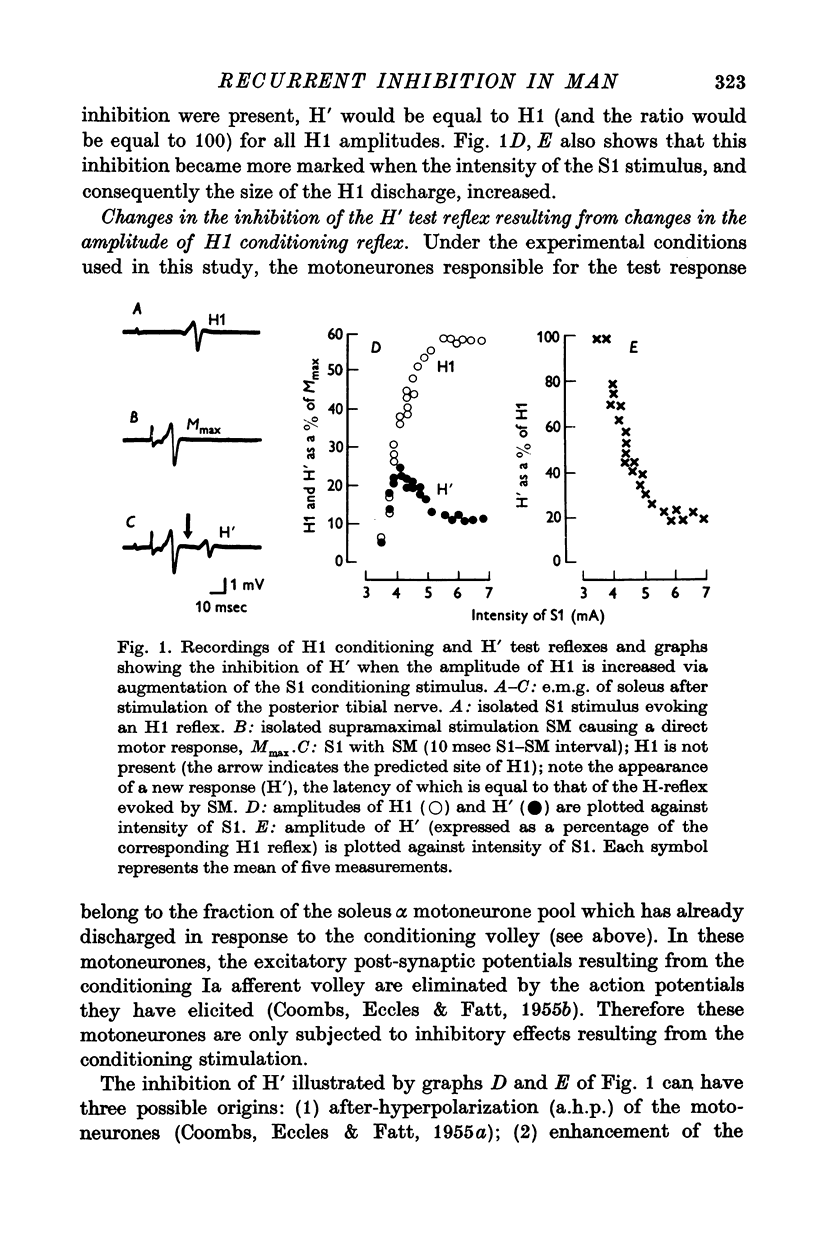
Abstract
1. The pattern of variations of a test H-reflex after a conditioning H-reflex was investigated in human subjects by an experimental design in which both reflexes involved the same soleus motoneurones. This was made possible by using a method based upon a collision in the motor axons between the orthodromic conditioning reflex volley and the antidromic volley elicited by a test stimulus supramaximal for the motor axons.
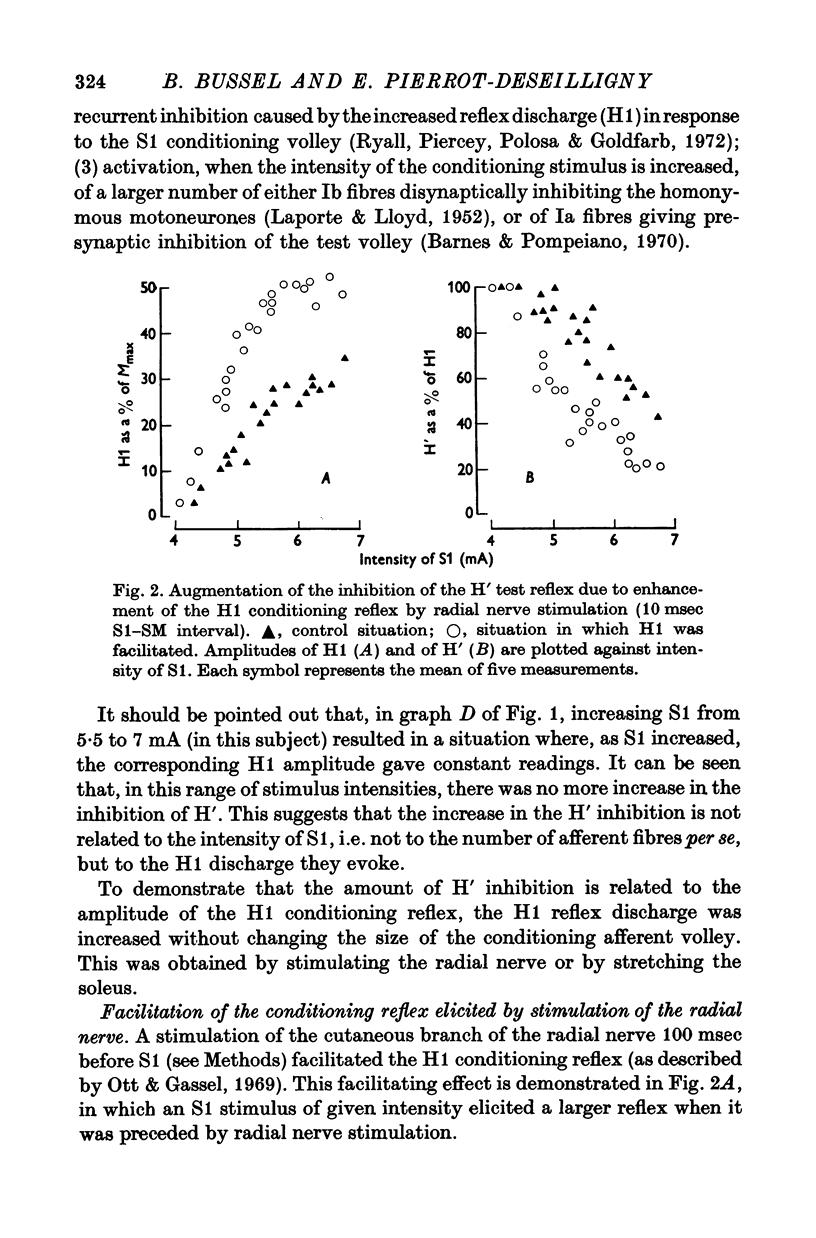
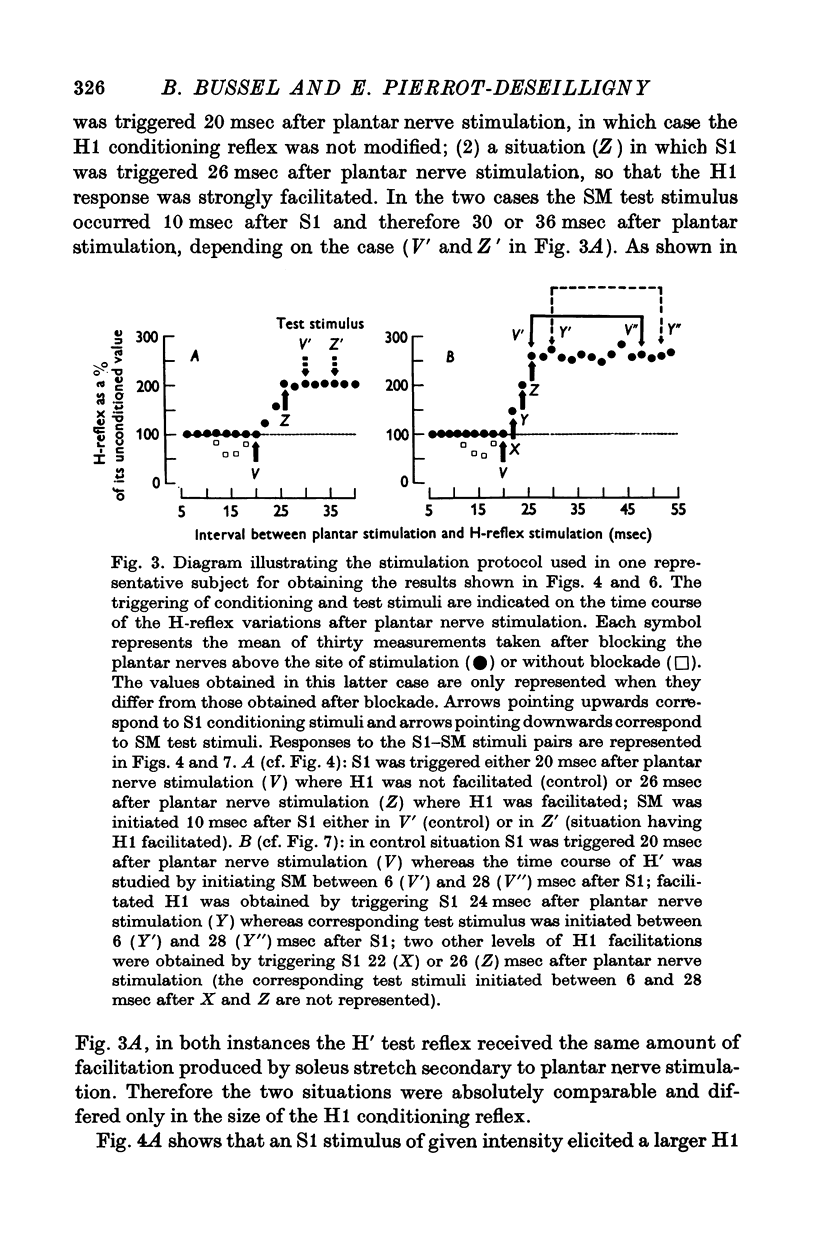
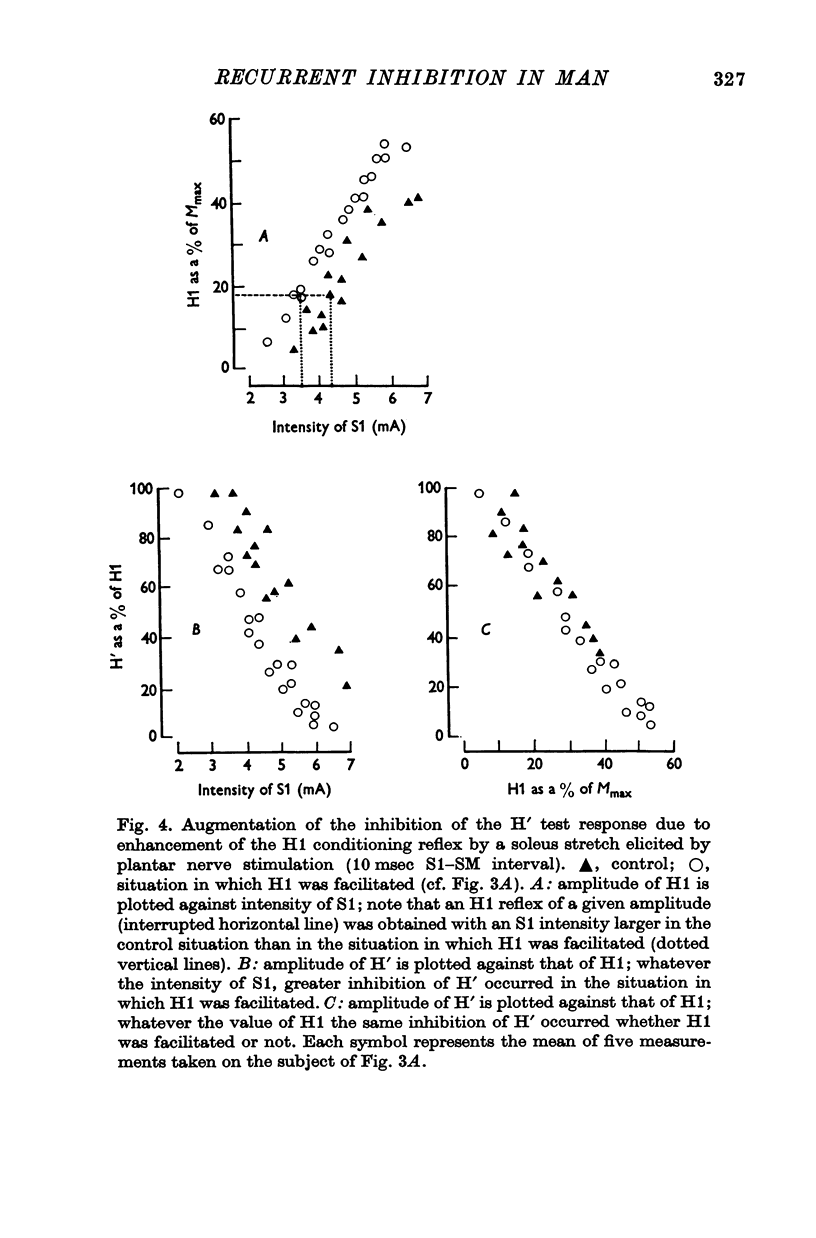
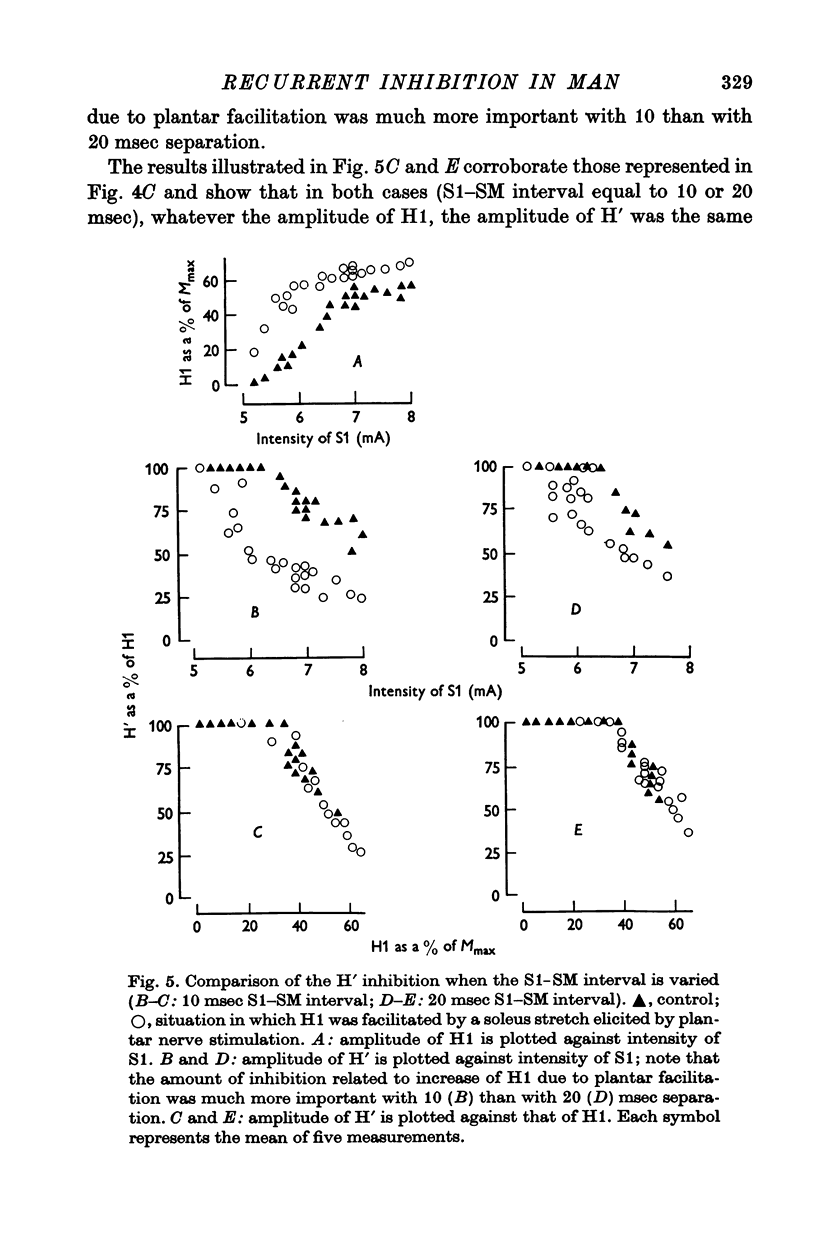
2. The variations of the test reflex amplitude seen when increasing the conditioning reflex discharge were studied. This was made possible by facilitating the conditioning reflex without changing the strength of the afferent volley. This facilitation was obtained through a soleus stretch elicited by a stimulation of the plantar nerves.
3. The amplitude of the test reflex depended only on the size of the conditioning reflex discharge.
4. As long as the conditioning reflex was of low amplitude, all the motoneurones responsible for the conditioning response could be activated by the test volley, even though these motoneurones were undergoing after-hyperpolarization. This indicates that, in man, the after-hyperpolarization of the most excitable motoneurones can be completely overcome by a large Ia afferent volley.
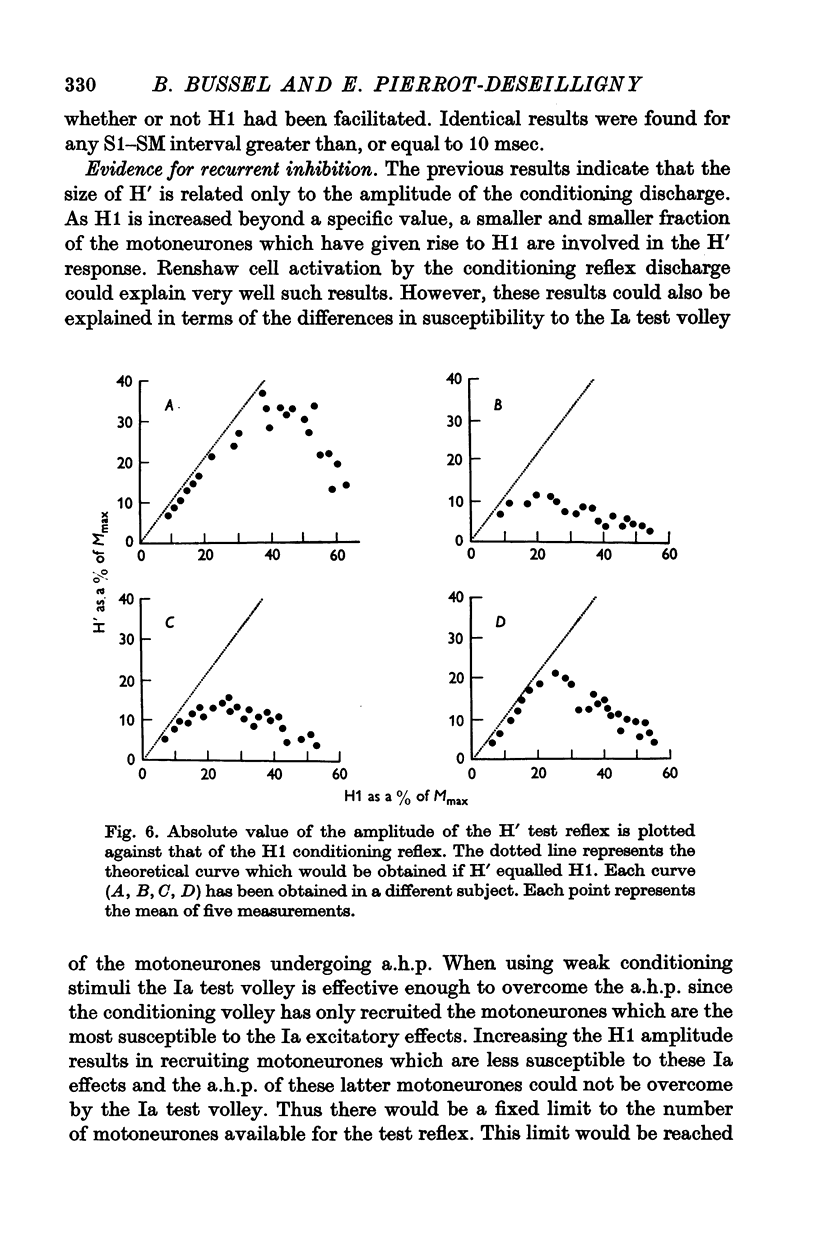
5. Increasing the conditioning reflex beyond a specific value resulted in an absolute decrease in the number of motoneurones involved in the test reflex. The amount of this decrease was related only to the amplitude of the conditioning reflex.
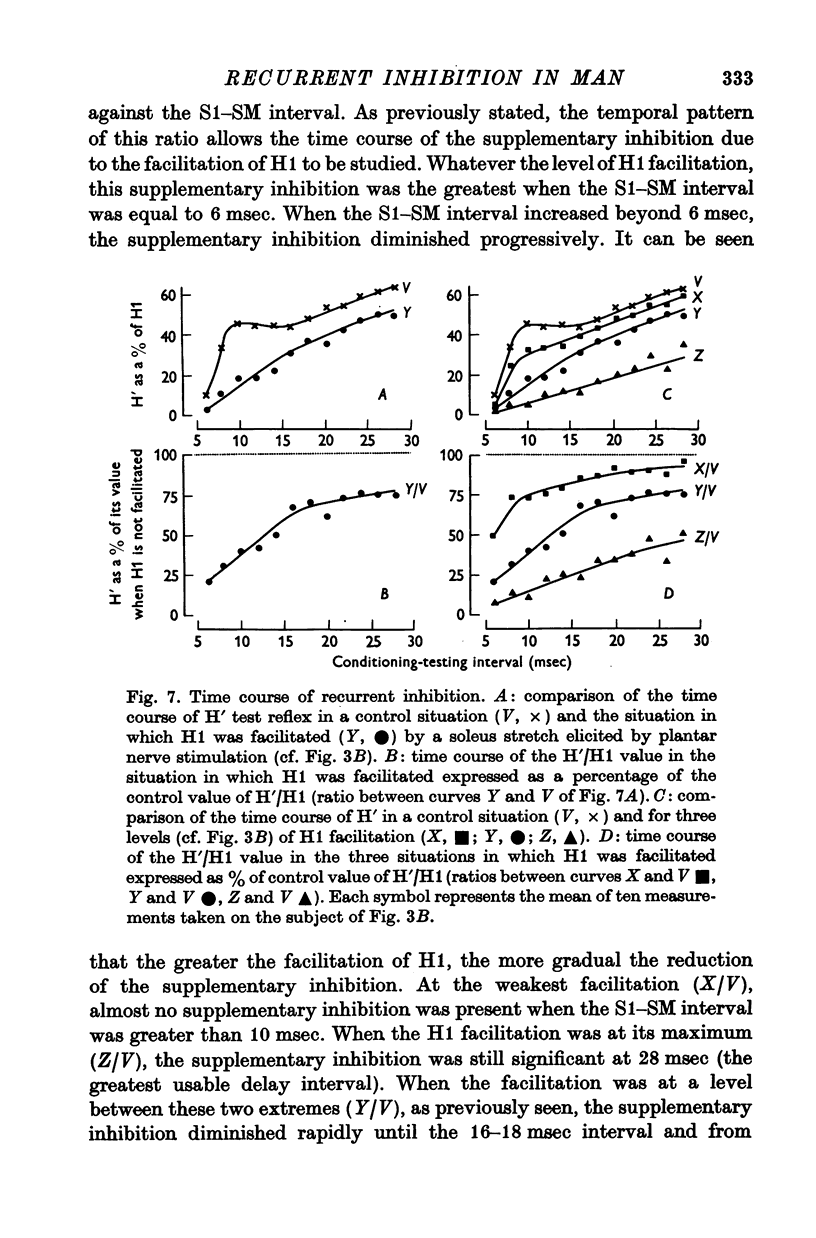
6. This inhibition decreased progressively as the time interval separating the test stimulus from the conditioning stimulus increased. The time course of this inhibition was studied with conditioning reflexes of different amplitudes. The duration of the inhibition increased with the size of the conditioning reflex.
7. These results strongly suggest that Renshaw cells excited by the conditioning reflex are responsible for this inhibition. The results are in agreement with observations made in animals on recurrent inhibition.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS V. B., WILSON V. J. Recurrent inhibition in the cat's spinal cord. J Physiol. 1959 May 19;146(2):380–391. doi: 10.1113/jphysiol.1959.sp006199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C. D., Pompeiano O. Presynaptic and postsynaptic effects in the monosyaptic reflex pathway to extensor motoneurons folowing vibration of synergic muscles. Arch Ital Biol. 1970 Apr;108(2):259–294. [PubMed] [Google Scholar]
- Buchthal F., Schmalbruch H. Contraction times and fibre types in intact human muscle. Acta Physiol Scand. 1970 Aug;79(4):435–452. doi: 10.1111/j.1748-1716.1970.tb04744.x. [DOI] [PubMed] [Google Scholar]
- Buchthal F., Schmalbruch H. Contraction times of twitches evoked by H-reflexes. Acta Physiol Scand. 1970 Nov;80(3):378–382. doi: 10.1111/j.1748-1716.1970.tb04801.x. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Group Ia synaptic input to fast and slow twitch motor units of cat triceps surae. J Physiol. 1968 Jun;196(3):605–630. doi: 10.1113/jphysiol.1968.sp008526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Motor unit types of cat triceps surae muscle. J Physiol. 1967 Nov;193(1):141–160. doi: 10.1113/jphysiol.1967.sp008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. Excitatory synaptic action in motoneurones. J Physiol. 1955 Nov 28;130(2):374–395. doi: 10.1113/jphysiol.1955.sp005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., ITO M. Distribution of recurrent inhibition among motoneurones. J Physiol. 1961 Dec;159:479–499. doi: 10.1113/jphysiol.1961.sp006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAPORTE Y., LLOYD D. P. C. Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol. 1952 Jun;169(3):609–621. doi: 10.1152/ajplegacy.1952.169.3.609. [DOI] [PubMed] [Google Scholar]
- MAGLADERY J. W., McDOUGAL D. B., Jr Electrophysiological studies of nerve and reflex activity in normal man. I. Identification of certain reflexes in the electromyogram and the conduction velocity of peripheral nerve fibers. Bull Johns Hopkins Hosp. 1950 May;86(5):265–290. [PubMed] [Google Scholar]
- Ott K. H., Gassel M. M. Methods of tendon jerk reinforcement. The role of muscle activity in reflex excitability. J Neurol Neurosurg Psychiatry. 1969 Dec;32(6):541–547. doi: 10.1136/jnnp.32.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Bussel B. Evidence for recurrent inhibition by motoneurons in human subjects. Brain Res. 1975 Apr 25;88(1):105–108. doi: 10.1016/0006-8993(75)90955-5. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Bussel B., Held J. P., Katz R. Excitability of human motoneurones after discharge in a conditioning reflex. Electroencephalogr Clin Neurophysiol. 1976 Mar;40(3):279–287. doi: 10.1016/0013-4694(76)90151-6. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Katz R., Bussel B. Influence of voluntary movement and posture on recurrent inhibition in human subjects. Brain Res. 1977 Apr 1;124(3):427–436. doi: 10.1016/0006-8993(77)90944-1. [DOI] [PubMed] [Google Scholar]
- Pompeiano O., Wand P., Sontag K. H. Excitation of Renshaw cells by orthodromic group Ia volleys following vibration of extensor muscles. Pflugers Arch. 1974 Jan 11;347(2):137–144. doi: 10.1007/BF00592395. [DOI] [PubMed] [Google Scholar]
- Ryall R. W., Piercey M. F. Excitation and inhibition of Renshaw cells by impulses in peripheral afferent nerve fibers. J Neurophysiol. 1971 Mar;34(2):242–251. doi: 10.1152/jn.1971.34.2.242. [DOI] [PubMed] [Google Scholar]
- Ryall R. W., Piercey M. F., Polosa C., Goldfarb J. Excitation of Renshaw cells in relation to orthodromic and antidromic excitation of motoneurons. J Neurophysiol. 1972 Jan;35(1):137–148. doi: 10.1152/jn.1972.35.1.137. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., TALBOT W. H., KATO M. INHIBITORY CONVERGENCE UPON RENSHAW CELLS. J Neurophysiol. 1964 Nov;27:1063–1079. doi: 10.1152/jn.1964.27.6.1063. [DOI] [PubMed] [Google Scholar]


