Abstract
1. The properties of the rat alveolar lining film have been studied by observing the behaviour in a hanging drop, under reduced or increased ambient pressure, of bubbles derived from the lung.
2. When such a bubble, covered by a metastable film of surfactant, is made to shrink, the material displaced from the surface usually remains in a form in which it can be re-adsorbed to the surface and retains its surpellic properties.
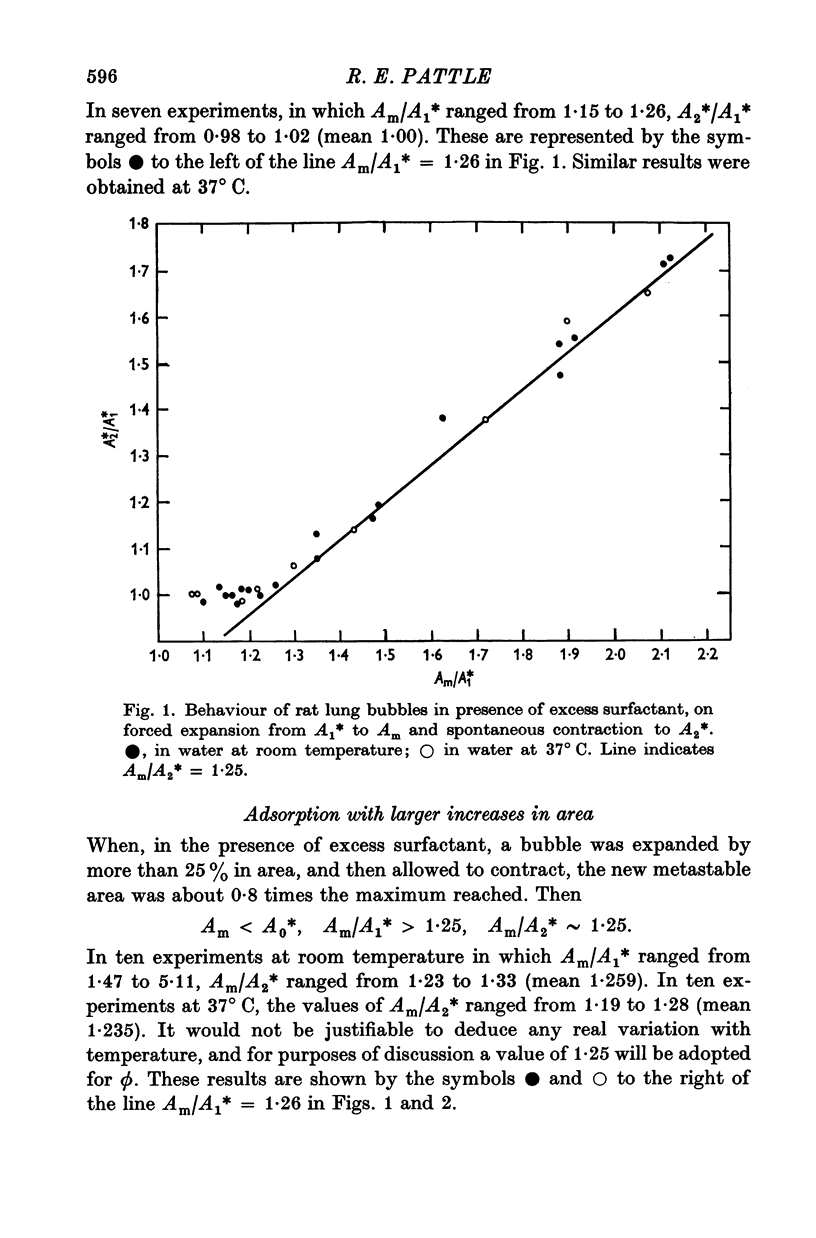
3. When an excess of surfactant is available for adsorption to the surface of such a bubble in water, an increase in area to about 1.25 (ϕ) times the metastable area is both necessary and sufficient for additional adsorption to the surface to take place.
4. No significant variation of the ratio ϕ with temperature between 22 and 37° C has been found.
5. It is concluded that during quiet breathing (involving a twofold change in lung volume in the rat) the variation in alveolar surface area is less than 25%. This finding is compatible with the extant morphometric data, but not with any assumption that the surface area is proportional to the 2/3 power of the gas volume.
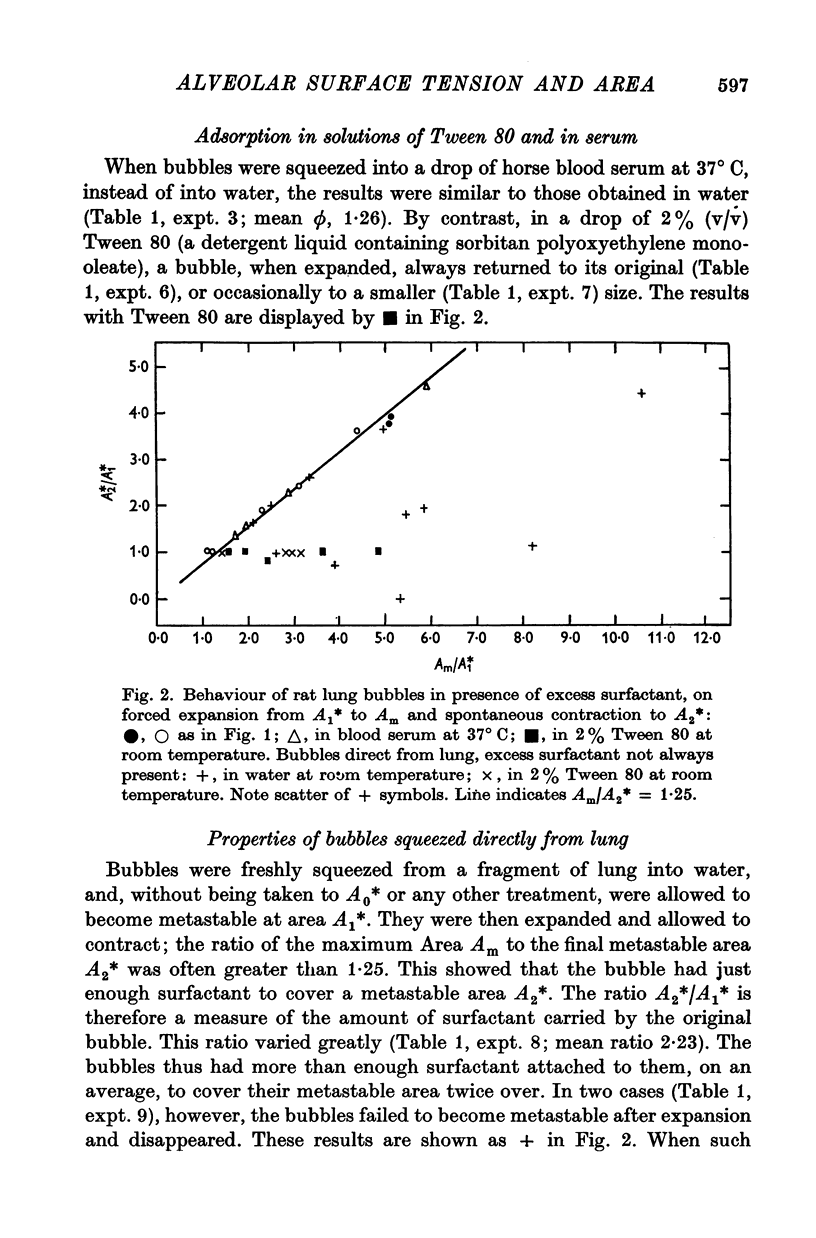
6. The behaviour of the bubbles in blood serum is similar to that in water. In a 2% solution of the detergent Tween 80, further adsorption of surfactant to the bubble surface does not take place.
7. The fact that bubbles obtained from the lung by instillation of a solution of Tween 80 have surfactant linings similar to those of bubbles obtained with water or saline demonstrates that the bubble lining layer consists of the original alveolar lining layer detached.
8. When a metastable bubble is stretched, it sometimes behaves as if some or all of its surfactant had been lost from the surface. The causes of this are unknown.
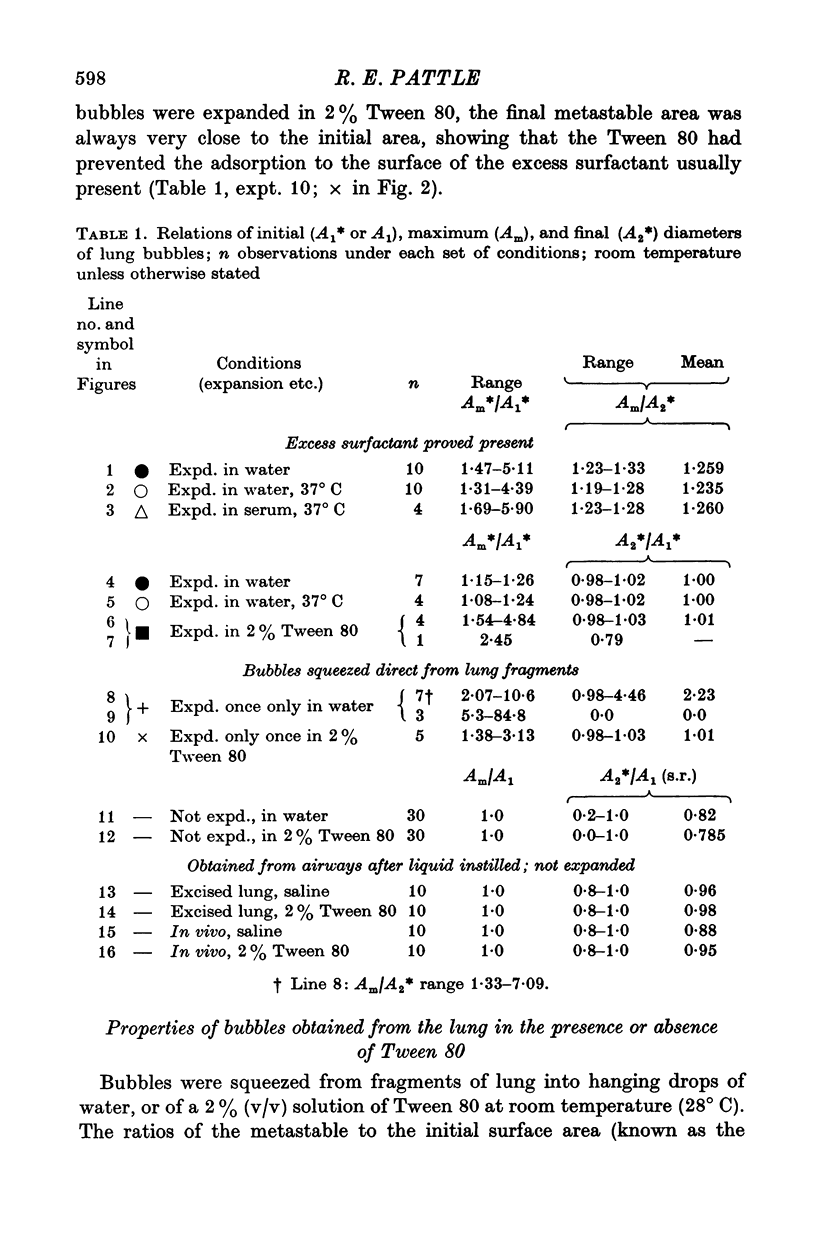
9. The amount of surfactant associated with metastable bubbles freshly squeezed from a fragment of lung varies from bubble to bubble; the amounts found have ranged from 1 to 4.5 times (mean, 2) that required to cover the original bubble area with a metastable film. This would be compatible with an alveolar lining film of very uneven thickness.
10. The maximum surface tension reached in the early stages of expansion of a lung bubble from the metastable state is about 34 mN/m.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery M. E. What is new in our understanding of perinatal pulmonary problems? Presidential address to the Society for Pediatric Research, May 19, 1973. Pediatr Res. 1973 Oct;7(10):842–845. doi: 10.1203/00006450-197310000-00008. [DOI] [PubMed] [Google Scholar]
- BENDIXEN H. H., HEDLEY-WHYTE J., LAVER M. B. IMPAIRED OXYGENATION IN SURGICAL PATIENTS DURING GENERAL ANESTHESIA WITH CONTROLLED VENTILATION. A CONCEPT OF ATELECTASIS. N Engl J Med. 1963 Nov 7;269:991–996. doi: 10.1056/NEJM196311072691901. [DOI] [PubMed] [Google Scholar]
- Bachofen H., Hildebrandt J., Bachofen M. Pressure-volume curves of air- and liquid-filled excised lungs-surface tension in situ. J Appl Physiol. 1970 Oct;29(4):422–431. doi: 10.1152/jappl.1970.29.4.422. [DOI] [PubMed] [Google Scholar]
- Brooks R. E. Lung surfactant: an alternate hypothesis. Am Rev Respir Dis. 1971 Oct;104(4):585–586. doi: 10.1164/arrd.1971.104.4.585. [DOI] [PubMed] [Google Scholar]
- CLEMENTS J. A., HUSTEAD R. F., JOHNSON R. P., GRIBETZ I. Pulmonary surface tension and alveolar stability. J Appl Physiol. 1961 May;16:444–450. doi: 10.1152/jappl.1961.16.3.444. [DOI] [PubMed] [Google Scholar]
- CROSFILL M. L., WIDDICOMBE J. G. Physical characteristics of the chest and lungs and the work of breathing in different mammalian species. J Physiol. 1961 Sep;158:1–14. doi: 10.1113/jphysiol.1961.sp006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland D. E. Fine structural study of gas secretion in the physoclistous swim bladder of Fundulus heteroclitus and Gadus callarias and in the euphysoclistous swim bladder of Opsanus tau. Z Zellforsch Mikrosk Anat. 1969;93(3):305–331. doi: 10.1007/BF00332659. [DOI] [PubMed] [Google Scholar]
- Dermer G. B. The fixation of pulmonary surfactant for electron microscopy. I. The alveolar surface lining layer. J Ultrastruct Res. 1969 Apr;27(1):88–104. [PubMed] [Google Scholar]
- Douglas F. G., Chong P. Y., Finlayson D. C. Effect of artificial ventilation on lung mechanics in dogs. J Appl Physiol. 1974 Sep;37(3):324–328. doi: 10.1152/jappl.1974.37.3.324. [DOI] [PubMed] [Google Scholar]
- Gil J., Reiss O. K. Isolation and characterization of lamellar bodies and tubular myelin from rat lung homogenates. J Cell Biol. 1973 Jul;58(1):152–171. doi: 10.1083/jcb.58.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J., Weibel E. R. Morphological study of pressure-volume hysteresis in rat lungs fixed by vascular perfusion. Respir Physiol. 1972 Jun;15(2):190–213. doi: 10.1016/0034-5687(72)90098-9. [DOI] [PubMed] [Google Scholar]
- Herxheimer H. The unsteady tidal air level and air trapping. J Physiol. 1975 Mar;246(2):49P–50P. [PubMed] [Google Scholar]
- Hills B. A. Effects of DPL at mercury-water interfaces and estimation of lung surface area. J Appl Physiol. 1974 Jan;36(1):41–44. doi: 10.1152/jappl.1974.36.1.41. [DOI] [PubMed] [Google Scholar]
- KLAUS M. H., CLEMENTS J. A., HAVEL R. J. Composition of surface-active material isolated from beef lung. Proc Natl Acad Sci U S A. 1961 Nov 15;47:1858–1859. doi: 10.1073/pnas.47.11.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. J., Clements J. A. Surface active materials from dog lung. II. Composition and physiological correlations. Am J Physiol. 1972 Sep;223(3):715–726. doi: 10.1152/ajplegacy.1972.223.3.715. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E. Properties, function and origin of the alveolar lining layer. Nature. 1955 Jun 25;175(4469):1125–1126. doi: 10.1038/1751125b0. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E. Properties, function, and origin of the alveolar lining layer. Proc R Soc Lond B Biol Sci. 1958 Feb 18;148(931):217–240. doi: 10.1098/rspb.1958.0015. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E. SURFACE LINING OF LUNG ALVEOLI. Physiol Rev. 1965 Jan;45:48–79. doi: 10.1152/physrev.1965.45.1.48. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E., THOMAS L. C. Lipoprotein composition of the film lining the lung. Nature. 1961 Mar 11;189:844–844. doi: 10.1038/189844a0. [DOI] [PubMed] [Google Scholar]
- Pattle R. E. Proceedings: Ratio of area at which surface tension of the lung lining is about 30 mN/m, to area at which surface tension is very low. J Physiol. 1976 Jun;258(1):32P–33P. [PubMed] [Google Scholar]
- Pattle R. E., Schock C., Battensby J. Some effects of anaesthetics on lung surfactant. Br J Anaesth. 1972 Nov;44(11):1119–1127. doi: 10.1093/bja/44.11.1119. [DOI] [PubMed] [Google Scholar]
- SUTNICK A. I., SOLOFF L. A. SURFACE TENSION REDUCING ACTIVITY IN THE NORMAL AND ATELECTATIC HUMAN LUNG. Am J Med. 1963 Jul;35:31–36. doi: 10.1016/0002-9343(63)90161-x. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Morphological basis of alveolar-capillary gas exchange. Physiol Rev. 1973 Apr;53(2):419–495. doi: 10.1152/physrev.1973.53.2.419. [DOI] [PubMed] [Google Scholar]
- Williams J. V., Tierney D. F., Parker H. R. Surface forces in the lung, atelectasis, and transpulmonary pressure. J Appl Physiol. 1966 May;21(3):819–827. doi: 10.1152/jappl.1966.21.3.819. [DOI] [PubMed] [Google Scholar]


