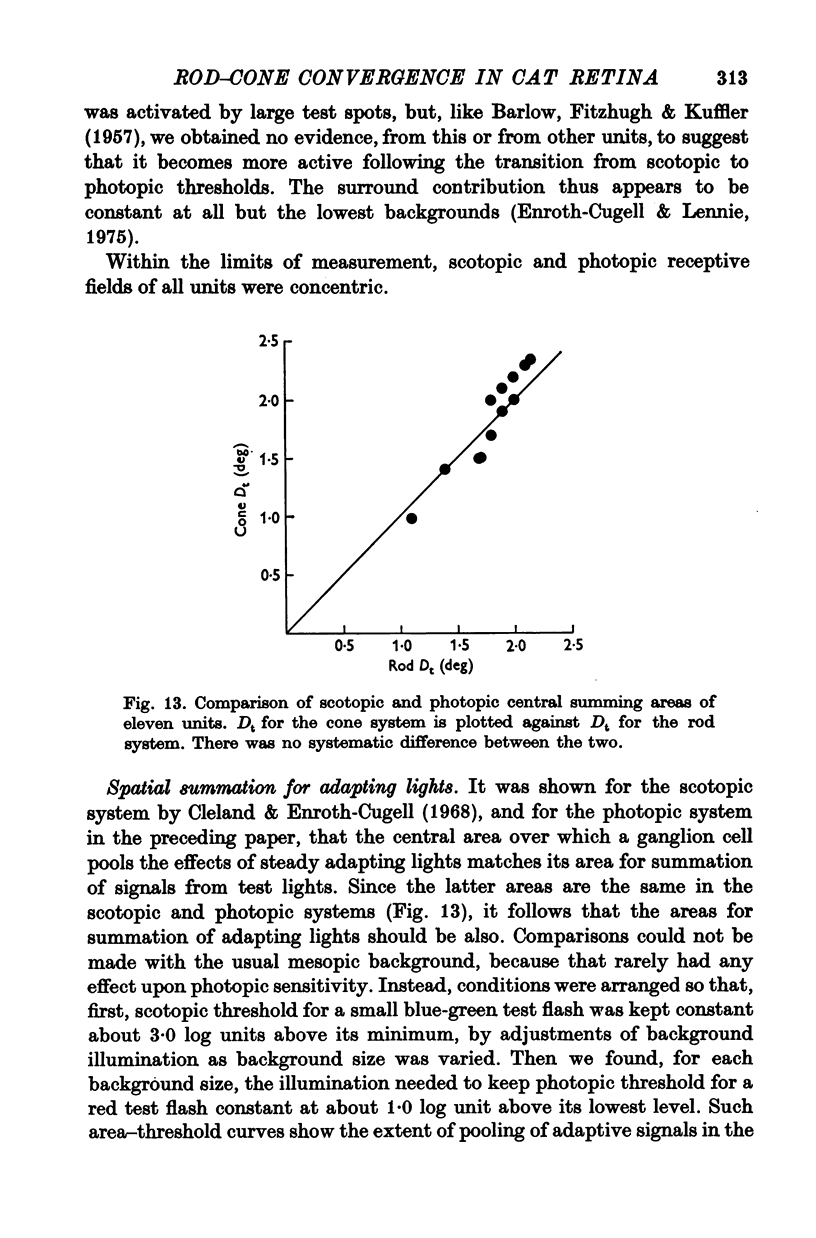
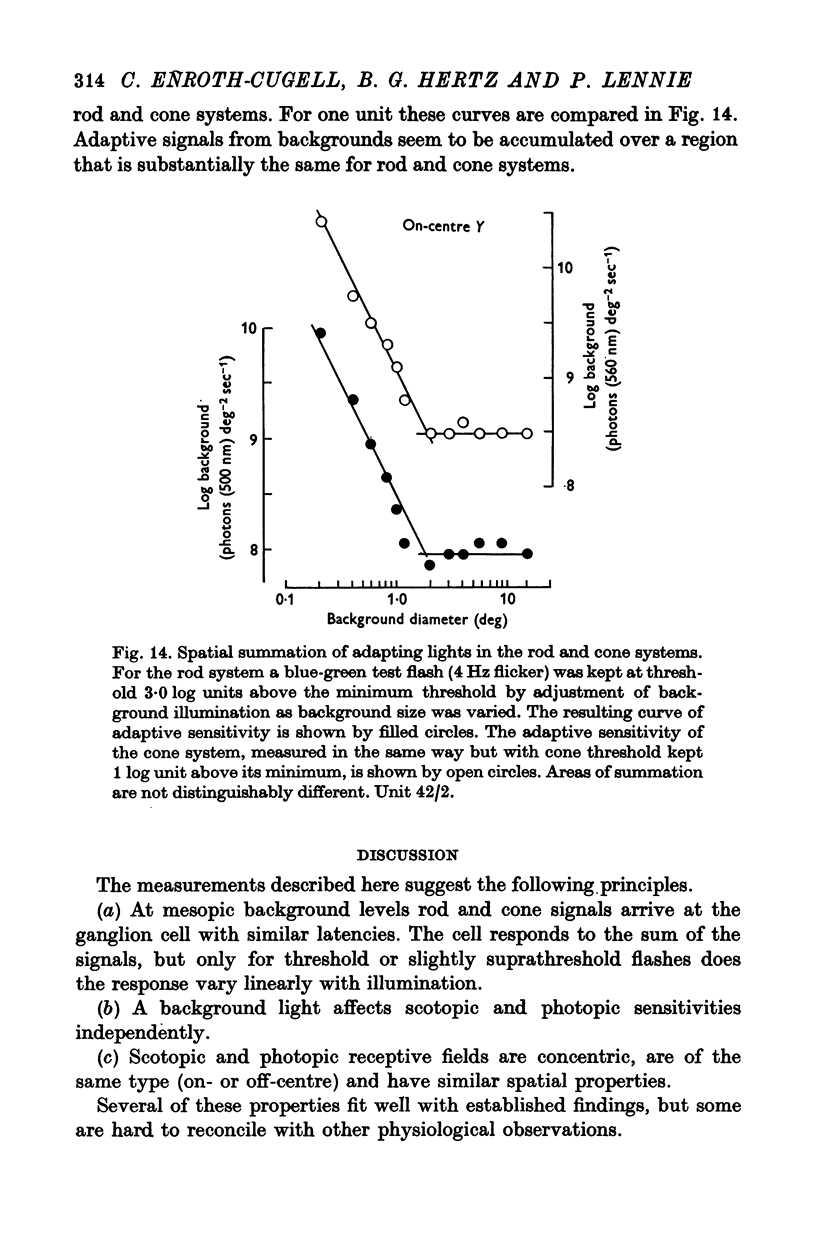
7. The scotopic and photopic receptive fields of a given ganglion cell always were of the same type, on- or off-centre, and, within the limits of measurement, the central regions of the receptive fields were concentric and both the same size.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. P., Hammond P. Mesopic increment threshold spectral sensitivity of single optic tract fibres in the cat: cone-rod interaction. J Physiol. 1970 Jul;209(1):65–81. doi: 10.1113/jphysiol.1970.sp009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. P., Hammond P. Suprathreshold spectral properties of single optic tract fibres in cat, under mesopic adaptation: cone-rod interaction. J Physiol. 1970 Jul;209(1):83–103. doi: 10.1113/jphysiol.1970.sp009157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONNER K. O., RUSHTON W. A. Retinal stimulation by light substitution. J Physiol. 1959 Dec;149:288–302. doi: 10.1113/jphysiol.1959.sp006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Rod saturation in the cat. Vision Res. 1971 Nov;11(11):1361–1364. doi: 10.1016/0042-6989(71)90020-4. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz G., Lennie P. Cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):273–296. doi: 10.1113/jphysiol.1977.sp011902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Lennie P. The control of retinal ganglion cell discharge by receptive field surrounds. J Physiol. 1975 Jun;247(3):551–578. doi: 10.1113/jphysiol.1975.sp010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Adaptation and dynamics of cat retinal ganglion cells. J Physiol. 1973 Sep;233(2):271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES A., BURLEIGH S., NEYLAND M. Electric responses to color shift in frog and turtle retina. J Neurophysiol. 1955 Nov;18(6):517–535. doi: 10.1152/jn.1955.18.6.517. [DOI] [PubMed] [Google Scholar]
- Flamant F., Stiles W. S. The directional and spectral sensitivities of the retinal rods to adapting fields of different wave-lengths. J Physiol. 1948 Mar 15;107(2):187–202. doi: 10.1113/jphysiol.1948.sp004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P., Link K. Rod and cone interaction in dark-adapted monkey ganglion cells. J Physiol. 1966 May;184(2):499–510. doi: 10.1113/jphysiol.1966.sp007928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. W., Abramov I. An analysis of spatial summation in the receptive fields of goldfish retinal ganglion cells. Vision Res. 1975 Jul;15(7):777–789. doi: 10.1016/0042-6989(75)90255-2. [DOI] [PubMed] [Google Scholar]
- MacLeod D. I. Rods cancel cones in flicker. Nature. 1972 Jan 21;235(5334):173–174. doi: 10.1038/235173a0. [DOI] [PubMed] [Google Scholar]
- Makous W., Boothe R. Cones block signals from rods. Vision Res. 1974 Apr;14(4):285–294. doi: 10.1016/0042-6989(74)90078-9. [DOI] [PubMed] [Google Scholar]
- Niemeyer G., Gouras P. Rod and cone signals in S-potentials of the isolated perfused cat eye. Vision Res. 1973 Aug;13(8):1603–1612. doi: 10.1016/0042-6989(73)90017-5. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Rushton W. A. Cancellation of rod signals by cones, and cone signals by rods in the cat retina. J Physiol. 1976 Jan;254(3):775–785. doi: 10.1113/jphysiol.1976.sp011258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H. Rod and cone contributions to S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1319–1329. doi: 10.1016/0042-6989(69)90069-8. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H. The rod after-effect in S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1345–1355. doi: 10.1016/0042-6989(69)90071-6. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Control of retinal sensitivity. II. Lateral interactions at the outer plexi form layer. J Gen Physiol. 1974 Jan;63(1):62–87. doi: 10.1085/jgp.63.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M. Influence of adaptation level on response pattern and sensitivity of ganglion cells in the cat's retina. J Physiol. 1972 Feb;221(1):93–104. doi: 10.1113/jphysiol.1972.sp009741. [DOI] [PMC free article] [PubMed] [Google Scholar]


