Abstract
Here we demonstrate that fruit from tomato (Lycopersicon esculentum) plants expressing Arabidopsis (Arabidopsis thaliana) H+/cation exchangers (CAX) have more calcium (Ca2+) and prolonged shelf life when compared to controls. Previously, using the prototypical CAX1, it has been demonstrated that, in yeast (Saccharomyces cerevisiae) cells, CAX transporters are activated when the N-terminal autoinhibitory region is deleted, to give an N-terminally truncated CAX (sCAX), or altered through specific manipulations. To continue to understand the diversity of CAX function, we used yeast assays to characterize the putative transport properties of CAX4 and N-terminal variants of CAX4. CAX4 variants can suppress the Ca2+ hypersensitive yeast phenotypes and also appear to be more specific Ca2+ transporters than sCAX1. We then compared the phenotypes of sCAX1- and CAX4-expressing tomato lines. The sCAX1-expressing tomato lines demonstrate increased vacuolar H+/Ca2+ transport, when measured in root tissue, elevated fruit Ca2+ level, and prolonged shelf life but have severe alterations in plant development and morphology, including increased incidence of blossom-end rot. The CAX4-expressing plants demonstrate more modest increases in Ca2+ levels and shelf life but no deleterious effects on plant growth. These findings suggest that CAX expression may fortify plants with Ca2+ and may serve as an alternative to the application of CaCl2 used to extend the shelf life of numerous agriculturally important commodities. However, judicious regulation of CAX transport is required to assure optimal plant growth.
Calcium (Ca2+) plays a fundamental role in plant membrane stability, cell wall stabilization, and cell integrity (Hirschi, 2004). Reduced Ca2+ in edible plant tissues negatively impacts total yield. Plant tissues low in Ca2+ are more susceptible than tissues with normal Ca2+ levels to some parasitic diseases during storage (Marschner, 1995). This is of particular concern in the case of fleshy fruits with their typically low Ca2+ levels. Application of Ca2+ to soils seems to be of questionable value (Lester and Grusak, 1999; Lopez-Lefebre et al., 2001); however, supplemental Ca2+ applied immediately before or just after harvest has been shown to maintain cell turgor, plasma membrane integrity, and fruit firmness and extend storage life (Gerasopoulos et al., 1996; Miklus and Beelman, 1996). In these treatments, application of Ca2+ increases total Ca2+ levels in the fruits by 20% to 40%; however, these procedures are labor intensive and often result in tissue damage and fungal infections.
The problems associated with low plant Ca2+ levels can be attributed to soil problems. For example, Ca2+ deficiencies are favored by very low soil pH and on soils high in magnesium and potassium. Probably the most recognizable Ca2+deficiency is blossom-end rot (BER) of tomato (Lycopersicon esculentum) fruits, which is induced by water stress (Bennett, 1993; Ho and White, 2005). At the time of fruit set, cells at the blossom end of fruits are injured. This is caused by insufficient Ca2+ translocation resulting in a dry-rot area on the expanding fruit.
One molecular-genetic approach to alter the Ca2+ levels in plants is to engineer high expression of Ca2+ transporters and Ca2+-binding proteins (Wyatt et al., 2002; Hirschi, 2004). Increased expression of a modified vacuolar Ca2+ antiporter, cation exchanger 1 (CAX1), in plants causes dramatic increases in Ca2+ when compared to vector control plants (Hirschi, 1999; Park et al., 2004, 2005). In a similar fashion, expression in tobacco (Nicotiana tabacum) of a wheat (Triticum aestivum) cation transporter LCT1 increases shoot Ca2+ levels (Antosiewicz and Hennig, 2004). Recent findings suggest that the corresponding changes in plant growth and development depend on the levels of transporter activity and the particular plant being modified.
The CAX1 cDNA that has been expressed in various plants was originally cloned through a yeast (Saccharomyces cerevisiae) suppression screen (Hirschi et al., 1996). We have identified a full-length CAX1 cDNA (previously termed long-CAX1) that is identical to the original clone except that it encodes a protein with an additional 36 amino acids at the N terminus (Pittman and Hirschi, 2001). This CAX1 cannot transport Ca2+ in a yeast biochemical assay, and we have shown that the N terminus functions as a regulatory region (Pittman et al., 2002). The original CAX1 is a partial-length cDNA that is deregulated for H+/Ca2+ antiport and is now termed N-terminally truncated CAX 1 (sCAX1). It is this activated form of CAX1 that when expressed in tobacco, carrots (Daucus carota), and potatoes (Solanum tuberosum) results in increased Ca2+ accumulation (Hirschi, 1999; Park et al., 2004, 2005). Recent work in yeast suggests that full-length CAX transporters may have some weak H+/Ca2+ transport capabilities (Cheng et al., 2005). In addition, various domains within the CAX transporters have been identified that confer substrate specificity. For example, the Ca2+ domain of CAX1 appears to be necessary for Ca2+ transport (Shigaki et al., 2001). An unexamined means of marginally increasing activity of H+/Ca2+ antiporters may be to express the entire CAX open reading frame or mutant CAX variants in plants.
Arabidopsis (Arabidopsis thaliana) appears to have up to 12 putative CAX transporters (Mäser et al., 2001). Like sCAX1, sCAX2 and sCAX4 can function in yeast as H+/Ca2+ exchangers (Hirschi et al., 1996; Cheng et al., 2002; Pittman et al., 2004b). Our preliminary biochemical analysis of sCAX1 and sCAX2 suggests transport of various metals (Mäser et al., 2001; Shigaki et al., 2003; Pittman et al., 2004b). However, the specific transport properties of numerous CAX transporters, including CAX4, have not been previously examined. Hence, efforts to specifically alter the Ca2+ levels in plants may be more efficient utilizing regulated expression of CAX transporters other than sCAX1.
Our primary focus here was to evaluate the potential for increasing the Ca2+ levels of tomatoes through expression of Arabidopsis H+/Ca2+ transporters and its potential impact on plant growth and development. Herein, utilizing yeast assays, we have characterized the transport properties of variants of CAX4. We also compare and contrast the effect of sCAX1 and CAX4 expression in tomato as a method to determine if utilizing full-length CAX expression may be a means to reduce deleterious phenotypes. This study suggests that modulation of Ca2+ transporters could make an important contribution toward increasing the value of various agriculturally important crops.
RESULTS
Transport Activity and Cation Selectivity Comparisons of CAX4 Variants
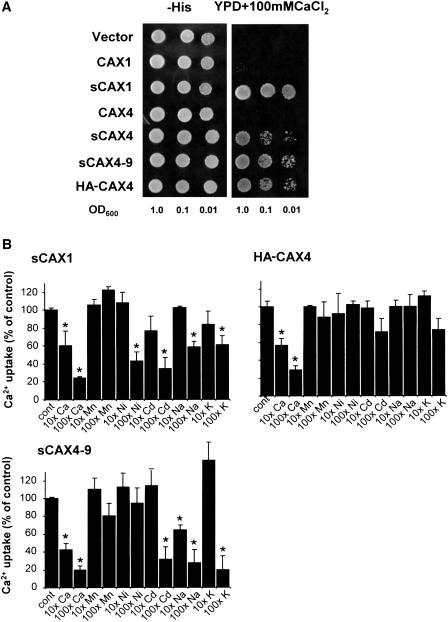
When expressed in yeast, the N-terminal region of CAX transporters acts as an autoinhibitory domain for H+/Ca2+ transport activity (Pittman et al., 2002, 2004b). To further examine the properties of CAX4 transporters with (CAX) and without (sCAX) the putative N-terminal autoinhibitory domain, we expressed these plasmids in a yeast strain deficient in vacuolar Ca2+ transporters and lacking functional calcineurin (Cunningham and Fink, 1996; Hirschi et al., 1996). This strain (K667) lacks the endogenous vacuolar Ca2+-ATPase PMC1 and vacuolar H+/Ca2+ antiporter VCX1 and thus is defective in vacuolar Ca2+ transport, making it unable to grow on high Ca2+ media (Cunningham and Fink, 1996). We examined the growth of yeast cells expressing empty vector control, sCAX1, and full-length CAX1 and CAX4 in media containing 50 mm CaCl2. Under these semipermissive conditions, the vector control and CAX4-expressing strains grew poorly; however, the full-length CAX1 and truncated sCAX1-expressing cells suppressed the Ca2+ sensitivity, although the suppression by CAX1 was weaker than sCAX1 (data not shown). We then tested those constructs and CAX4 variants (Cheng et al., 2002) on higher Ca2+-containing media (100 mm CaCl2). One chimeric CAX4 construct has a 37-amino acid N-terminal truncation and contains the 9-amino acid CAX1 Ca2+ domain (Shigaki et al., 2001; sCAX4-9), while the other form contains a triple copy of the epitope tag hemagglutinin (HA) fused to the N terminus of full-length CAX4 (HA-CAX4). As shown in Figure 1A, CAX4 variant-expressing cells can strongly suppress the Ca2+ sensitivity in a similar manner to sCAX1.
Figure 1.
Substrate characteristics of CAX4 variants expressed in yeast. A, Suppression of Ca2+ sensitivity of the pmc1 cnb vcx1 yeast mutant (K667) by CAX1, sCAX1, CAX4, sCAX4, sCAX4-9, and HA-CAX4. Saturated liquid cultures of K667 containing various plasmids were diluted to the cell densities, as indicated, and then spotted onto selection medium (−His) and yeast-extract peptone dextrose (YPD) medium containing 100 mm CaCl2. B, Inhibition of Ca2+ uptake by sCAX1, HA-CAX4, and sCAX4-9 into yeast vacuolar membrane vesicles in the presence of other metals. ΔpH-dependent uptake of 10 μm 45Ca2+, estimated as the difference between uptake with and without 5 μm FCCP, was measured in the absence (control) or presence of 10 × (100 μm) or 100 × (1 mm) of nonradioactive CaCl2, MnCl2, CdCl2, ZnCl2, NiCl2, NaCl, or KCl after 10 min. Ca2+ uptake values are shown following subtraction of the FCCP background values and expressed as percentages of the control in the absence of any excess nonradiolabeled metals. The data represent means of two to four replications from two to three independent membrane preparations, and the bars indicate se. An asterisk indicates significant difference from the control (P ≥ 0.001).
The Ca2+ transport activities of CAX4 and sCAX4 are too weak and cannot give measurable 45Ca2+ transport in yeast (Cheng et al., 2002; data not shown), so to further infer the transport properties of CAX4, competition transport experiments were performed using the deregulated CAX4 variants HA-CAX4 and sCAX4-9. This allowed us to compare cation selectivity between sCAX1 and these CAX4 variants. ΔpH-dependent 10 μm 45Ca2+ uptake was measured at a single 10-min time point into yeast vacuolar-enriched membrane vesicles isolated from K667 strains expressing sCAX1, sCAX4-9, and HA-CAX4. Ca2+ uptake (10 μm) determined in the absence of excess nonradioactive metal (control) was compared with uptake determined in the presence of two concentrations (10× and 100×) of nonradioactive metals CaCl2, MnCl2, CdCl2, ZnCl2, NiCl2, NaCl, and KCl (Fig. 1B). Inhibition of Ca2+ uptake by nonradioacitve Ca2+ was used as an internal control, and as expected Ca2+ uptake by each CAX transporter was strongly inhibited by excess Ca2+. Nonradioactive Ca2+, particularly the 10× concentration, did not completely inhibit Ca2+ uptake, further highlighting the low Ca2+ affinity of the CAX transporters. Ca2+ uptake by sCAX1 and sCAX4-9 was inhibited by Cd2+, Na+, and K+, but this inhibition was only significant at the higher concentrations. Interestingly, HA-CAX4 Ca2+ transport was not inhibited by any of the metals tested (Fig. 1B). These results indicate that CAX4 may be more specific for Ca2+ than sCAX1 or the sCAX4 variants that contain the Ca2+ domain of CAX1.
CAX Expression in Tomato
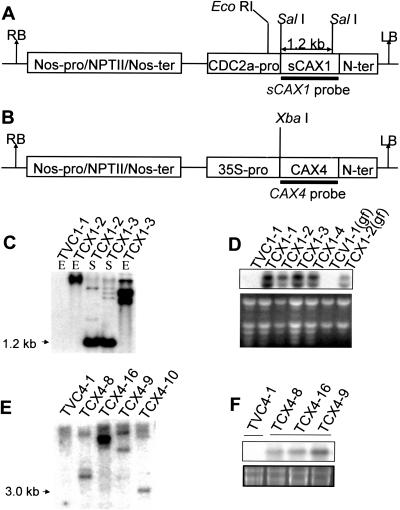
The phenotypes of transgenic plants expressing sCAX1, in conjunction with the biochemical properties of sCAX1 in yeast, suggest that expression of this transporter can alter Ca2+ homeostasis in tomatoes (Hirschi et al., 1996; Hirschi, 1999). Given the Ca2+ deficiency-like symptoms caused by sCAX1 expression in tobacco using the cauliflower mosaic virus (CaMV) 35S promoter (Hirschi, 1999), we opted to express sCAX1 under the control of the cdc2a promoter (Doerner et al. 1996; Fig. 2A). In Arabidopsis, cdc2a transcript levels are correlated with the competence to divide; however, the expression of this Arabidopsis promoter has not been detailed in tomato. In our preliminary analysis, we found that cdc2a∷sCAX1 plants expressed less than half the amount of sCAX1 RNA as compared to 35S∷sCAX1 plants (data not shown). Initially we transformed cdc2a∷sCAX1 into tomato cv Red Cherry, a BER-tolerant variety. We use the abbreviation TCX1 (for tomato sCAX1) to identify plants expressing the cdc2a∷sCAX1 construct. A tomato vector control (TVC1)-expressing line is representative of the empty vector controls used in this study. We generated eight TCX1-expressing lines and eight TVC1-expressing lines. Initially, we examined the transgenic tomato lines by Southern analysis. TCX1 lines contain various copy numbers of the sCAX1 expression construct (Fig. 2C). The line termed TCX1-2 contained a single insertion. The RNA gel blot documented that sCAX1 transcripts accumulated in all TCX1 lines. We consistently saw two transcripts using the cdc2a promoter and only one with the 35S∷sCAX1 tomatoes (Fig. 2D; data not shown). We attribute the two transcripts to multiple transcriptional initiation sites on the vector. The lower band of the two corresponded to the band seen in 35S∷sCAX1 plants (data not shown). The inability to detect a tomato CAX1 homolog in the TVC lines by either Southern or northern blots may be due to the stringency of hybridization used in this study.
Figure 2.
Molecular analyses of sCAX1- and CAX4-expressing tomatoes. A and B, T-DNA regions of pcdc2A∷sCAX1 (A) and pCaMV35S∷CAX4 (B). RB, Right border; LB, left border; Nos-pro, nopaline synthase promoter; NPTII, neomycin phosphotransferase; Nos-ter, nopaline synthase terminator; CDC2a-pro, cell division cycle promoter; 35S-pro, CaMV 35S promoter; CAX4, cation exchanger 4; N-ter, nopaline synthase terminator. C and E, Southern-blot analysis of transgenic tomato plants. Five to 10 μg of tomato genomic DNA were digested with EcoRI or SalI (for pcdc2A∷sCAX1) and with XbaI (for pCaMV35S∷CAX4), and hybridized with the sCAX1 cDNA probe (C) and CAX4 cDNA probe (E), respectively. D and F, Northern-blot analysis of transgenic tomato plants. Ten micrograms of total RNA from expanded leaves and green fruit (gf) were hybridized with the sCAX1 cDNA probe (D) and CAX4 cDNA probe (F), respectively. Ethidium bromide-stained rRNA (bottom) is shown as a loading control.
The 35S∷CAX4 construct, in which CAX4 was driven by the CaMV 35S promoter (Fig. 2B), was transformed into tomato (cv Rubion). Ten independent CAX4-expressing lines were generated. We randomly selected four transgenic lines, and the stable integration and transmission of the 35S∷CAX4 chimera in the genome of T1 TCX4 tomatoes was confirmed by Southern-blot analysis (Fig. 2E). The lines we have termed TCX4-8, TCX4-9, and TCX4-10 appeared to contain more than one integration event, while line TCX4-16 had a single-copy insertion. RNA gel blot documented that CAX4 transcripts accumulated in all of the transgenic lines (Fig. 2F; data not shown).
Three T1 transgenic lines (TCX4-8, TCX4-9, and TCX4-16) showing a low copy number of the CAX4 gene were selected and subjected to further analysis of Ca2+ accumulation and shelf life in CAX4-expressing fruits.
sCAX1 Expression Altered Tomato Growth
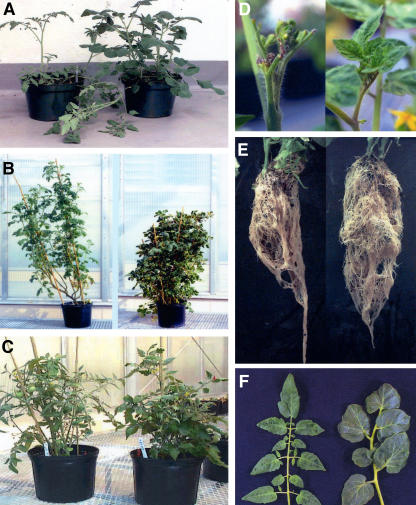
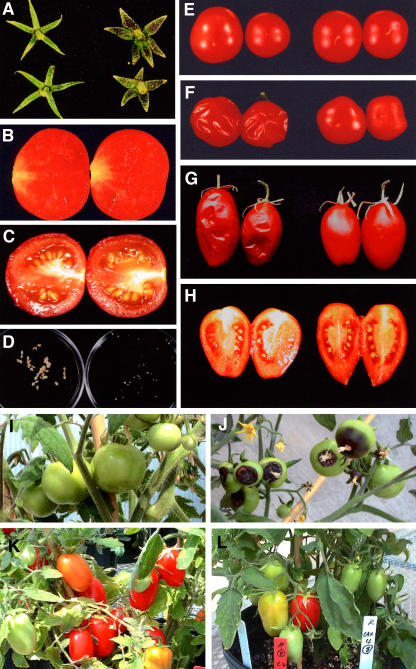
The expression of sCAX1 caused all the tomato plants to be more compact and sturdier throughout their lives (Fig. 3, A and B). sCAX1 expression caused necrotic lesions to form on primary transformants (Fig. 3D, left). This phenotype was apparent on all the sCAX1-expressing transformants, while none of the vector control lines displayed this phenotype. With the addition of Ca2+ to the growth media, the primary sCAX1-expressing transformants no longer had extensive apical burning (Fig. 3D, right). The sCAX1-expressing plants grown in soil also manifested mild Ca2+ deficiency-like symptoms; again, this phenotype could be significantly suppressed by adding Ca2+ to the watering solution. Examination of the root structure showed a 40% increase in the root mass in all of the sCAX1-expressing plants (root weight at day 40 was 34 ± 3 g for TCX1 lines, right, and 25 ± 3 g for TVC1 lines, left; Fig. 3E). Examination of plant height for the sCAX1-expressing plants (plant height from soil surface to the upper leaf; 105 ± 15 cm for TCX1 lines) found that they were approximately 50-cm shorter than vector control plants (165 ± 23 cm for TVC1 lines) after 5 months of growth in soil (Fig. 3B). The mature sCAX1-expressing plants appeared to have thicker leaves (0.4 ± 0.05 mm for TCX1 lines, right, and 0.2 ± 0.05 mm for TVC1 lines, left; Fig. 3F). The morphology of the sepals of sCAX1-expressing lines (right) was also altered compared to vector control lines (left; Fig. 4A). The fruit set was delayed by approximately 4 to 5 weeks in six of the sCAX1-expressing lines; the other two lines did not produce fruit. The overall shape of the transgenic fruit was indistinguishable from that of vector controls (Fig. 4, B, C, and E). However, the seed size was significantly reduced in the sCAX1-expressing lines compared to vector control lines (Fig. 4, B–D). One out of six sCAX1-expressing primary transformants was capable of making viable seed, in contrast to most of the vector control lines. Another striking phenotype of the sCAX1-expressing lines was a significantly increased occurrence of BER. The incidence of BER was higher in all of the sCAX1-expressing lines (75% ± 14% for TCX1 lines and 8% ± 5% for TVC1 lines; Fig. 4, I and J). cdc2a∷sCAX1 was also transformed into tomato cv FM9, another BER-tolerant variety, to determine whether the sCAX1-induced phenotypes were not a consequence of the Red Cherry variety used in the study. Severe BER symptoms were also observed for the FM9 variety expressing sCAX1 in addition to the other changes in plant morphology that were found in sCAX1-expressing Red Cherry tomato lines (data not shown).
Figure 3.
Phenotypes of sCAX1- and CAX4-expressing tomato plants. A, Growth of TVC1-1 vector control (left) and the sCAX1-expressing TCX1-2 plant (right) after 3 weeks in soil. B, Growth of vector control (left) and sCAX1-expressing TCX1-2 plant (right) after 5 months in soil. C, Growth of vector control (left) and CAX4-expressing TCX4-16 plant (right) after 4 months in soil. D, sCAX1-expressing TCX1-2 tomato grown in soil for 2 months without application of exogenous Ca2+ (left) and with the addition of Ca2+ (right). E, Root structure of vector control (left) and sCAX1-expressing TCX1-2 plant (right). F, Leaf phenotype of vector control (left) and sCAX1-expressing TCX1-4 plant (right).
Figure 4.
Phenotypes of sCAX1- and CAX4-expressing tomato fruits. A, Sepals of vector control (left) and sCAX1-expressing plants (right). B and C, Tomato longitudinal section of sCAX1-expressing tomato fruit (B) and vector control (C). D, Seeds of vector control (left) and sCAX1-expressing plants (right). E, Fruit from vector control (left) and sCAX1-expressing TCX1-2 and TCX1-4 plants (right) 20 d after breaker stage. F, Fruits from vector control (left) and sCAX1-expressing TCX1-2 and TCX1-4 plants (right) 40 d after breaker stage. G, Fruits from vector control (left) and CAX4-expressing TCX4-9 and TCX4-16 plants (right) 30 d after breaker stage. H, Tomato longitudinal section of vector control (left) and CAX4-expressing tomato fruit (right). I and J, BER of tomato fruits from vector control (I) and sCAX1-expressing tomato plant (J). The incidences of BER were mainly seen in the sCAX1-expressing plants. K and L, BER of tomato fruits from vector control (K) and CAX4-expressing tomato plant (L). No incidences of BER were mainly seen in the CAX4-expressing plants.
All subsequent experiments with sCAX1-expressing Red Cherry tomato lines were done on primary transformants that had similar phenotypes. The cdc2a∷sCAX1-expressing primary transformants (TCX1-2 and TCX1-4), which have a single copy of CAX1, were used for all other analysis.
Phenotypes of CAX4-Expressing Tomatoes
While the sCAX1-expressing lines were sensitive to Ca2+ deficiency and showed Ca2+ deficiency-like symptoms that were suppressed by addition of Ca2+, the CAX4-expressing lines were not sensitive to Ca2+ deficiency and did not require any additional Ca2+ supplementation for normal growth. In addition, both CAX4-expressing lines and wild-type control grew similarly on one-half strength Murashige and Skoog medium, Ca2+-depleted one-half strength Murashige and Skoog medium, and the one-half strength Murashige and Skoog medium supplemented with various ion metals, such as NaCl and MgCl2 (data not shown). In TCX4 plants, CAX4 expression did not perturb the morphology, growth (Fig. 3C), or fruit set (Fig. 4, K and L). Moreover, the fruit set was not delayed, all CAX4 transformants were capable of making viable seed (Fig. 4H), and the total fruit yield of CAX4 plants was indistinguishable from the wild-type control (data not shown). As the expression of sCAX1 caused a significant increase in BER occurrence in supposed BER-tolerant tomato varieties (Red Cherry and FM9), we chose to express CAX4 in a BER-susceptible tomato variety (cv Rubion) in order to examine the effect of CAX4 expression on BER induction. The incidence of BER was equivalent in the CAX4-expressing lines (BER ratio; 15% ± 5% for TCX4 lines) and vector control lines (BER ratio; 18% ± 6% for TVC4 lines).
H+/Ca2+ Antiport in sCAX1-Expressing Tomatoes
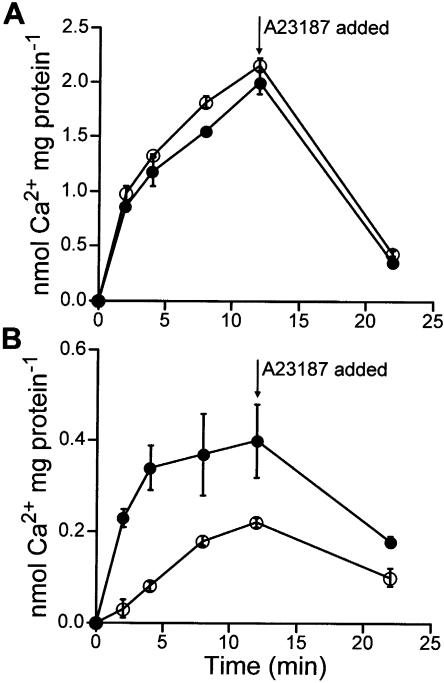
Expression of sCAX1 in yeast restores H+/Ca2+ antiport activity to yeast strains deficient in this transporter (Hirschi et al., 1996). In plants, expression of sCAX1 in tobacco also causes increased H+/Ca2+ antiport (Hirschi, 1999). H+/Ca2+ antiport activity was measured in tonoplast-enriched vesicles from sCAX1 transgenic and vector control tomato roots to confirm that expression of sCAX1 resulted in increased ΔpH-dependent Ca2+ transport. The sCAX1 transcript was found to be constitutively expressed throughout the tomato plant in all lines (data not shown); therefore, root tissue was used to determine antiport activity. A proton gradient (acid inside vesicles) was formed by activation of the Mg2+ATP-dependent H+-ATPase. H+/Ca2+ antiport activity measured in the presence of the P-type ATPase inhibitor, vanadate, was significantly higher in vesicles from the TCX1-2 line compared to the TVC1 (Fig. 5B). A TVC line accumulated Ca2+ to approximate steady-state concentrations of 0.22 ± 0.01 nmol Ca2+ mg protein−1 (Ca2+ uptake in the presence of vanadate without carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone [FCCP] was 0.93 ± 0.01 nmol Ca2+ mg protein−1 and in the presence of vanadate with FCCP was 0.71 ± 0.01 nmol Ca2+ mg protein−1), while TCX1-2 accumulated Ca2+ to concentrations of 0.39 ± 0.09 nmol Ca2+ mg protein−1 (Ca2+ uptake in the presence of vanadate without FCCP was 1.2 ± 0.08 nmol Ca2+ mg protein−1 and in the presence of vanadate with FCCP was 0.81 ± 0.02 nmol Ca2+ mg protein−1). Ca2+ transport activity measured in the absence of vanadate and in the presence of the protonophore FCCP was identical in vesicles from both plants (Fig. 5A).
Figure 5.
Ca2+ uptake activity into tonoplast-enriched vesicles from tomato root. A and B, Time courses of Mg2+ATP-energized 10 μm 45Ca2+ uptake were performed in the presence of 0.1 mm NaN3, 10 mm KCl, 3 mm ATP, and 3 mm MgSO4. Ca2+-ATPase activity (A) was determined in the absence of orthovanadate and the presence of 5 μm FCCP. ΔpH-dependent H+/Ca2+ antiport activity (B) was determined in the presence of 0.2 mm orthovanadate as the difference between Ca2+ uptake in the absence and presence of 5 μm FCCP. The Ca2+ ionophore A23187 (5 μm) was added at the time indicated. Black circles, sCAX1-expressing TCX1-2 plant; white circles, vector controls. Results are the average (± se) of six replicate experiments using two independent membrane preparations.
Ca2+ Accumulation in CAX-Expressing Tomato Plants
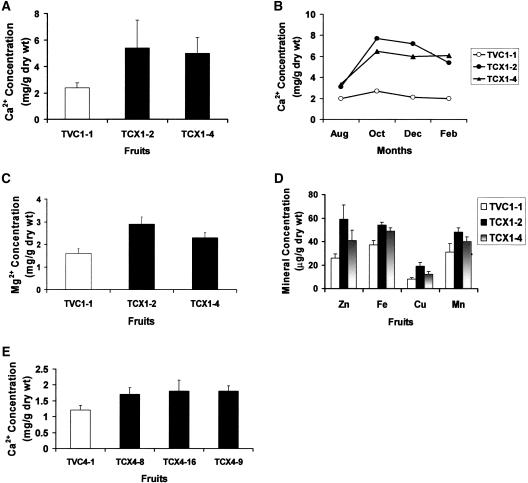
Altered H+/Ca2+ antiport activity in tonoplast-enriched membrane vesicles from sCAX1-expressing tomato plants indicated perturbed Ca2+ transport properties. To ascertain whether sCAX1 expression altered ion levels, Ca2+ levels and other minerals were measured in the transgenic plants. The leaves of all sCAX1-expressing plants showed at least a 20% increase in Ca2+ levels (data not shown), while initial measurements of fruit Ca2+ levels showed that three (TCX1-2, -3, and -4) of the sCAX1-expressing lines had significant alteration in Ca2+ (Fig. 6A). Over time the TCX1-2 and TCX1-4 fruits contained more than twice the Ca2+ levels as vector control plants that were supplemented with Ca2+ (Fig. 6B). TCX1-2 and TCX1-4 fruits also contained increased levels of Cu2+, Fe2+, Mg2+, Mn2+, and Zn2+ (Fig. 6, C and D). Similar changes were seen in the other sCAX1-expressing line (TCX1-3), which contains multiple copies of sCAX1 (data not shown).
Figure 6.
Concentrations of Ca2+ and other minerals in fruits of sCAX1- and CAX4-expressing plants. A to E, Fruit Ca2+ and mineral analysis was performed at 20 d after breaker stage. Total Ca2+ (A, B, and E) and mineral contents (C and D) of fruits (pooled at least five-fruit batches) were determined by inductively coupled plasma emission spectrophotometer. Data represent the values obtained from pools containing at least five fruit over a 6-month period (B), and the means (±sd) of four independent analyses (A and C–E). E, Concentrations of Ca2+ in fruits of CAX4-expressing plants.
All of the CAX4-expressing T1 tomatoes (TCX4-8, -9, and -16) contained significantly more Ca2+ (40%–50% increase) than vector controls (Fig. 6E). No significant increase of other minerals (Cu2+, Fe2+, Mg2+, Mn2+, and Zn2+) was observed with any of the lines analyzed (data not shown). In addition, no significant differences were observed for Na+ and K+ levels in both sCAX1- and CAX4-expressing lines compared to wild-type lines (data not shown).
Increased Fruit Firmness and Prolonged Shelf Life in CAX-Expressing Plants
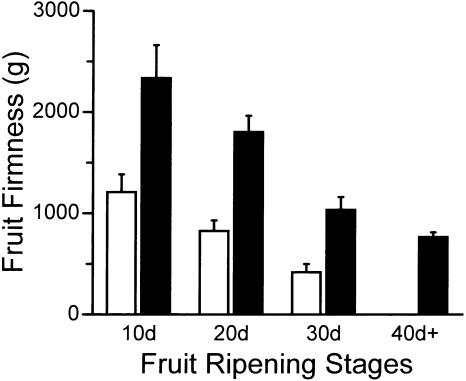
In some agriculturally important crops, addition of exogenous Ca2+ increases fruit firmness and shelf life (Lester and Grusak, 1999; Lopez-Lefebre et al., 2001). We were interested in determining whether the increased Ca2+ in the transgenic fruit would cause increased firmness in the TCX1 lines. As shown in Figure 7, the decline in fruit firmness that is associated with fruit ripening was greatly delayed in sCAX1-expressing plants. During the first 30 d after the breaker stage, the TCX1 fruits were twice as firm as the controls. After 40 d, tomato fruits harvested from sCAX1-expressing plants maintained their structural integrity, while the vector control lines were significantly shrunken (Fig. 4F). Similar changes were seen in the sCAX1-expressing line (TCX1-3) containing multiple copies of the transgene (data not shown).
Figure 7.
Firmness analysis in fruits of sCAX1-expressing plants. Fruits were harvested at 10 (10d), 20 (20d), 30 (30d), and 40 (40d) days after the first color change (the breaker stage). Four fruits were used for each measurement. Firmness was determined using a texture analyzer. Values shown are the means (±sd) of four fruits. White bars, control; black bars, fruits of sCAX1-expressing plants.
To determine whether the increased Ca2+ in the transgenic fruit increased firmness and prolonged shelf life in the TCX4 lines, we measured the mean separations at specific time points (i.e. 5, 10, 15, 20, 25, 30, 35, and 40 d). A significant change was observed in break date and treatment (Table I). At 30 d after the breaker stage, the TCX4 fruits harvested from CAX4-expressing plants maintained their structural integrity, while the vector control fruits were significantly shrunken (Fig. 4G). Overall, the decline in fruit firmness that is associated with fruit ripening was slightly delayed in CAX4-expressing plants (data not shown), and the shelf life of CAX4-expressing T2 tomatoes extended the time until shrinkage about 5 d compared to the vector controls (Table I).
Table I.
Shelf life analysis of CAX4-expressing tomato fruits
The letters (a, b, c, and d) denote statistically significant differences between TCX4 fruits and control fruits. One balanced design (12 plots) and one unbalanced design (eight plots) containing each of 20 fruits a plot were used for shelf life analysis by a randomized-complete block design with three replications. Data were analyzed by ANCOVA, and mean separations was based on the Tukey-Kramer procedure at α = 0.05.
| CAX4-Expressing Lines (Treatment)
|
na
|
Shrinkage Rating Scalesb after Breaker Stage
|
||||||
|---|---|---|---|---|---|---|---|---|
| Break Date
| ||||||||
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | ||
| TVC4-1 (Control) | 120 | 0.0a | 0.2a | 0.8a | 1.3a | 1.9a | 2.5a | 3.0a |
| TCX4-8 | 60 | 0.0a | 0.1b | 0.6b | 1.2b | 1.7b | 2.3b | 2.8b |
| TCX4-9 | 100 | 0.0a | 0.0b | 0.4c | 0.9c | 1.4d | 1.9d | 2.4d |
| TCX4-16 | 120 | 0.0a | 0.0b | 0.6b | 1.1b | 1.6c | 2.1c | 2.6c |
Total number of fruits tested. Fruits were harvested at mature green stage and ripened in air at 22°C to 24°C.
Shrinkage rating scales were as follows: rate 1, signs of shrinkage; rate 2, ≤25%; rate 3, 25% to 50%; and rate 4, >50%.
Sugar Concentration and Ethylene Production
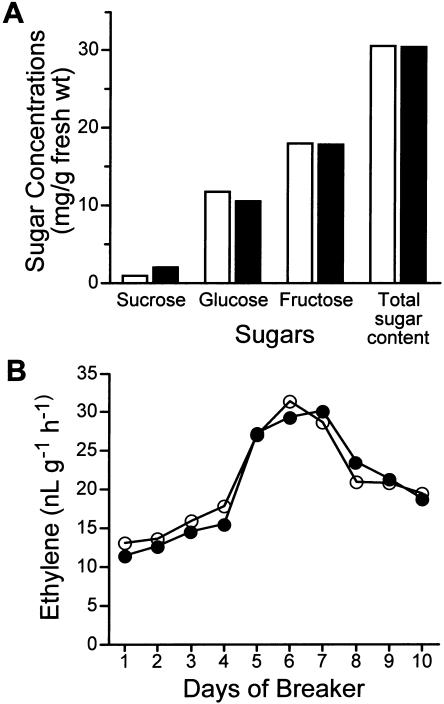
Because the phenotypes were the most prevalent in the sCAX1-expressing plants, we measured sugar and ethylene levels in these transgenic fruit during ripening. The sugar concentration and ethylene production in the sCAX1-expressing lines were comparable to those of the vector control lines (Fig. 8, A and B). Again, similar results were obtained in two sCAX1-expressing lines having a single copy (TCX1-4) and multiple copies (TCX1-3) of the transgene (data not shown). Like the sCAX1-expressing fruit, the CAX4-expressing fruit has no alteration in sugar concentration compared to wild-type (data not shown).
Figure 8.
Sugar and ethylene production in fruits of sCAX1-expressing plants. A, Concentration of total sugars in fruits of sCAX1-expressing plants. Control (30.60 mg g−1 fresh weight and sCAX1-expressing fruits (30.54 mg g−1 fresh weight) were harvested at 20 d after breaker stage, and the concentration of soluble sugars in fruits (pooled four-fruit batches) were analyzed by HPLC. Values (Suc, Glc, and Fru) shown are the means of three injections on the HPLC. White bars, control; black bars, fruits of sCAX1-expressing plants. B, Ethylene production was assayed during a 10-d ripening period after initiation of the first color change. Data shown are the means of values obtained from three fruits. White circles, control; black circles, fruits of sCAX1-expressing plants.
DISCUSSION
We selected tomato as the vegetable in which to express H+/Ca2+ transporters as a means to increase Ca2+ content. Three tomato cultivars, Red Cherry and FM9, fresh market tomatoes reported to have some tolerance to BER (K. Crosby, Texas Agricultural Experiment Station, personal communication), and Rubion, a processing tomato completely susceptible to BER (R. Johns, Seminis, personal communication), were selected in this study. Ca2+ deficiency is the most common nutritional problem affecting tomatoes, which comprise the second-largest vegetable crop in the United States, after potatoes. A lack of Ca2+, water, or both can cause BER in tomatoes (Ho and White, 2005). In addition, tomatoes are highly susceptible to postharvest decay, and must be handled with special care to avoid wounds, bruises, and other injuries that would serve as ports of entry for various pathogens. To ensure firmness on arrival after shipping, tomatoes are picked at the mature green stage, which precludes extended vine ripening. Here we have demonstrated the ability to increase Ca2+ levels in tomatoes, which may be a means to deliver more Ca2+ to consumers, adjust growth constraints, and vary the harvesting practices of an agriculturally important commodity.
Previous studies have demonstrated that expression of Ca2+-signaling components, such as a Ca2+ transporter or a Ca2+-binding protein, can be used to increase Ca2+ levels in various plants (Wyatt et al., 2002; Hirschi, 2004). In this study, we have expressed sCAX1 driven by the cell cycle promoter cdc2a (Doerner et al., 1996; Fig. 2A). To our knowledge, the Arabidopsis cdc2a promoter has not been previously used in tomato and here we show the first preliminary examination of its use in this plant. The cdc2a-driven sCAX1 expression was found to be constitutively expressed in all tomato tissues analyzed (data not shown), suggesting that sCAX1 H+/Ca2+ antiport activity would be equally enhanced in all tissues compared to control plants. We therefore chose to measure antiport activity from root tissue rather than tomato fruit as it is technically much more difficult to isolate intact membrane vesicles from fruit, particularly when ripe, rather than from other tissues. Roots from TCX1 plants showed a 44% increase in tonoplast-enriched H+/Ca2+ transport (Fig. 5B) and no change in Ca2+-ATPase activity (Fig. 5A). These results show we have not measurably perturbed another Ca2+ transport mechanism on the root vacuolar membrane.
The cdc2a∷sCAX1-expressing tomatoes without additional Ca2+ supplementation in the soil demonstrate symptoms of Ca2+ deficiencies, particularly apical burning (Fig. 3D, left). The alterations in plant size, leaf morphology, fruit set, and ripening (Figs. 3 and 4) further emphasize the importance of regulated Ca2+ transport in plant growth and development. Several of these phenotypes, particularly the apical burning, are similar to those of sCAX1-expressing tobacco plants (Hirschi, 1999). Thus, despite the increased Ca2+ in both tobacco and tomato plants, they are suffering from Ca2+ deficiency symptoms (see below). Ca2+ supplementation in the soil (Fig. 3D, right) was required to obtain maximal growth of sCAX1-expressing tomato plants (Fig. 3, D and F). The roots of the cdc2a∷sCAX1-expressing tomatoes appeared more vigorous than those of the control plants. These findings implicate the levels of Ca2+ in strongly influencing root growth (Picchioni et al., 2001). This is interesting when one considers that 35S∷sCAX1 expression in tobacco decreases root mass (Hirschi, 1999). Our findings suggest that the use of the cdc2a promoter mitigated some of the most severe symptoms associated with high-level expression of sCAX1. Some cation/H+ antiporters are known to have a role in regulating cytoplasmic or vacuolar pH (Yamaguchi et al., 2001). We have not analyzed the effect of CAX expression on pH levels in the tomato, and it will be interesting to see whether any morphological changes to the plants are due to altered pH homeostasis.
Unexpectedly, one of the most deleterious changes in fruit development caused by sCAX1 expression in the tomato cultivars, Red Cherry and FM9 (both cultivars have been reported to have tolerance to BER), was the dramatically increased incidence of BER (Fig. 4J). Evidence for Ca2+ deficiency as the primary cause of BER has been derived from observations that the blossom end has the lowest content of Ca2+ within tomato fruits (Adams and Ho, 1993; Nonami et al., 1995). BER is generally associated with the disintegration and increased ion permeability of cells, resulting in loss of turgor and cell fluids invading the intercellular air space, thus causing the watery appearance in the early stages (Shear, 1975; Simon, 1978). However, despite the increased Ca2+ in tomato fruits, the increased incidence of BER in sCAX1-expressing tomatoes is perplexing. We expect that the increased Ca2+ in the tomato fruit is due to a significant increase in vacuolar Ca2+ level within the rapidly expanding fruit due to the enhanced and unregulated vacuolar Ca2+/H+ antiport activity. Therefore, one possible explanation for the increased BER is that sCAX1-expressing lines have altered Ca2+ homeostasis between cytosolic, apoplastic, and vacuolar Ca2+ pools, with decreases in cytosolic and apoplastic Ca2+ levels. It has been suggested that this may disrupt Ca2+-signaling processes, membrane integrity, and normal cell wall development, leading to cell death and thus the occurrence of BER (Ho and White, 2005). Future work will therefore need to determine whether there is indeed reduced cytosolic and/or apoplastic Ca2+ levels in the sCAX1-expressing tomato fruit. An alternative explanation for increased occurrence of BER might be that expression of sCAX1 in vegetative tissues results in a localized Ca2+ deficiency in early fruit development.
While the sCAX1-expressing Red Cherry and FM9 (BER-tolerant cultivars) have increased incidence of BER, no significant difference was observed with the CAX4-expressing Rubion (a cultivar susceptible to BER) when compared to vector controls (Fig. 4, K and L). This may be due in part to the more modest increase in tomato fruit Ca2+ levels seen with TCX4 lines compared to the TCX1 lines. Moreover, CAX4 expression did not perturb the morphology, growth (Fig. 3C), or fruit set (Fig. 4L). Future work will be focused on the mechanisms of CAX expression and BER development.
The deleterious changes in plant growth caused by cdc2a∷sCAX1 expression in tomato plants (e.g. Fig. 3, B and E) suggest that further modulation of the expression of H+/Ca2+ transporters is needed. Rather than alter the expression of the transporter, another approach is to turn down the CAX-mediated Ca2+ transport throughout the plant by posttranslational down-regulation. Here we have used full-length CAX4 containing the entire putative N-terminal autoinhibitory domain. CAX4 is 54% identical to CAX1 at the amino acid level, and previous work suggests that repositioning of the N terminus in this transporter confers Ca2+ transport in yeast assays (Fig. 1A; Cheng et al., 2002). When HA-CAX4 is expressed in yeast, competition experiments suggest that this variant is a more specific Ca2+ transporter than sCAX1 or the variant of CAX4 that contains the Ca2+ domain of CAX1 (sCAX4-9). However, it is possible that the presence of the HA tag could disrupt the selectivity or the regulatory properties of CAX4. Transport experiments with sCAX4 should determine whether the HA tag does actually affect CAX4 activity. Unfortunately we were unable to obtain transport measurements for sCAX4 in the yeast system so alternative approaches will be required to address this question in the future. Our working hypothesis is that in planta CAX4 is an H+/Ca2+ transporter. Interestingly, we attempted several times to generate transgenic lines expressing either sCAX4-9 or HA-CAX4 but were unable to produce viable plants (data not shown).
While the cdc2a∷sCAX1 tomato plants have increased Ca2+ when compared to vector controls, they also display increased levels of several other ions, particularly Mg2+, Zn2+, Fe2+, and Mn2+ (Fig. 6). Metal competition experiments infer that sCAX1 can transport Cd2+ as well as Ca2+ but not Mn2+ (Fig. 1B; Shigaki et al., 2001). Competition experiments also suggest that sCAX1 cannot transport Fe2+ and Cu2+ (data not shown), while direct transport measurements have shown that wild-type sCAX1 cannot efficiently transport Ni2+, Co2+, and Mn2+ but has some ability to transport Cd2+ and Zn2+ in addition to Ca2+ (Shigaki et al., 2005). Similar direct transport experiments will be needed to further determine the transport properties of CAX4 and CAX4 variants. Meanwhile, in agreement with the yeast uptake assays using HA-CAX4, the CAX4-expressing plants have increased levels only of Ca2+. The lack of deleterious morphological phenotypes in the CAX4-expressing plants may correlate with the absence in accumulation of various transition metals. Even at micromolar concentrations some transition metals can be particularly toxic (Marschner, 1995); therefore, some of the phenotypes in the sCAX1-expressing plants could also be due in part to toxicity induced by Fe2+, Zn2+, or Mn2+ accumulation. It is also worth noting that increased H+/Ca2+ transport activity may activate other proton-mediated transporters (Cheng et al., 2005) to cause alterations in plant growth.
While all the sCAX1-expressing lines showed increased Ca2+ levels in the leaves (data not shown), only a portion of the lines showed changes in Ca2+ levels in the fruit. This may be because we did not assay fruit from older plants for all the lines. Our data suggest that fruit derived from older sCAX1-expressing plants contain more Ca2+ (Fig. 6B). Alternatively, the sCAX1 transporter may have to be highly expressed in particular cells in order to facilitate increased Ca2+.
Currently, most Americans obtain their dietary Ca2+ from milk-related products; fruits like tomatoes do not contribute significantly to Ca2+ intake (Fleming and Heimback, 1994). In other areas of the world, communities obtain a majority of their total dietary Ca2+ from the consumption of fruits and vegetables (Weaver et al., 1999). Increasing the endogenous levels of Ca2+ in commonly consumed fruits should help yield improved dietary Ca2+ intakes within many population groups. However, bioavailability studies are needed to determine to what extent these mineral changes in fruit translate into improved Ca2+ bioavailability and nutritional quality.
Applications of CaCl2 are used as a means to increase the firmness of various fruits prior to shipment. Since little translocation of Ca2+ occurs from leaves to growing fruits, direct Ca2+ application on the surface is recommended. However, late-season application of Ca2+ to apples (Malus domestica), pears (Pyrus communis), and other commodities is often avoided due to the costs and the potential of damaging the fruit or accelerating postharvest fungal infections. We have demonstrated here that expression of CAX transporters in tomatoes can be used to increase the firmness and shelf life of tomato fruit (Figs. 4, F and G, and 7). Interestingly, sCAX1 expression does not appear to alter the sugar concentration of the tomatoes (Fig. 8). These findings imply that CAX-expressing tomatoes can be left on the vine to ripen longer, enabling them the potential to offer improved flavor while retaining the firmness necessary to withstand the rigors of shipping. Exogenous applications of Ca2+ to fruit have been shown to be associated with decreased fruit respiration rate and ethylene production (Faust and Shear, 1972; Lieberman and Wang, 1982). However, an increase in fruit Ca2+ levels by enhanced activity of vacuolar Ca2+/H+ antiport does not appear to decrease ethylene production (Fig. 8B). The mechanisms by which Ca2+ effects ethylene biosynthesis are not fully understood, but it is conceivable that cytosolic Ca2+ signals may have a role in regulating ethylene production (Njoroge et al., 1998). While exogenous application of Ca2+ to tomato fruit will likely cause an initial increase in cytosolic Ca2+ and a decrease in ethylene, increase in fruit Ca2+ by sCAX1 overexpression will increase vacuolar Ca2+ levels but may prevent any substantial increase in cytosolic Ca2+, thus preventing a decrease in ethylene production.
Tomato fruit is a climacteric fruit in which ripening is initiated by increased production of ethylene (Adams-Phillips et al., 2004; Giovannoni, 2004). In this study we have observed that the fruit from sCAX1-expressing plants ripen more slowly (Figs. 4F and 7) despite ethylene production being unperturbed (Fig. 8B). Previous studies have also demonstrated alteration in tomato fruit ripening without an alteration in ethylene biosynthesis. The tomato mutants Green-ripe, Never-ripe (Nr), and Nr-2 do not fully ripen, yet ethylene synthesis is unchanged compared to wild-type fruit (Lanahan et al., 1994; Barry et al., 2005). It was suggested that these altered-ripening phenotypes are due to ethylene insensitivity. In fact, in the Nr mutant, this insensitivity is due to a mutation in an ethylene receptor (Wilkinson et al., 1995). Studies suggest that Ca2+ is involved in signaling pathways regulating fruit ripening. For example, it has been shown that Ca2+ is required for ethylene-dependent processes (Raz and Fluhr, 1992) and a tomato Ca2+-dependent protein kinase-related kinase, LeCRK1, which is regulated during ripening has been identified (Leclercq et al., 2005). The results from our work suggest that altering the Ca2+ homeostasis of the fruit by sCAX1 expression can affect the regulation of fruit ripening. Future work will need to be directed at the actual signaling and developmental events that are altered in the CAX-expressing plants, such as whether the plants are ethylene-insensitive like the Nr mutants.
In addition to the possible effect of increased Ca2+ on fruit ripening pathways, the excess Ca2+ may also enhance fruit firmness due to improved cell wall integrity. Cell wall-associated Ca2+ maintains cell wall integrity by generating cross-links with nonesterified pectins in the primary cell wall and middle lamella (Jarvis, 1984; Poovaiah et al., 1988). Homogalacturonan, one of the main polymers of pectin, can form cross-links with Ca2+ by the binding of Ca2+ with carboxylate ions, to form parallel and antiparallel chains, causing the wall to be more rigid. The presence of Ca2+-pectin bridges prevents the access of cell wall hydrolytic enzymes, thus inhibiting cell wall expansion (Jauneau et al., 1994; Konno et al., 1999). Further work is required to demonstrate that cell wall-associated Ca2+ levels are indeed enhanced in CAX-expressing plants compared to wild type.
Increased Ca2+ levels have been shown to alter the severity of several plant pathogens (Marschner, 1995). In the sCAX1-expressing tomatoes, we see no alterations to infection by Pseudomonas syringae pv tomato and Xanthomonas campestris pv vesicatoria or Botrytis (data not shown). Further tests must be performed in various environmental conditions to firmly establish that we have not altered the susceptibility to pathogens. However, it is easy to envision that increasing the firmness of the tomato fruits could delay the penetration of a particular pathogen.
In this report, we have demonstrated the ability to increase Ca2+ levels in tomatoes through heightened activity of a Ca2+ transporter. We have demonstrated here that expression of the H+/Ca2+ transporters can increase fruit Ca2+ levels as well as firmness.
MATERIALS AND METHODS
Yeast Growth, Vacuolar Membrane Isolation, and Transport Measurements
The yeast (Saccharomyces cerevisiae) strain K667 (cnb1∷LEU2 pmc1∷TRP1 vcx1Δ) (Cunningham and Fink, 1996) was used to express various CAX cDNA constructs in the yeast shuttle vector pHGpd (Nathan et al., 1999). Yeast transformation using the lithium acetate/polyethylene glycol transformation method, and isolation of yeast vacuolar-enriched membrane vesicles, were performed as described previously (Pittman et al., 2004a). Measurements of 45CaCl2 uptake into yeast membrane vesicles and metal competition experiments were performed as described previously (Shigaki et al., 2003).
Plant Material, Transformation, and Growth Conditions
Seeds of tomato (Lycopersicon esculentum) Mill. cultivars Red Cherry, FM9, and Rubion were surface sterilized. Seeds were germinated on a Murashige and Skoog (1962) inorganic salt medium with 30 g L−1 Suc, pH 5.7, and solidified using 8 g L−1 TC agar (Sigma). Tomato transformation was performed via Agrobacterium-mediated transformation method using cotyledon and hypocotyl explants as described (Park et al., 2003). Cultures were maintained at 25°C under a 16-h photoperiod. After 6 to 8 weeks (subcultured once at 3–4 weeks), regenerated shoots were transferred to rooting medium for 6 more weeks (for sCAX1-expressing transgenic lines, 3 mL of 2 mm CaCl2 were added to rooting medium to suppress the growth defects of sCAX1-expressing tomato once at 4 weeks), then established in soil. All plants were watered as needed. Once a week they were watered with Miracle-Gro for tomato (Scotts Miracle-Gro Products). The temperature of the greenhouse was maintained within a range of 25°C to 30°C. For maximal growth, sCAX1-expressing transgenic lines in the greenhouse were watered to saturation with 2 mm CaCl2 twice a week for the first 8 weeks.
Bacterial Strain and Plasmids
Agrobacterium tumefaciens LBA 4404 octopine (Hoekema et al., 1983) was used for the transformations. The plasmid pcdc2A∷sCAX1 or pCaMV35S∷CAX4 was introduced into A. tumefaciens using the freeze-thaw method (Holsters et al., 1978). The sCAX1 open reading frame was cloned into the nos/nptII/nos-ter/cdc2a /nos-ter expression vector, which was obtained from John Celenza (Doerner et al., 1996).
DNA Isolation and Southern-Blot Analysis
Tomato genomic DNA was extracted from leaf tissue as previously described (Paterson et al., 1983). DNA (5–10 μg) was digested with SalI or EcoRI and separated by electrophoresis and blotted onto a nylon membrane (Zeta-probe GT membrane, Bio-Rad Laboratories) according to the manufacturer's instructions. The probe for the sCAX1 gene was isolated from a NotI (1.4 kb) restriction fragment of the p039 plasmid (Hirschi et al., 1996), and the probe for the CAX4 gene was isolated from a SacI-XbaI (1.3 kb) restriction fragment of the pBlue-CAX4 plasmid (Cheng et al., 2002). The membranes were prehybridized overnight at 65°C in 7% SDS and 0.25 m Na2HPO4, and then hybridized overnight at 65°C in the same solution containing the probe labeled with 32P-dCTP using NEBlot kit (NEB BioLabs). Membranes were washed twice for 30 min each with 20 mm Na2HPO4 and 5% SDS at 65°C and then washed twice again for 30 min each with 20 mm Na2HPO4 and 1% SDS at 65°C. Membranes were exposed to x-ray film at −80°C.
RNA Isolation and Northern-Blot Analysis
Total RNA was extracted from green fruit tissues and leaves using RNeasy plant kits (Qiagen) according to the manufacturer's instructions. Total RNA (7 μg) was separated on a 1.2% agarose gel containing 1.5% formaldehyde, blotted onto a Zeta-Probe GT membrane according to the manufacturer's instructions. Hybridization and washing were as previously described in Southern-blot analyses.
Plant Measurements
The heights of plants (from soil surface to the upper leaf) were measured after 5 months of growth in soil. The means (±sd) of three independent sCAX1-expressing lines were compared to the means (±sd) of three control lines. At this stage, five leaves of similar age from each of these three transgenic lines and three control lines were sampled, and leaf thickness was measured under a microscope (model 475050, Zeiss). Pictures of the leaf shape of these plant lines were taken to record the phenotypes.
Root Measurements
Root mass was measured by taking three TCX1-expressing lines and three TVC1-expressing lines and after 2 months of growth in soil gently removing the soil from the roots using water. The intact plants were then transferred to hydroponic growth conditions and allowed to grow for 14 additional days as previously described (Hirschi, 1999). The roots were then harvested and dried as previously described (Hirschi, 1999).
Endomembrane Isolation and Ca2+ Transport Assay
Roots of 4-week-old soil-grown TVC1 and TCX1-2 plants were homogenized at 4°C and microsomal pellets obtained (Hirschi, 1999). Endomembrane-enriched vesicles were then prepared as previously described (Pittman et al., 2002). The 45Ca2+ transport assay was performed as previously described (Pittman and Hirschi, 2001) except 5 μm FCCP was used instead of gramicidin.
Ca2+ and Mineral Analysis
Fruit Ca2+ and mineral analysis was performed at 20 d after breaker stage, and the fruits (pooled at least five-fruit batches) were dried at 70°C for 4 d. A total of 0.25 g (dry weight) of fruits was digested for analysis (Feagley et al., 1994). Ca2+ and mineral contents per gram of dry weight were determined by inductively coupled plasma emission spectrophotometer (Spectro).
Firmness Analysis
Fruits were harvested at 10, 20, 30, and 40 d after the first color change (the breaker stage). Four fruits were used for each measurement. Firmness was determined using a TA-XTZi texture analyzer (Texture Technologies). A speed of 2 mm s−1 was used to compress fruit by 4 mm with a circular probe of 4.5 cm in diameter.
Shelf Life Analysis
After segregation analysis on T2 seeds from self-pollinated T1 plant lines (showing a segregation pattern of 3:1 on kanamycin medium), fruit from each of 10 homozygous T2 lines was selected. Fruit was harvested at the mature green stage and ripened at 22°C to 24°C. One balanced design (12 plots) and one unbalanced design (8 plots) containing each of 20 fruits per plot were used for shelf life analysis by a randomized complete block design with three replications. After the breaker stage, the mean separations were performed at special break date values of 5, 10, 15, 20, 25, 30, 35, and 40 d. In this study, shrinkage rating scales were as follows: rate 1 (signs of shrinkage), rate 2 (≤25%), rate 3 (25%–50%), and rate 4 (>50%). Data were analyzed by ANCOVA, and mean separations was based on the Tukey-Kramer procedure at α = 0.05. Observation on fruit number and percentage of incidences of BER were recorded on all fruits from all plant lines selected in this study.
Ethylene Production Analysis
Ethylene production was assayed during a 10-d ripening period after the start of the first color change. Three fruits were used for each measurement. Individual fruits were placed into sealed containers at room temperature for 1 h, and then 1-mL gas samples were withdrawn. Gas samples were analyzed via gas chromatography (model 8500 gas chromatograph, Perkin-Elmer) using a 5% carbowax column (1.8 m × 2.1 mm) and a flame-ionization detection system.
Sugar Analysis
Standard sugar analysis was performed (Hamilton et al., 1997). Ten grams of fruits (pooled in four-fruit batches) at 20 d after the first color change were cut and blended with 80% ethyl alcohol and filtered.
Acknowledgments
We are thankful to Leonard Pike, George Sundin, and Larry Barnes for discussions and for providing biological reagents. We are also grateful to Chris Hundley for help with statistical analysis.
This work was supported by the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service (grant no. 2001–34402–10543), Designing Foods for Health, and the National Institutes of Health (grant no. 1R01 DK 062366).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sunghun Park (s-park4@tamu.edu).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.066266.
References
- Adams P, Ho LC (1993) Effects of environment on the uptake and distribution of calcium in tomato and on the incidence of blossom-end rot plant and soil. Plant Soil 154: 127–132 [Google Scholar]
- Adams-Phillips L, Barry C, Giovannoni J (2004) Signal transduction systems regulating fruit ripening. Trends Plant Sci 9: 331–338 [DOI] [PubMed] [Google Scholar]
- Antosiewicz DM, Hennig J (2004) Overexpression of LCT1 in tobacco enhances the protective action of calcium against cadmium toxicity. Environ Pollut 129: 237–245 [DOI] [PubMed] [Google Scholar]
- Barry CS, McQuinn RP, Thompson AJ, Seymour GB, Grierson D, Giovannoni JJ (2005) Ethylene insensitivity conferred by the Green-ripe and Never-ripe 2 ripening mutants of tomato. Plant Physiol 138: 267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett WF (1993) Nutrient Deficiencies and Toxicities in Crop Plants. APS Press, St. Paul
- Cheng NH, Pittman JK, Shigaki T, Hirschi KD (2002) Characterization of CAX4, an Arabidopsis H+/Ca2+ antiporter. Plant Physiol 128: 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR (1996) Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+-ATPases in Saccharomyces cerevisiae. Mol Cell Biol 16: 2226–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner P, Jorgensen J-E, You R, Steppuhn J, Lamb C (1996) Control of root growth and development by cyclin expression. Nature 380: 520–523 [DOI] [PubMed] [Google Scholar]
- Faust M, Shear CB (1972) The effect of calcium on the respiration of apples. J Am Soc Hortic Sci 97: 437–439 [Google Scholar]
- Feagley SE, Valdez MS, Hudnall WH (1994) Papermill sludge, phosphorous, potassium, and lime effect on clover grown on a mine soil. J Environ Qual 23: 759–765 [Google Scholar]
- Fleming KH, Heimback JT (1994) Consumption of calcium in the US: food sources and intake levels. J Nutr 124: 1426S–1430S [DOI] [PubMed] [Google Scholar]
- Gerasopoulos D, Chouliaras V, Lionakis S (1996) Effects of preharvest calcium chloride sprays on maturity and storability of Hayward kiwifruit. Postharvest Biol Technol 7: 65–72 [Google Scholar]
- Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 16: S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton BK, Leonard MP, Yoo KS (1997) Clonal variations of pungency, sugar content, and bulb weight of onions due to sulphur nutrition. Sci Hortic (Amsterdam) 71: 131–136 [Google Scholar]
- Hirschi KD (1999) Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell 11: 2113–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD (2004) The calcium conundrum: both versatile nutrient and specific signal. Plant Physiol 136: 2438–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Zhen R-G, Cunningham KW, Rea PA, Fink GR (1996) CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA 93: 8782–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LC, White PJ (2005) A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Ann Bot (Lond) 95: 571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekema A, Hirsch PR, Hooykaas PJJ, Schillperoort RA (1983) A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303: 179–180 [Google Scholar]
- Holsters M, De Waele D, Depicker A, Messens E, Van Montagu M, Schell J (1978) Transfection and transformation of A. tumefaciens. Mol Gen Genet 163: 181–187 [DOI] [PubMed] [Google Scholar]
- Jarvis MC (1984) Structure and properties of pectin gels in plant cell walls. Plant Cell Environ 7: 153–164 [Google Scholar]
- Jauneau A, Cabin-Flaman A, Verdus MC, Ripoll C, Thellier M (1994) Involvement of calcium in the inhibition of endopolygalacturonase activity in epidermis cell wall of Linum usitatissimum. Plant Physiol Biochem 32: 839–846 [Google Scholar]
- Konno H, Nakashima S, Maitani T, Katoh K (1999) Alteration of pectic polysaccharides in cell walls, extracellular polysaccharides, and glycan-hydrolytic enzymes of growth-restricted carrot cells under calcium deficiency. Physiol Plant 107: 287–293 [Google Scholar]
- Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ (1994) The Never Ripe mutation blocks ethylene perception in tomato. Plant Cell 6: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq J, Ranty B, Sanchez-Ballesra M-T, Li Z, Jones B, Jauneau A, Pech J-C, Latché A, Ranjeva R, Bouzayen M (2005) Molecular and biochemical characterization of LeCRK1, a ripening-associated tomato CDPK-related kinase. J Exp Bot 56: 25–35 [DOI] [PubMed] [Google Scholar]
- Lester GE, Grusak MA (1999) Postharvest application of calcium and magnesium to honeydew and netted muskmelons: effects on tissue ion concentrations, quality, and senescence. J Am Soc Hortic Sci 124: 545–552 [Google Scholar]
- Lieberman M, Wang SY (1982) Influence of calcium and magnesium on ethylene production by apple tissue slices. Plant Physiol 69: 1150–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lefebre LR, Rivero RM, Garcia PC, Sanchez E, Ruiz JM, Romero L (2001) Effect of calcium on mineral nutrient uptake and growth of tobacco. J Sci Food Agric 81: 1334–1338 [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants. Academic Press, New York
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D, et al (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126: 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklus MB, Beelman RB (1996) CaC12 treated irrigation water applied to mushroom crops (Agaricus bisporus) increases Ca concentration and improves postharvest quality and shelf life. Mycologia 88: 403–409 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Nathan DF, Vos MH, Lindquist S (1999) Identification of SSF1 and HCH1 as multicopy suppressors of a Saccharomyces cerevisiae Hsp90 loss-of-function mutation. Proc Natl Acad Sci USA 96: 1409–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoroge CK, Kerbel EL, Briskin DP (1998) Effect of calcium and calmodulin antagonists on ethylene biosynthesis in tomato fruits. J Sci Food Agric 76: 209–214 [Google Scholar]
- Nonami H, Fukuyama T, Yamamoto M, Yang L, Hashimoto Y (1995) Blossom-end rot of tomato plants may not be directly caused by calcium deficiency. Acta Hortic 395: 107–114 [Google Scholar]
- Park SH, Kang T-S, Kim C-K, Han J-S, Kim S, Smith RH, Pike LM, Hirschi KD (2005) Genetic manipulation for enhancing calcium content in potato tuber. J Agric Food Chem 53: 5598–5603 [DOI] [PubMed] [Google Scholar]
- Park SH, Kim C-K, Pike LM, Smith RH, Hirschi KD (2004) Increased calcium in carrots by expression of an Arabidopsis H+/Ca2+ transporter. Mol Breed 14: 275–282 [Google Scholar]
- Park SH, Morris JL, Park JE, Hirschi KD, Smith RH (2003) Efficient and genotype-independent Agrobacterium-mediated tomato transformation. J Plant Physiol 160: 1253–1257 [DOI] [PubMed] [Google Scholar]
- Paterson AH, Brubaker CL, Wendel JF (1983) A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol Biol Rep 11: 122–127 [Google Scholar]
- Picchioni GA, Valenzuela-Vazquez M, Armenta-Sanchez S (2001) Calcium-activated root growth and mineral nutrient accumulation of Lupinus havardii: ecophysiological and horticultural significance. J Am Soc Hortic Sci 126: 631–637 [Google Scholar]
- Pittman JK, Cheng NH, Shigaki T, Kunta M, Hirschi KD (2004. a) Functional dependence on calcineurin by variants of the Saccharomyces cerevisiae vacuolar Ca2+/H+ exchanger Vcx1p. Mol Microbiol 54: 1104–1116 [DOI] [PubMed] [Google Scholar]
- Pittman JK, Hirschi KD (2001) Regulation of CAX1, an Arabidopsis Ca2+/H+ antiporter: identification of an N-terminal autoinhibitory domain. Plant Physiol 127: 1020–1029 [PMC free article] [PubMed] [Google Scholar]
- Pittman JK, Shigaki T, Cheng NH, Hirschi KD (2002) Mechanism of N-terminal autoinhibition in the Arabidopsis Ca2+/H+ antiporter CAX1. J Biol Chem 277: 26452–26459 [DOI] [PubMed] [Google Scholar]
- Pittman JK, Shigaki T, Marshall JL, Morris JL, Cheng NH, Hirschi KD (2004. b) Functional and regulatory analysis of the Arabidopsis thaliana CAX2 cation transporter. Plant Mol Biol 56: 959–971 [DOI] [PubMed] [Google Scholar]
- Poovaiah BW, Glenn GM, Reddy ASN (1988) Calcium and fruit softening: physiology and biochemistry. Hortic Rev (Am Soc Hortic Sci) 10: 107–152 [Google Scholar]
- Raz V, Fluhr R (1992) Calcium requirement for ethylene-dependent responses. Plant Cell 4: 1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear CB (1975) Calcium-related disorders of fruits and vegetables. HortScience 10: 361–365 [Google Scholar]
- Shigaki T, Barkla BJ, Miranda-Vergara MC, Zhao J, Pantoja O, Hirschi KD (2005) Identification of a crucial histidine involved in metal transport activity in the Arabidopsis cation/H+ exchanger CAX1. J Biol Chem 280: 30136–30142 [DOI] [PubMed] [Google Scholar]
- Shigaki T, Cheng NH, Pittman JK, Hirschi KD (2001) Structural determinants of Ca2+ transport in the Arabidopsis Ca2+/H+ antirporter CAX1. J Biol Chem 276: 43152–43159 [DOI] [PubMed] [Google Scholar]
- Shigaki T, Pittman JK, Hirschi KD (2003) Manganese specificity determinants in the Arabidopsis metal/H+ antiporter CAX2. J Biol Chem 278: 6610–6617 [DOI] [PubMed] [Google Scholar]
- Simon EW (1978) The symptoms of calcium deficiency in plants. New Phytol 80: 1–15 [Google Scholar]
- Weaver CM, Proulx WR, Heaney R (1999) Choices for achieving adequate dietary calcium with a vegetarian diet. Am J Clin Nutr 70: 543S–548S [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HC (1995) An ethylene-inducible component of signal transduction encoded by never-ripe. Science 270: 1807–1809 [DOI] [PubMed] [Google Scholar]
- Wyatt SE, Tsou P-L, Robertson D (2002) Expression of the high-capacity calcium-binding domain of calreticulin increases bioavailable calcium stores in plants. Transgenic Res 11: 1–10 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Fukada-Tanaka S, Inagaki Y, Saito N, Yonekura-Sakakibara K, Tanaka Y, Kusumi T, Iida S (2001) Genes encoding the vacuolar Na+/H+ exchanger and flower coloration. Plant Physiol 42: 451–461 [DOI] [PubMed] [Google Scholar]