Abstract
1. Cortex-free adrenal glands previously labelled with the isotope 45Ca have been perfused with Locke or modified Locke solution to assess Ca2+ movements under different conditions.
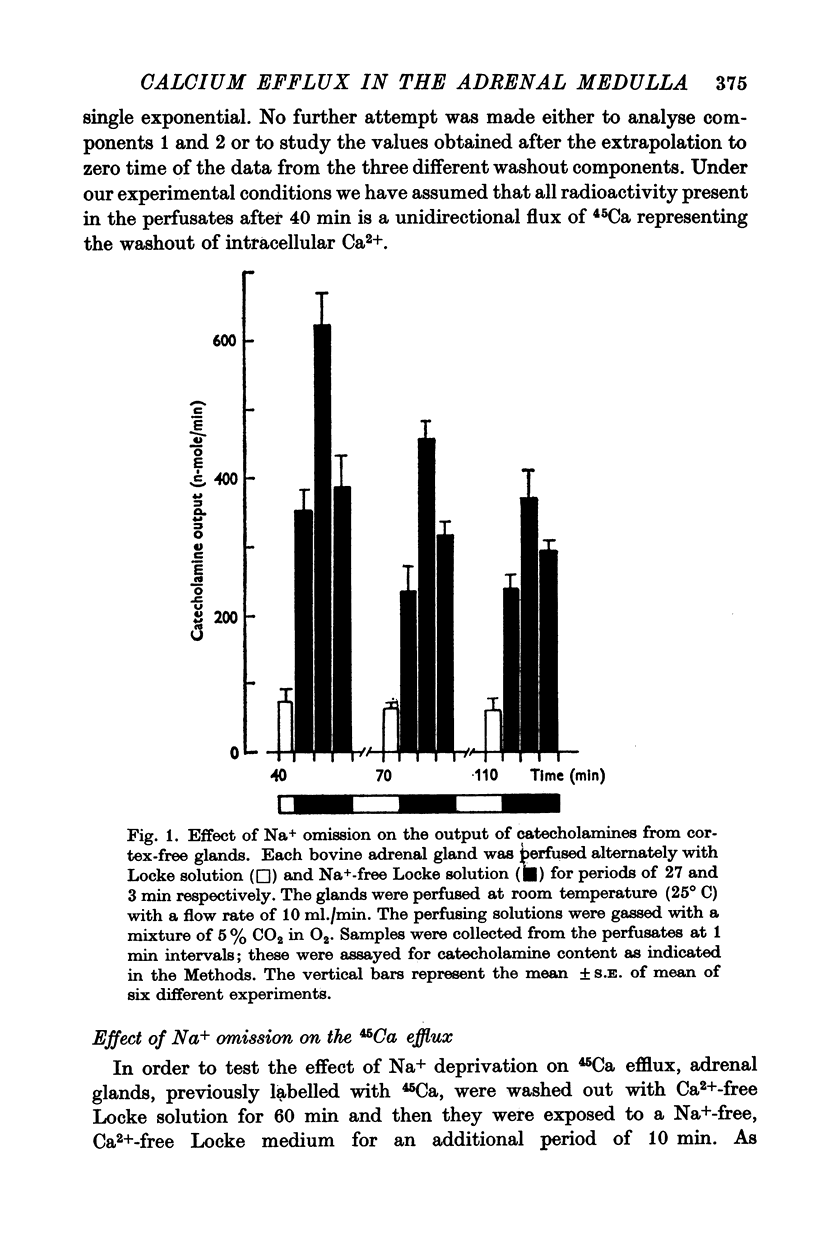
2. Substitution of Na+ by either sucrose or choline during perfusion with Ca2+-free Locke solution induced a significant and sustained decrease in the 45Ca efflux. Concomitant with this effect there was an increase in the output of catecholamines from the perfused gland.
3. In the presence of Ca2+ (2·2 mM) in the perfusion fluid, Na+ omission induced an increase in the 45Ca efflux. This increase was significantly reduced if 3 × 10-4 M methoxyverapamil (D-600) was present in the perfusion fluid. However, the increased catecholamine output in response to Na+ deprivation remained unchanged.
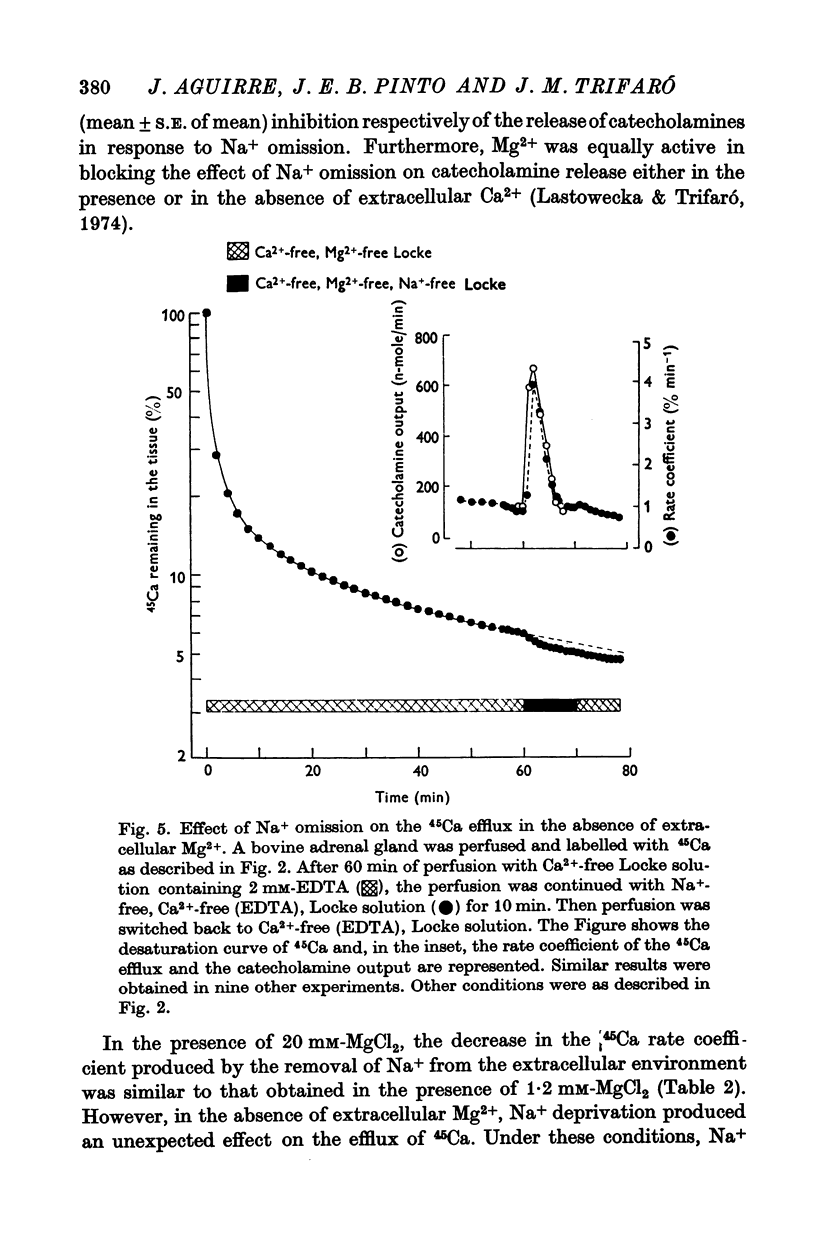
4. Excess of Mg2+ (20 mM) in the extracellular medium blocked the increase in catecholamine output in response to Na+ omission. However, the decrease in the 45Ca efflux produced by Na+ deprivation in the presence of this high concentration of Mg2+ was similar to that observed in the presence of 1·2 mM-Mg2+.
5. In the absence of Mg2+ in the extracellular medium, substitution of Na+ by either sucrose or choline induced a sharp and transient increase in the 45Ca efflux rate coefficient. This increased 45Ca efflux, which has similar time course as the enhanced catecholamine output, was not affected by the presence of 3 × 10-4 M methoxyverapamil.
6. In the absence of Mg2+, the graded substitution of Na+ in the perfusion medium by sucrose enhanced the efflux of 45Ca. This increase in the 45Ca outward movement was linearly related to the logarithm of the extracellular Na+ concentration.
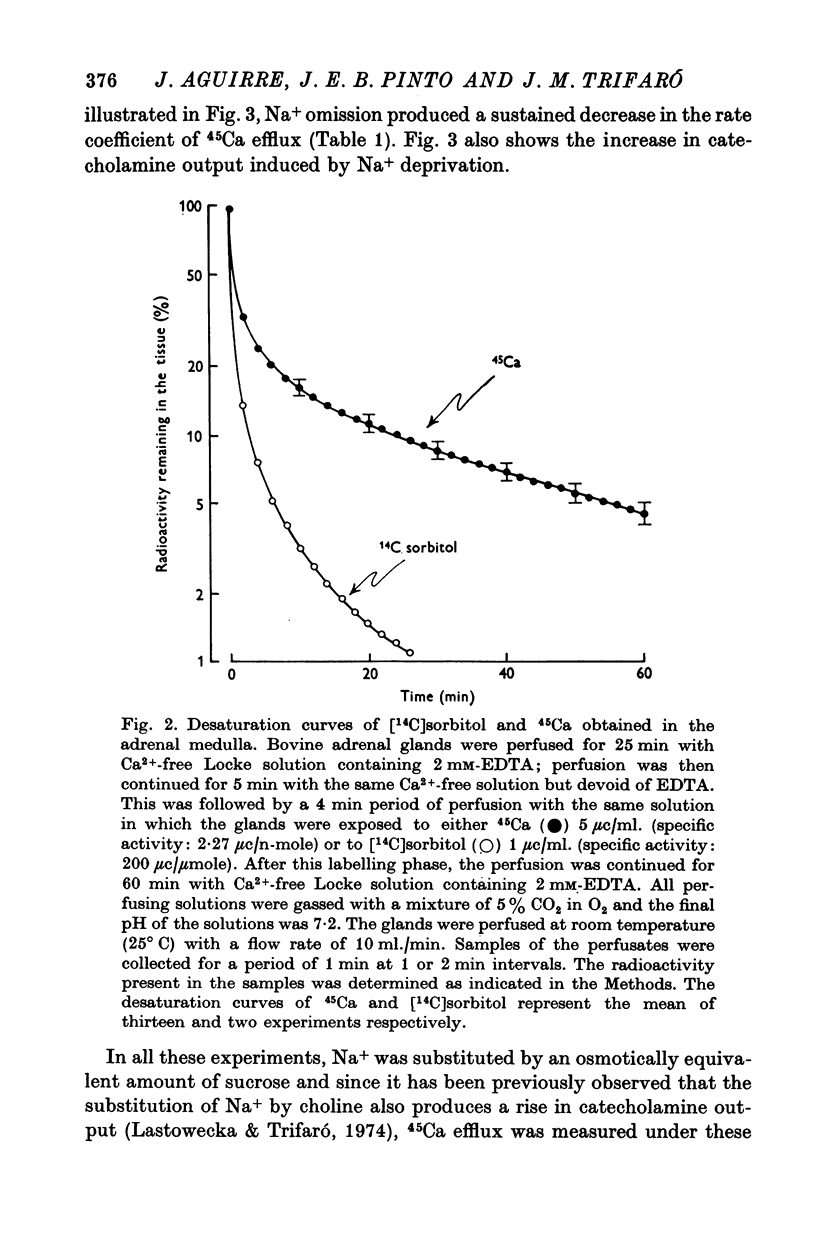
7. After perfusion of glands with Ca2+-free Locke solution, the reintroduction of Ca2+ (2·2 mM) into the perfusion fluid produced an increase in the 45Ca efflux. This was accompanied by a discharge of catecholamines.
8. Although Mg2+ (20 mM) was effective in blocking catecholamine release, this divalent cation did not modify the increase in the 45Ca efflux produced by Ca2+ reintroduction.
9. In contrast to these later observations, methoxyverapamil (3 × 10-4 M) was effective in inhibiting both increases in catecholamine output and 45Ca efflux in response to Ca2+ reintroduction.
10. It is concluded from these experiments that (a) Ca2+ movements in the adrenal medulla may involve both Na+-Ca2+ and Ca2+-Ca2+ exchange mechanisms; (b) the omission of Na+ from the extracellular environment produces not only an increase in the output of catecholamines but it may increase the intracellular levels of Ca2+ and that this may result in an increased Ca2+ efflux when Mg2+ is omitted from the perfusion fluids, and that (c) the competition between Ca2+ and Mg2+ during the secretory process may involve an intracellular site.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Ashley C. C., Ellory J. C., Hainaut K. Calcium movements in single crustacean muscle fibres. J Physiol. 1974 Oct;242(1):255–272. doi: 10.1113/jphysiol.1974.sp010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol. 1975 Dec;253(2):593–620. doi: 10.1113/jphysiol.1975.sp011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Grau J. D., Nordmann J. J. Effects on the isolated neurohypophysis of agents which affect the membrane permeability to calcium. J Physiol. 1973 Jun;231(2):96P–98P. [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein A., Grün G., Tritthart H., Byon K. Uterus-Relaxation durch hochaktive Ca plus,plus-antagonistische Hemmstoffe der elektro-mechanischen Koppelung wie Isoptin (Verapamil, Iproveratril), Substanz D 600 und Segontin (Prenylamin). Versuche am isolierten Uterus virgineller Ratten. Klin Wochenschr. 1971 Jan;49(1):32–41. doi: 10.1007/BF01494064. [DOI] [PubMed] [Google Scholar]
- Griffey M. A., Conaway H. H., Whitney J. E. Insulin secretion induced by Na+ deprivation. Endocrinology. 1974 Nov;95(5):1469–1472. doi: 10.1210/endo-95-5-1469. [DOI] [PubMed] [Google Scholar]
- Haugaard N., Haugaard E. S., Lee N. H. Role of magnesium, phosphate and ATP in the regulation of calcium uptake by rat-liver mitochondria. Proc K Ned Akad Wet C. 1969;72(1):1–15. [PubMed] [Google Scholar]
- Kanno T., Cochrane D. E., Douglas W. W. Exocytosis (secretory granule extrusion) induced by injection of calcium into mast cells. Can J Physiol Pharmacol. 1973 Dec;51(12):1001–1004. doi: 10.1139/y73-153. [DOI] [PubMed] [Google Scholar]
- Lastowecka A., Trifaró J. M. The effect of sodium and calcium ions on the release of catecholamines from the adrenal medulla: sodium deprivation induces release by exocytosis in the absence of extracellular calcium. J Physiol. 1974 Feb;236(3):681–705. doi: 10.1113/jphysiol.1974.sp010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C. J., van Breemen C., Casteels T. The action of lanthanum and D600 on the calcium exchange in the smooth muscle cells of the guinea-pig Taenia coli. Pflugers Arch. 1972;337(4):333–350. doi: 10.1007/BF00586650. [DOI] [PubMed] [Google Scholar]
- Nielsen S. P., Petersen O. H. Transport of calcium in the perfused submandibular gland of the cat. J Physiol. 1972 Jun;223(3):685–697. doi: 10.1113/jphysiol.1972.sp009869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J. E., Trifaró J. M. The different effects of D-600 (methoxyverapamil) on the release of adrenal catecholamines induced by acetylcholine, high potassium or sodium deprivation. Br J Pharmacol. 1976 May;57(1):127–132. doi: 10.1111/j.1476-5381.1976.tb07662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisner A. M., Hava M. The role of adenosine triphosphate and adenosine triphosphatase in the release of catecholamines from the adrenal medulla. IV. Adenosine triphosphate-- activated uptake of calcium by microsomes and mitochondria. Mol Pharmacol. 1970 Jul;6(4):407–415. [PubMed] [Google Scholar]
- Russell J. T., Thorn N. A. Calcium and stimulus-secretion coupling in the neurohypophysis. II. Effects of lanthanum, a verapamil analogue (D600) and prenylamine on 45-calcium transport and vasopressin release in isolated rat neurohypophyses. Acta Endocrinol (Copenh) 1974 Jul;76(3):471–487. [PubMed] [Google Scholar]
- Trifaro J. M., Dworkind J. Phosphorylation of the membrane components of chromaffin granules: synthesis of diphosphatidylinositol and presence of phosphatidylinositol kinase in granule membranes. Can J Physiol Pharmacol. 1975 Jun;53(3):479–492. doi: 10.1139/y75-068. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Duerr A. C. Isolation and characterization of a Golgi-rich fraction from the adrenal medulla. Biochim Biophys Acta. 1976 Jan 14;421(1):153–167. doi: 10.1016/0304-4165(76)90179-3. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Poisner A. M., Douglas W. W. The fate of the chromaffin granule during catecholamine release from the adrenal medulla. I. Unchanged efflux of phospholipid and cholesterol. Biochem Pharmacol. 1967 Nov;16(11):2095–2100. doi: 10.1016/0006-2952(67)90006-8. [DOI] [PubMed] [Google Scholar]
- Winkler H., Schöpf J. A., Hörtnagl H. Bovine adrenal medulla: subcellular distribution of newly synthesised catecholamines, nucleotides and chromogranins. Naunyn Schmiedebergs Arch Pharmacol. 1972;273(1):43–61. doi: 10.1007/BF00508079. [DOI] [PubMed] [Google Scholar]


