Abstract
1. Intracellular micro-electrode recordings of acinar cell membrane potential and resistance were made from the mouse pancreas superfused in vitro. The acinar cells under investigation were stimulated by micro-iontophoretic ACh application from an extracellular AChCl-filled micro-electrode.
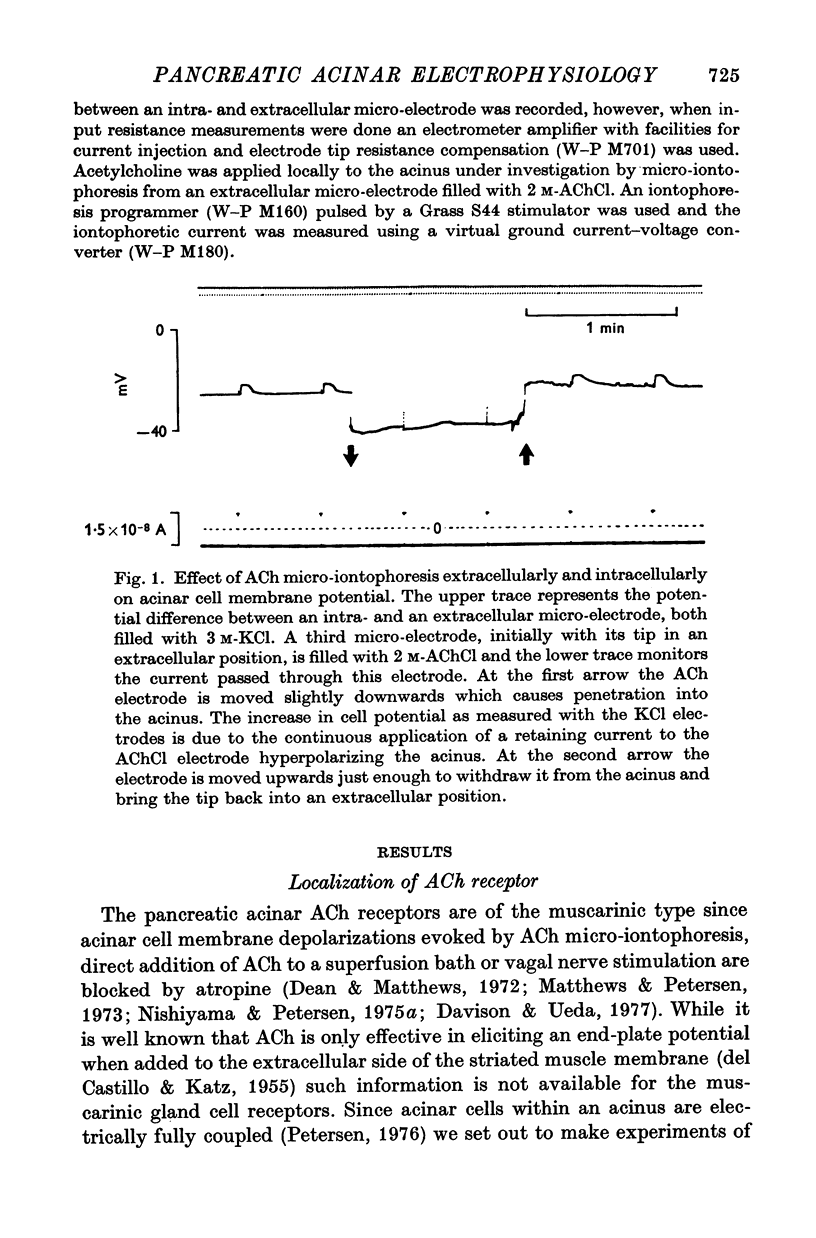
2. Passing short-lasting ejecting current pulses through the AChCl-electrode caused acinar cell depolarization when the electrode was in an extracellular position not far (< 50 μm) from an acinus impaled by a KCl micro-electrode. After insertion of the AChCl electrode into a neighbouring acinar cell, electrically coupled to the acinar cell already impaled by the KCl-electrode, ejecting ACh current pulses only affected the membrane potential in a direct electrical manner whereas there was no sign of an effect of ACh on the membrane potential.
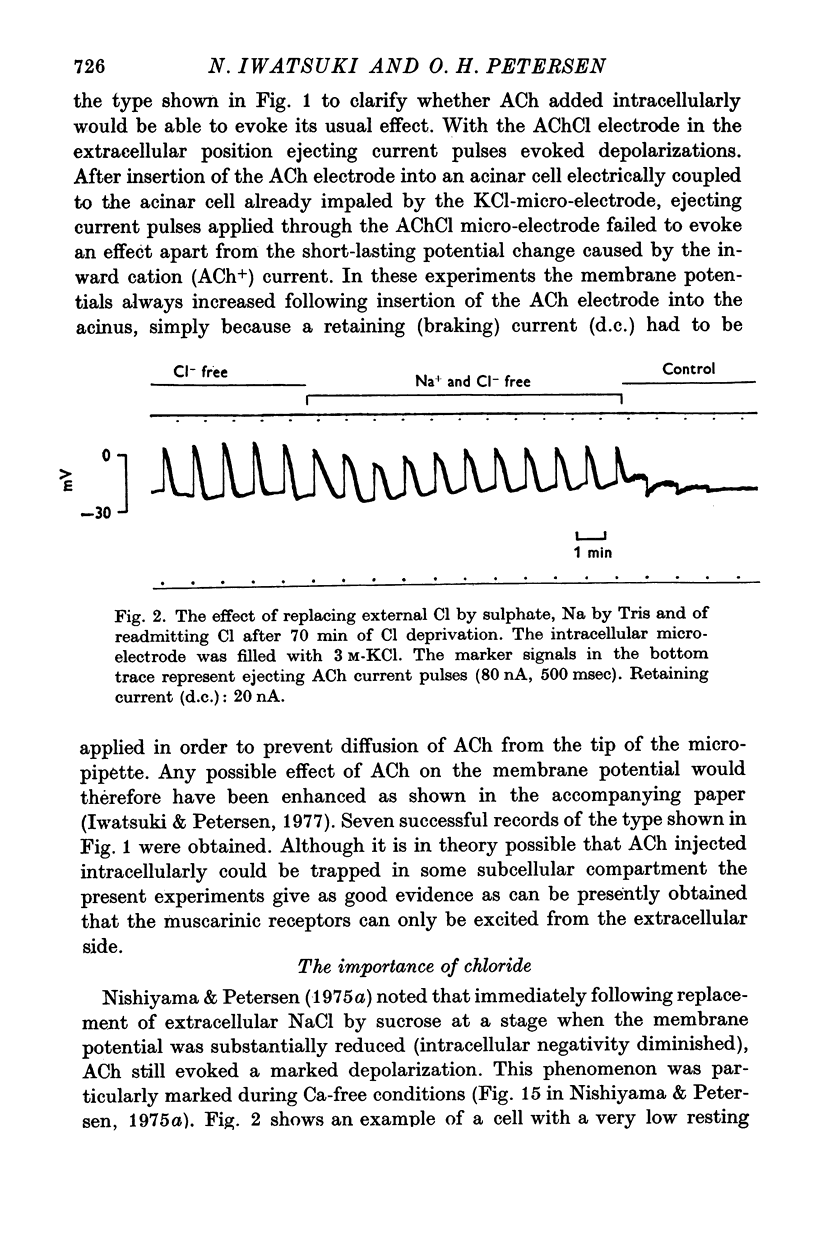
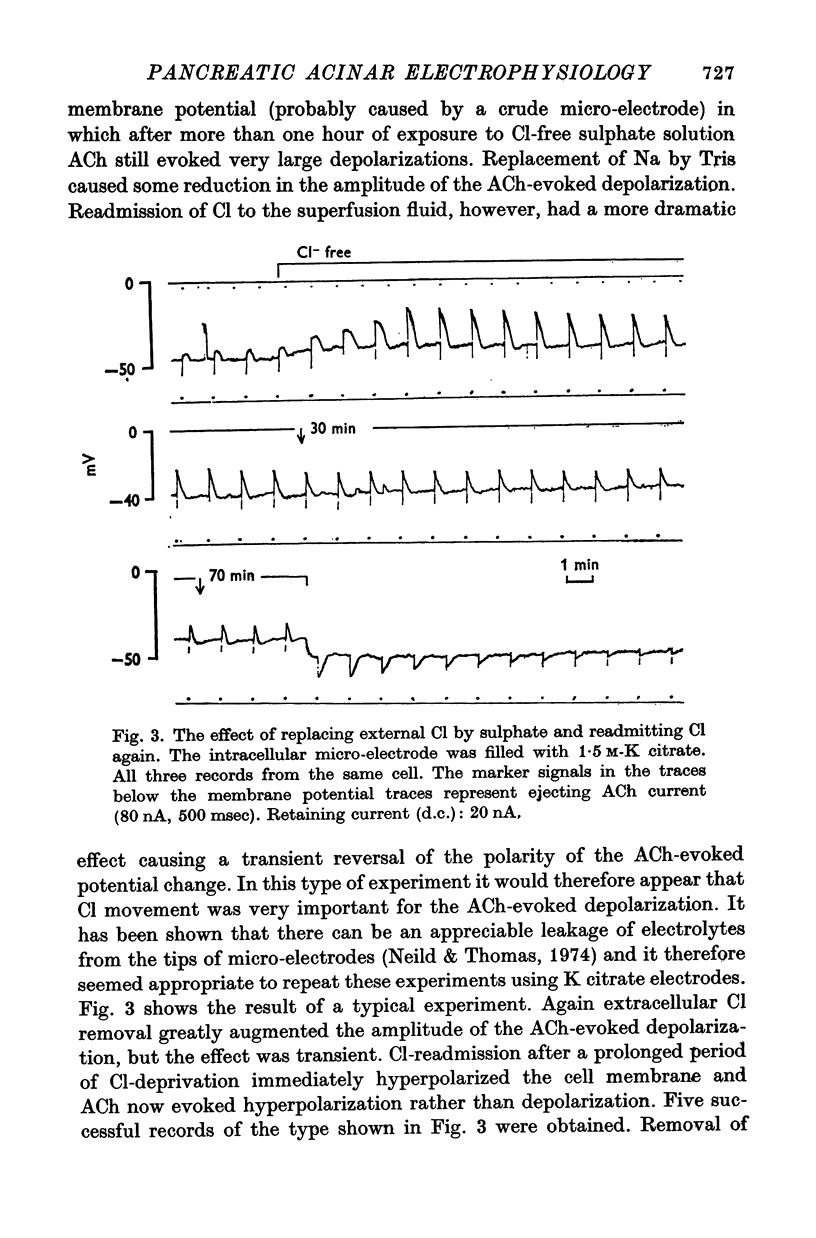
3. Replacing extracellular chloride by sulphate caused a marked increase in the amplitude of the ACh-evoked depolarization. If the membrane potential was recorded with a KCl electrode ACh continued to evoke very large depolarizations even after more than 1 hr exposure to Cl-free solution. If the membrane potential was recorded with a K-citrate electrode the effect of Cl-removal was only transient. Removal of Na+ during exposure to Cl-free solution reduced the amplitude of the ACh-evoked depolarization somewhat. Readmission of Cl after more than 1 hr of Cl deprivation caused an immediate reversal of the ACh effect into a hyperpolarization.
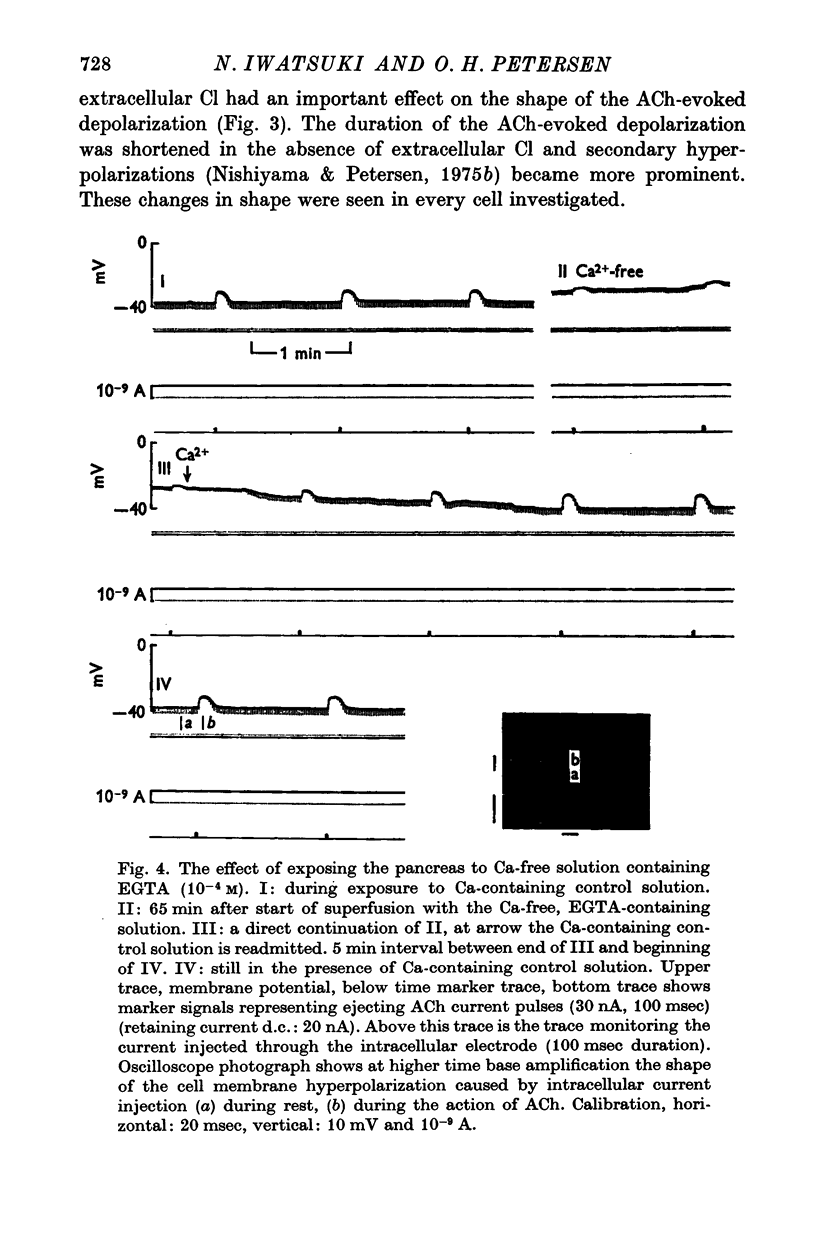
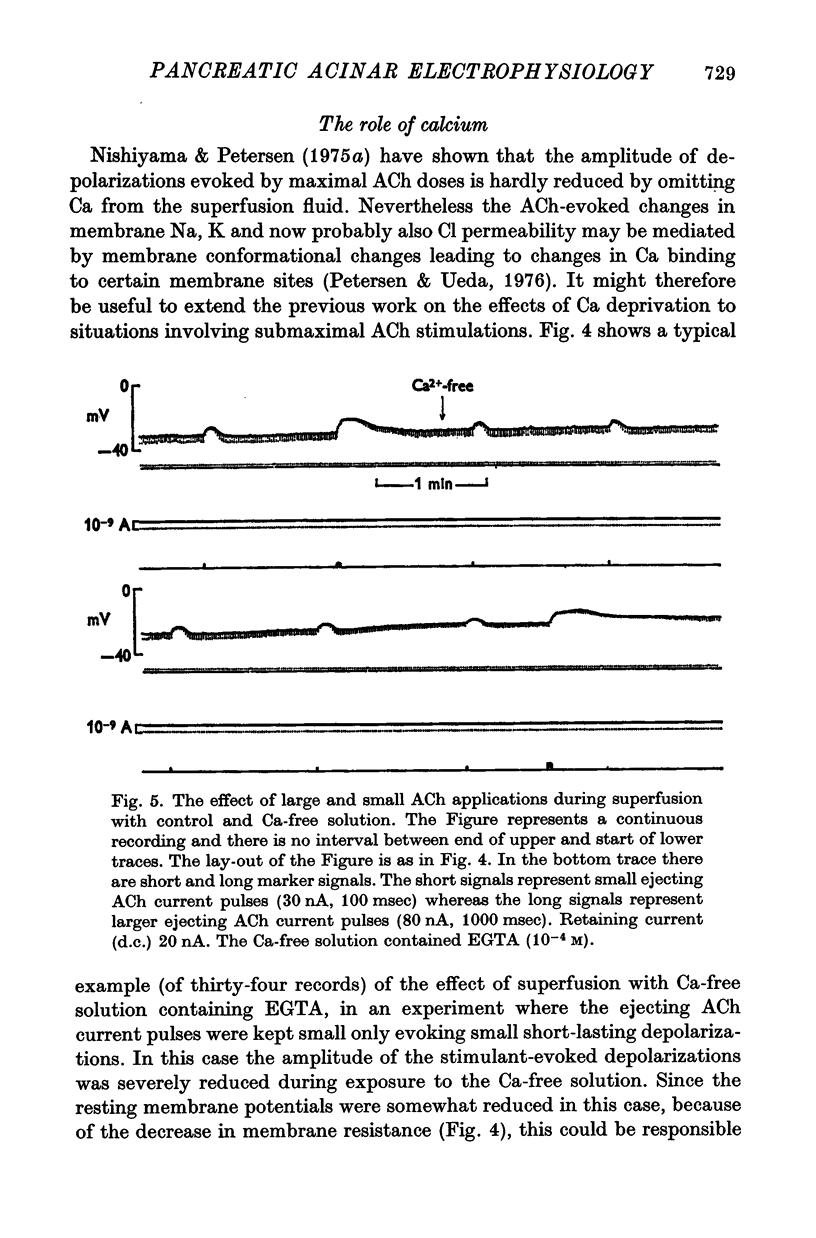
4. Removal of extracellular Ca2+ caused a marked reduction in the amplitude of small depolarizations evoked by just suprathreshold doses of ACh, whereas there was very little effect on larger depolarizations evoked by maximal or supramaximal ACh ejections. The effect of Ca removal was fully reversible. Addition of Mn after Ca-deprivation was as efficient as Ca in restoring normal electrophysiological responses to small doses of ACh.
5. The acinar cell membrane seems only to be responsive to ACh added to the extracellular side and ACh probably causes an increase in membrane Cl permeability in addition to the previously described effects on Na and K permeability. Ca may be important in determining ACh receptor sensitivity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison J. S., Ueda N. Depolarization of rat pancreatic acinar cells by cervical vagal stimulation [proceedings]. J Physiol. 1977 Mar;266(1):39P–40P. [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Pancreatic acinar cells: measurement of membrane potential and miniature depolarization potentials. J Physiol. 1972 Aug;225(1):1–13. doi: 10.1113/jphysiol.1972.sp009926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W. Generation of end-plate potentials. Physiol Rev. 1976 Jan;56(1):177–247. doi: 10.1152/physrev.1976.56.1.177. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: the acetylcholine equilibrium potential and its ionic dependency. J Physiol. 1977 Aug;269(3):735–751. doi: 10.1113/jphysiol.1977.sp011926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H. Pancreatic acinar cells: ionic dependence of the membrane potential and acetycholine-induced depolarization. J Physiol. 1973 Jun;231(2):283–295. doi: 10.1113/jphysiol.1973.sp010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O., Thomas R. C. Intracellular chloride activity and the effects of acetylcholine in snail neurones. J Physiol. 1974 Oct;242(2):453–470. doi: 10.1113/jphysiol.1974.sp010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Biphasic membrane potential changes in pancreatic acinar cells following short pulses of acetylcholine stimulation. Proc R Soc Lond B Biol Sci. 1975 Dec 16;191(1105):549–553. doi: 10.1098/rspb.1975.0144. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: ionic dependence of acetylcholine-induced membrane potential and resistance change. J Physiol. 1975 Jan;244(2):431–465. doi: 10.1113/jphysiol.1975.sp010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J Physiol. 1974 Apr;238(1):145–158. doi: 10.1113/jphysiol.1974.sp010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. Electrophysiology of mammalian gland cells. Physiol Rev. 1976 Jul;56(3):535–577. doi: 10.1152/physrev.1976.56.3.535. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Ueda N. Pancreatic acinar cells: the role of calcium in stimulus-secretion coupling. J Physiol. 1976 Jan;254(3):583–606. doi: 10.1113/jphysiol.1976.sp011248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEYER L. H., SCHNEYER C. A. Electrolyte and inulin spaces of rat salivary glands and pancreas. Am J Physiol. 1960 Oct;199:649–652. doi: 10.1152/ajplegacy.1960.199.4.649. [DOI] [PubMed] [Google Scholar]


