Abstract
The function of HKT1 in roots is controversial. We tackled this controversy by studying Na+ uptake in barley (Hordeum vulgare) roots, cloning the HvHKT1 gene, and expressing the HvHKT1 cDNA in yeast (Saccharomyces cerevisiae) cells. High-affinity Na+ uptake was not detected in plants growing at high K+ but appeared soon after exposing the plants to a K+-free medium. It was a uniport, insensitive to external K+ at the beginning of K+ starvation and inhibitable by K+ several hours later. The expression of HvHKT1 in yeast was Na+ (or K+) uniport, Na+-K+ symport, or a mix of both, depending on the construct from which the transporter was expressed. The Na+ uniport function was insensitive to external K+ and mimicked the Na+ uptake carried out by the roots at the beginning of K+ starvation. The K+ uniport function only took place in yeast cells that were completely K+ starved and disappeared when internal K+ increased, which makes it unlikely that HvHKT1 mediates K+ uptake in roots. Mutation of the first in-frame AUG codon of HvHKT1 to CUC changed the uniport function into symport. The expression of the symport from either mutants or constructs keeping the first in-frame AUG took place only in K+-starved cells, while the uniport was expressed in all conditions. We discuss here that the symport occurs only in heterologous expression. It is most likely related to the K+ inhibitable Na+ uptake process of roots that heterologous systems fail to reproduce.
Living cells need to accumulate large amounts of K+ for osmotic and charge balance adjustments. Although, from a chemical point of view, Na+ could perform these functions, high Na+ concentrations are toxic for many cellular processes, and Na+ exclusion from the cell is as crucial as K+ uptake in all types of cells that are growing in Na+-rich media (Rodríguez-Navarro, 2000). Plant cells follow this universal rule and plant roots have the function of providing the entire amount of K+ needed by the whole plant, while restricting the movement of Na+ to the xylem sap. This restriction prevents the possibly lethal Na+ accumulation in leaves that would inevitably follow water evaporation. Regarding Na+ tolerance, plants have large vacuoles where Na+ can undertake osmotic functions without producing toxic effects (Apse et al., 1999; Zhang and Blumwald, 2001), but the Na+ efflux systems are less effective than in animal cells, as a consequence of their adaptation to the oligotrophic conditions that prevail in many terrestrial environments (Benito and Rodríguez-Navarro, 2003).
To understand the relationships of plants with K+ and Na+ and how K+ and Na+ move into and inside the plant, a solid and broad understanding of the function of the K+ and Na+ transporters is required. This understanding has been pursued for a long time, but, recently, interest in the plant K+ and Na+ transporters has increased because of the technological importance of constructing crop plants that are more tolerant to salinity. The use of salty water, which prevails in many agricultural conditions, is an important cause of reductions in crop productivity and a threat to food security (Rhoades et al., 1992).
Despite all this technological interest, present knowledge about K+ and Na+ transporters in plants is still fragmentary and confusing. The best example of this situation is the HKT1 transporter of wheat (Triticum aestivum), which was the first K+ transporter that was identified in plants (Schachtman and Schroeder, 1994; Rubio et al., 1995) after inward-rectifying K+ channels (Anderson et al., 1992; Sentenac et al., 1992). HKT1 was originally characterized as the K+-H+ symporter that mediated the high-affinity K+ uptake in wheat roots (Schachtman and Schroeder, 1994), but it was later found to cotransport Na+-K+ when expressed in yeast (Saccharomyces cerevisiae) or Xenopus oocytes (Rubio et al., 1995). Although this cotransport has never been shown to operate in the roots of any cereal (Maathuis et al., 1996; Walker et al., 1996; Hayes et al., 2001), the notion that HKT1 is a high-affinity root K+ transporter still persists (Horie and Schroeder, 2004). Another proposal is that high-affinity K+ uptake is mediated by HAK transporters (Santa-María et al., 1997; Bañuelos et al., 2002), whereas HKT1 and orthologous transporters in other species are the high-affinity Na+ uptake systems of the roots of cereals (Garciadeblás et al., 2003). Consistent with this notion, the involvement of wheat HKT1 in Na+ uptake, but not in K+ uptake, has been shown in plants in which the HKT1 expression was partially silenced (Laurie et al., 2002). However, if the relevant function of HKT1 is Na+ uptake, it should be possible to express this transporter in a heterologous system and find the plant function, high-affinity Na+ uptake, that is inhibited by low concentrations of K+ (Rains and Epstein, 1967a, 1967b; Garciadeblás et al., 2003). Alternatively, HKT1, instead of being involved in root cation uptake (Rubio et al., 1996; Wang et al., 1998; Laurie et al., 2002), could mediate internal Na+ fluxes, as the Arabidopsis (Arabidopsis thaliana) HKT1 transporter (Mäser et al., 2002a; Berthomieu et al., 2003). In this case, another still-unknown HKT transporter would mediate root Na+ uptake (Garciadeblás et al., 2003).
This controversy about the function of wheat HKT1 in roots is a challenging situation that needs to be resolved because current science should be able to explain the function of a transporter that was cloned 10 years ago. With the aim of solving the controversy, we have cloned and studied the HKT1 barley (Hordeum vulgare) homolog. Barley was selected because it has many genetic similarities to wheat (Laurie and Devos, 2002; Bennetzen and Ma, 2003), the presence of the HKT1 transporter has been described (Wang et al., 1998), and it is a diploid plant. It is worth observing that HKT1 was isolated (Schachtman and Schroeder, 1994) from hexaploid wheat (genomes A, B, and D) and the probable existence of three duplicate versions of HKT1 in the same root cells could be a source of difficulty when analyzing the HKT1 function in this species.
We report here that yeast expression of the HvHKT1 cDNA can result in either a Na+ (or K+) uniporter or in a Na+-K+ symporter, depending on the constructs used for inserting the HvHKT1 cDNA into the yeast expression vector. The symporter was expressed exclusively in K+-starved cells, while the uniporter was also expressed in cells growing under normal conditions. Because only the uniport function was identified in barley roots, we suspect that the symport function is an artifact of expressing HKT1 in yeast. Mutational analysis suggests that the artifact may be produced by a sequence context or secondary structure of the mRNA that is involved in an alternative initiation of translation in the plant and that the yeast cells do not understand.
RESULTS
High-Affinity Na+ Uptake in the Roots of Barley Seedlings
The roots of K+-starved barley seedlings exhibit high-affinity Na+ uptake that is inhibited by K+ and in no cases stimulated by K+ (Rains and Epstein, 1967a, 1967b; Garciadeblás et al., 2003). This high-affinity uptake could not be detected in seedlings with a normal K+ content (e.g. grown in the presence of 3 mm K+) and only appeared as a consequence of K+ starvation. In order to learn more about high-affinity Na+ uptake, we tackled the study of Na+ uptake during the induction period, when the seedlings still had a normal K+ content. The events that describe the evolution of high-affinity Na+ fluxes in a typical experiment with barley seedlings grown at 3 mm K+ and transferred to a K+-free medium are the following (the effects of K+ starvation can be accelerated if the K+-free medium is renewed very frequently in order to keep K+ permanently at very low concentrations): (1) High-affinity Na+ uptake appeared in less than 2 h (Fig. 1A), and very soon the uptake rate reached the value that was shown by 7-d-old seedlings grown permanently in the absence of K+ (Fig. 1C); (2) high-affinity Na+ uptake was neither enhanced nor inhibited by K+ at the beginning of the K+ starvation period (Fig. 1A), but sensitivity to K+ soon appeared, increasing as the starvation period progressed (Fig. 1B); (3) after 24 to 48 h, the inhibition by K+ was as strong as in 7-d-old seedlings grown permanently in the absence of K+ (Fig. 1C; Garciadeblás et al., 2003).
Figure 1.
High-affinity Na+ uptake in barley roots and the effect of K+. Plots show the depletion of the Na+ added to the external medium by excised roots of 7-d-old barley seedlings grown at 3 mm K+ and transferred to K+-free MES buffer for different lengths of time. The experiments were started by adding either Na+, at the concentration shown, or Na+ plus 100 μm K+, approximately. The absence of Na+ efflux was checked in control experiments by adding K+, at 100 μM, but not Na+, and determining the Na+ concentration in the testing medium (black triangles). A, Roots starved for 2 h; in the experiment without K+ added, the K+ concentration varied from 5 to 7 μM. B, Roots starved for 4 h; in the experiment without K+ added, the concentration of K+ was 1 μM. C, Seven-day-old seedlings grown permanently in the absence of K+; in the experiment without K+ added, the K+ concentration was <0.5 μM. In the experiment shown in A, the roots lost K+ and for this reason the external K+ was 5 to 7 μM. A similar experiment carried out in the presence of yeast cells to maintain 0.3 μm K+ proved that the presence of 5 to 7 μm K+ had no effect on Na+ uptake.
To investigate whether, at any time, an HKT transporter was mediating K+ uptake, we used two types of experiments. First, we tried to find a condition in which either K+ uptake was enhanced by Na+ or Na+ uptake was enhanced by K+, but failed to find any of those effects at any time during the starvation period. Second, we compared Rb+ uptake with K+ uptake, expecting to find differences if HKT1 was transporting K+ because the K+ transporter HAK1 does not discriminate between K+ and Rb+ (Santa-María et al., 1997), while HKT1 does not transport Rb+ (Rubio et al., 1995). Again, we could not find a condition in which the K+ uptake rate was higher than the rate of Rb+ uptake.
To sum up, our experiments revealed that high-affinity Na+ uptake (tests at 100–50 μm) in barley roots was mediated by two transporters or by a single transporter that could be in two different states, insensitive and inhibitable by K+. In addition, they ruled out the presence of an Na+-K+ symporter and suggested that K+ uptake was mediated exclusively by HvHAK1 (Santa-María et al., 1997).
Cloning of the HvHKT1 cDNA and Gene
By using primers deduced from the wheat HKT1 sequence and standard reverse transcription (RT)-PCR methods, we cloned a cDNA from barley roots, HvHKT1, whose sequence was 78% identical to the wheat HKT1 sequence. This cDNA could encode a protein with an amino acid sequence 92.5% identical to that of the wheat HKT1 transporter, and 67.3% and 69.0% identical to OsHKT1 and OsHKT2, respectively. A Gly residue in the first membrane-pore-membrane (MPM) motif of the wheat HKT1 transporter (the structure of HKT transporters is made up of four MPM motifs, as discussed elsewhere [Durell and Guy, 1999]), which is supposed to be critical for K+ selectivity (Mäser et al. 2002b), was conserved in the barley transporter. By PCR amplification using primers identical to 5′ and 3′ sequences external to the open reading frame of the cDNA and DNA purified from barley roots, we cloned the HvHKT1 gene. By comparing the sequences of the gene and cDNA, a gene structure of three exons and two introns was deduced. The introns were situated in the positions previously described for the rice (Oryza sativa) HKT genes (Garciadeblás et al., 2003).
The sequence similarity between HvHKT1 and wheat HKT1 cDNAs and the absence of other barley clones with high similarity to HvHKT1 (see below) indicated that the expression studies previously published in barley (Wang et al., 1998) corresponded to the gene that we had cloned.
Functional Expression of HvHKT1 in Yeast
The HvHKT1 cDNA was inserted into the yeast expression vector pYPGE15 (Brunelli and Pall, 1993) and transformed into a yeast trk1 trk2 mutant that is defective for Na+ and K+ uptake (Madrid et al., 1998; Haro et al. 1999). Then, the resulting strain was used for testing Na+ or K+ uptake when the two cations, at micromolar concentrations (50–100 μm), were added either independently or together (the recipient yeast strain does not transport K+ or Na+ in these conditions). Surprisingly, we found that the kinetics of cation uptake exhibited by the expression of HvHKT1 in yeast was variable, depending on the construct: Na+ (or K+) uniport, Na+-K+ symport, or something that could be defined as a uniport that was enhanced by the addition of the other cation (a function that is intermediate between uniport and symport). The term uniport is used here only to denote that Na+ or K+ are transported independently, which does not formally exclude Na+-H+ or K+-H+ symports. Similarly, we use the term symport to denote that Na+ or K+ was not taken up when added independently and was taken up at equal rates when both cations were added together.
Many constructs gave rise to the function that was intermediate between the pure uniport and symport functions, but we identified several that expressed almost pure uniport or symport functions. Out of these, we selected two with different polylinker fragments joining the PGK1 promoter and the 5′ end of the cDNA (starting 20-bp upstream of the first in-frame ATG triplet) as models for the expression of the two functions. The short-linking fragment (SLF) construct gave rise to an Na+ (or K+) uniport (the effect of adding the two cations together had a small enhancing effect on Na+ uptake), whereas the long-linking fragment (LLF) construct gave rise to an Na+-K+ symport (the rate of uniport activity of this construct was 5 to 10 times lower than the symport activity; Fig. 2). As previously reported for HKT1 (Schachtman and Schroeder, 1994; Rubio et al., 1995), HvHKT1 suppressed the K+ requirements of the trk1 trk2 yeast mutant (Fig. 2B). Interestingly, the SLF construct expressing the uniport function was toxic and the yeast transformants lost transport activity gradually after several transfers to fresh media. This has already been observed in the OsHKT1 transporter, which is also a Na+ uniporter (Garciadeblás et al., 2003). On the contrary, the LLF construct expressing the symport function was innocuous and the transformants did not lose the symport function.
Figure 2.
Types of transport exhibited by K+-starved yeast cells transformed with two constructs of HvHKT1. A, Scheme showing the LLF and SLF constructs, which differ in an XbaI fragment of 59 nt; the XbaI recognition sequences are in bold; the 20 nt preceding the first in-frame ATG belong to the HvHKT1 cDNA; not-in-frame ATG triplets in the LLF constructs and stop codons are highlighted. B, Suppression of the defective growth of the trk1 trk2 yeast mutant at low K+ by the two constructs of the HvHKT1 cDNA; serial dilutions of a suspension of yeast cells were inoculated in media with different K+ concentrations; the SLF construct shows toxicity at high K+. C, High-affinity Na+ and K+ uptake in yeast cells transformed with the two constructs of the HvHKT1 cDNA; Na+ and K+ were added independently or together to the suspension of yeast cells that had been K+ starved for 4 h and the decrease in the external concentrations of the added cations was recorded. Abbreviations of the experimental conditions: K+ or Na+, Depletion of K+ or Na+ in experiments with a single cation; K+ (+Na+) or Na+ (+K+), depletion of K+ or Na+, respectively, in an experiment with K+ and Na+.
Rb+ alone was not transported by transformants with either of the two constructs when tested at micromolar concentrations. However, in transformants with both the SLF and LLF constructs, the addition of Na+ triggered Rb+ uptake (Fig. 3). Moreover, with the LLF construct, Rb+ triggered Na+ uptake, and Na+ and Rb+ were taken up exactly at the same rate (Fig. 3). In contrast, with the SLF construct, Na+ was taken up in the absence of other cations (Fig. 2) and Rb+ stimulated, but not triggered, Na+ uptake (compare Figs. 2 and 3). In other words, with the SLF construct, the transporter functioned simultaneously as a Na+ uniporter and a Na+-Rb+ symporter.
Figure 3.
High-affinity Rb+ uptake mediated by the LLF and SLF constructs of HvHKT1. Rb+ and Na+ were added independently or together to the suspension of yeast cells and the decrease in the external concentrations was recorded. Abbreviations of the experimental conditions: Rb+ or Na+, Depletion of Rb+ or Na+ in experiments with a single cation; Rb+ (+Na+) or Na+ (+Rb+), depletion of Rb+ or Na+, respectively, in an experiment with Rb+ and Na+.
Molecular Basis of the Uniport and Symport Functions of HvHKT1
The only possible explanation for the uniport and symport modes of Na+ and K+ uptake that were mediated by the products of the SLF and LLF constructs of HvHKT1 was that the two constructs expressed two transporters that were physically different. A possible explanation for this was a construct-dependent splicing of an unpredicted intron in either of the two constructs. In the PGK1 fragment in plasmid pYPGE15, there is an ATG triplet followed by an in-frame stop codon that is common to the two constructs. No other ATG triplet exists in the SLF construct, but in the LLF construct there are two additional ATG triplets that are not in frame with the HvHKT1 coding region but that could also be used for the initiation of translation after splicing (Fig. 2A). After checking that the reported initiation of transcription of the PGK1 promoter (Rathjen and Mellor, 1990) applied to our constructs, we designed a series of primers and PCR experiments that proved that the prevalent mRNAs were complete from the initiation of translation up to a point that corresponded with the end of the first MPM motif of the transporter. We especially checked that, in both the SLF and LLF constructs, the amplified cDNA contained the first in-frame ATG triplet of the HvHKT1 coding region.
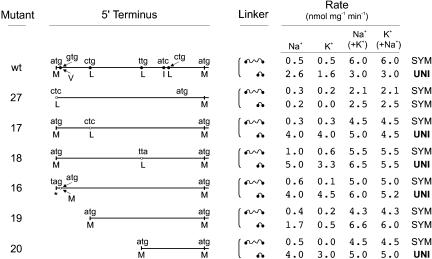
The next experiments were then carried out to test whether the initiation of translation was different in the SLF and LLF constructs. For this purpose, we mutated the first in-frame ATG triplet of HvHKT1 to CTC (HvHKT1-27), finding that this mutation produced symporters in the two constructs (Fig. 4). This result indicated that the uniport was mediated by a protein whose translation was initiated at the first in-frame AUG, while the symport was mediated by a shorter protein (observe in Fig. 2A that the in-frame stop triplet that is 24 nucleotides [nt] upstream of the first in-frame ATG triplet prevents the synthesis of a protein with an N terminus longer than the sequence recorded in Fig. 4A). The second in-frame ATG triplet in HvHKT1 encoded a Met residue situated at position 63, at the end of the first M fragment of the first MPM motif. Although it was unlikely that a transporter lacking the first M fragment of the first MPM motif was still functional, we deleted the first 186 nt of the open reading frame of HvHKT1 cDNA (HvHKT1-15) and produced two constructs, SLF and LLF, identical to those shown in Figure 2, except that Met-1 in the new constructs corresponded to Met-63 in the original ones. Transformants of the yeast mutant with these two constructs failed to exhibit any type of Na+ or K+ uptake.
Figure 4.
High-affinity K+ and Na+ uptake by yeast cells transformed with wild-type cDNA and several mutants of HvHKT1. A, Sequence of the 5′ terminus of the HvHKT1 coding region; the two in-frame ATG triplets are highlighted with a black background, the in-frame non-ATG triplets that have been mutated are highlighted in gray. B, Schematic representation of the mutations and initial rates of uptake both in the LLF and SLF constructs; the initial rates of uptake were calculated from experiments that were identical to those shown in Figure 2C. Abbreviations of the experimental conditions are as in Figure 2C.
These results indicated that the symport function that was produced by the LLF construct was mediated by a protein whose translation was initiated at a non-AUG codon located between the first and second in-frame AUG codons. In an attempt to find out the initiation of translation in this construct, we fused to the 3′ end of the HvHKT1 cDNA an in-frame DNA fragment that added a His tail to the protein and cloned the modified cDNA into the SLF and LLF constructs. Both constructs were active, exhibiting ion transport activities identical to those of the original clones (uniport-symport), except for 40% larger Vmax. However, despite these encouraging results, the purification of the proteins proved to be difficult, and we were unable to obtain a reliable amino acid sequence of the protein produced by the LLF construct.
Tackling the problem of the initiation of translation of the protein that functioned as a symport by mutational analyses was difficult because the number of non-AUG codons to test was high (Fig. 4A). However, we selected some in-frame triplets differing only in 1 nt from ATG (the selected triplets encoded the residues Val-4, Leu-16, Leu-39, Ile-46, and Leu-48) and created triplets that differed 2 nt from ATG, expecting that the corresponding codons in the mRNA would not be able to serve for initiation of translation (Drabkin and Rajbhandary, 1998). All these mutations did not change the functions observed in yeast cells with reference to wild-type cDNA and failed to reveal the exact n terminus generated by the LLF construct of HvHKT1 (Fig. 4 shows a scheme of the mutations and records the results with one of these mutants, HvHKT1-17; similar results were obtained with the other four mutants). Considering the possibility that a promiscuous initiation of translation was neutralizing the effects of individual mutations, we continued our analysis introducing deletions in the 5′ coding sequence of HvHKT1. Two mutants affecting the CTG triplet encoding Leu-16 gave further support to the hypothesis of the non-AUG initiation of translation. In the first mutant (HvHKT1-28), we deleted the first 45 nt of the coding sequence, thus locating the CTG triplet in place of the first in-frame ATG. The second mutant (HvHKT1-19) was identical, except for the addition of a mutation that changed the CTG triplet to ATG (Fig. 4). Both mutations in the SLF and LLF constructs gave rise to Na+-K+ symport when transformed into yeast cells. However, deletion of 114 nt (up to the TTG triplet corresponding to Leu-39) and creation of an ATG translation start (HvHKT1-20) again gave rise to the symport-uniport functions, but further deletions and creations of ATG translation starts were not functional (HvHKT1-22 and -24). The important suggestions of these results is that an HvHKT1 protein lacking the first 15 amino acids is a symporter and that the CUG codon that encodes Leu-16 in the conceptual translation of the gene was probably used for the initiation of translation in HvHKT1-28. They also suggest that yeast cell translation of the HvHKT1 mRNA from the LLF construct may be very complex, including the possibility of producing heteromeric transporters that are made up of proteins of different lengths. Indeed, this is the best explanation for the results obtained with the HvHKT1-20 mutant.
The Wheat HKT1 Transporter Behaves as HvHKT1
The wheat HKT1 has been taken as a K+ transporter model (Véry and Sentenac, 2003; Horie and Schroeder, 2004), whereas the barley transporter had not been studied previously. Therefore, it was of great interest to demonstrate that TaHKT1 behaved as HvHKT1, and that our findings were not a peculiarity of barley and applied to other cereals. Supporting the latter notion, the mutant yeast strain transformed with the SLF and LLF constructs of TaHKT1 in plasmid pYPGE15 transported Na+ and K+ as described for HvHKT1. Moreover, selected mutants of TaHKT1 behaved as the mutants constructed in HvHKT1 (Fig. 5).
Figure 5.
High-affinity K+ and Na+ uptake by yeast cells transformed with wild-type cDNA and several mutants of TaHKT1. Schematic representations and recordings are as in Figure 4B. The sequence of the 5′ terminus of the open reading frame that encodes TaHKT1 is not identical to that of HvHKT1, but the few differences do not affect the selected triplets.
The Symport Is Expressed Only in Special Conditions
All the experiments reported so far were carried out in yeast cells that were starved for 4 h in a K+-free medium (K+-starved cells) in order to decrease their K+ content and cellular pH (Ramos et al., 1990), which in combination allow a rapid and large uptake of cations. Another way to decrease the K+ content of yeast cells and obtain cells with the capacity to take up large amounts of cations is rapid treatment with NaN3 (10 min) and subsequent washing of the drug. This method differs from K+ starvation in that the cellular proteins were all produced before the cells felt the stress of the K+-free medium (Ramos and Rodríguez-Navarro, 1986; Haro and Rodríguez-Navarro, 2002, 2003). When NaN3 treatment was applied to the yeast strains transformed with HvHKT1, the cells transformed with the SLF construct exhibited the aforementioned uniport function, although at a slightly lower rate, but the LLF construct cells did not transport either Na+ or K+. To rule out an unpredicted inhibition of metabolism by NaN3, we prepared standard K+-starved cells expressing the LLF construct, treated them with NaN3, and tested Na+ and K+ uptake, finding that their regular Na+-K+ symport was not affected by the treatment with NaN3. These results indicated that the LLF HvHKT1-transformed cells expressed a functional transporter exclusively during K+ starvation.
If the symporter was expressed when translation was initiated at a non-AUG codon and this occurred exclusively in K+-starved cells, it could be predicted that mutants HvHKT1-27 and HvHKT1-28, in which the first in-frame AUG had been eliminated, would not exhibit transport in normal cells treated with NaN3, regardless of whether the construct was SLF or LLF. These experiments were carried out and the results confirmed the prediction. In contrast, mutant HvHKT1-19, which expressed symporter functions from the two constructs, presumably initiating translation at the AUG codon generated by mutation, exhibited the symport functions both in K+-starved and NaN3-treated cells (Table I). These results suggested that non-AUG initiation of translation was dependent on the conditions imposed by K+ starvation.
Table I.
Functional expression of the HvHKT1 transporter in yeast cells grown at high K+
Yeast cells were grown at high K+ and then treated with NaN3 to decrease their K+ content and cellular pH to bring the cells to a state in which their capacity for transporting Na+ and K+ is high. In all the recorded cases, transport is positive in K+-starved cells and NaN3 treatment of these cells did not decrease their transport capacity. Mutants and the types of transport that they express, uniport or symport, are described in Figure 4.
| Transporter | Construct | Transport |
|---|---|---|
| HvHKT1 | SLF | Yes |
| HvHKT1 | LLF | No |
| HvHKT1-27 | SLF | No |
| HvHKT1-27 | LLF | No |
| HvHKT1-28 | SLF | No |
| HvHKT1-28 | LLF | No |
| HvHKT1-19 | SLF | Yes |
| HvHKT1-19 | LLF | Yes |
A Second Copy of HvHKT1 Apparently Does Not Exist
High-affinity Na+ uptake in the roots of barley seedlings was found to be K+ insensitive or inhibitable by K+, depending on the conditions of the seedlings (Fig. 1). The expression of HvHKT1 in the yeast mutant from the SLF construct produced a K+-insensitive Na+ uptake (Fig. 2) similar to that found in roots, but under no conditions did HvHKT1 reproduce the K+-inhibitable Na+ uptake in yeast. This posed the question of whether another HvHKT gene, perhaps an almost identical copy of HvHKT1, could encode the transporter that mediated the K+-inhibitable Na+ uptake. In rice, OsHKT2 is an almost exact copy of OsHKT1 that exists in cultivars of the indica subspecies (Horie et al., 2001; Garciadeblás et al., 2003). Therefore, to investigate this possibility, we designed a RT-PCR approach in which we used several pairs of primers that corresponded to sequences conserved in the HvHKT1, TaHKT1, and OsHKT1 cDNAs, as well as others that corresponded to protein fragments that are conserved in all HKT transporters. We cloned more than 100 HvHKT cDNA fragments from the roots of K+-starved seedlings, which are the plants exhibiting the K+-inhibitable Na+ uptake. In 90% of these isolations, the fragments corresponded to HvHKT1, demonstrating that the frequency of isolation of HvHKT1 cDNA fragments was much higher than fragments of other HvHKT cDNAs. HvHKT1 fragments were amplified even when the sequences of the used primers were identical or more similar to non-HvHKT1 than to the HvHKT1 cDNA sequences. The second most frequently isolated fragment exhibited a high similarity to OsHKT6, which is a transporter that does not belong to group I and is not associated with high-affinity Na+ uptake (Garciadeblás et al., 2003).
From these results, we concluded that HvHKT1 was the only HKT gene that was highly expressed in the roots of K+-starved barley seedlings and that, consequently, both K+-insensitive and K+-inhibitable Na+ influxes were both mediated by a single product or alternative products of the HvHKT1 gene.
Among all possible post-translational modifications that could change the activity of HvHKT1, phosphorylation could easily be tested by mutational analysis and expression in yeast. Replacement of Ser residues by Asp mimics a phosphorylated Ser, while replacement by Leu prevents the phosphorylation. According to the structure proposed for HKT transporters (Durell and Guy, 1999; Kato et al., 2001), HvHKT1 has 15 Ser, eight Thr, and three Tyr residues facing the internal milieu of the cell. However, several of them have a much higher probability of phosphorylation than others by network prediction programs (Blom et al., 1999). We selected five Ser residues (68, 186, 396, 419, and 528) with high probability of phosphorylation at http://www.cbs.dtu.dk/services/NetPhos/, constructed the mutants that replaced each of them by either Leu or Asp, and tested the function of the mutated transporter in yeast. In all cases, the mutations did not show any effect. Although these results are not conclusive, they make the phosphorylation hypothesis less likely.
The Uniport Is under Strict Kinetic Regulation by the Cellular Conditions
In the experiments of Na+ and K+ uptake with K+-starved yeast cells transformed with HvHKT1 (Fig. 2), we had observed that the uptake of cations ceased before the cells had reached the normal level of cation content. In fungal cells, cation uptake increases the internal pH due to the exchange of internal H+ for external K+ or Na+ and, consequently, the H+ pump decreases its pumping rate (Blatt and Slayman, 1987; Rodríguez-Navarro, 2000). Due to this, uptake ceases, but this occurs when the normal cation content is reached. Therefore, the simplest explanation for the early cessation of uptake was that the increase of the Na+ and K+ content in the cells exerted kinetic control over its own transport. To test this possibility, we prepared K+-starved cells of the clone transformed with the SLF-HvHKT1 construct and allowed them to take up either Na+ or K+ for a short period of time before the actual test of cation uptake. The results showed clearly that the previous uptake of approximately 100 nmol mg−1 of Na+ or K+ (this amount is approximately one-third of that needed to reach the level of cells growing in a medium without K+ restrictions) inhibited almost completely the uptake of the loaded cation without showing a significant effect on the other cation (Fig. 6). The main conclusion that can be drawn from these results is that the transporter expressed by the SLF-HvHKT1 construct in cells with medium or high K+ content is an Na+ uniporter with a null capacity to mediate K+ uptake.
Figure 6.
Inhibition of the Na+ or K+ uniport by the internal concentration of the corresponding cation. The cells were allowed to take up 100 nmol mg−1 of Na+ (A) or K+ (B), approximately, before initiating the actual uptake test. Other conditions and abbreviations are as in the SLF construct in Figure 2C.
DISCUSSION
Two HKT1 Functions Are Expressed in Heterologous Systems
The study of many constructs revealed that the yeast expression of HvHKT1 exhibited a variable mechanism of transport. In most of them, yeast cells took up Na+ and K+ when added independently and the uptake of each of them was enhanced when the other cation was present. In contrast, the SLF and LLF constructs reported here exhibited almost pure uniport and symport functions, respectively (Fig. 2). This suggested that the same cDNA produced two basic transport functions, uniport and symport, and that intermediate transport functions could be explained by the simultaneous production of the two basic functions in different proportions. The production of many different transporters with functions that are midway between the uniport and symport cannot be formally ruled out, but seems unlikely.
A stoichiometry of 2 K+ to 1 Na+ has been proposed for HKT1, with experimental data of 1.7 to 1 in Xenopus oocytes and 2.1 to 1 in K+-starved yeast cells (Rubio et al., 1995). However, this implies a transporter with three binding sites, which contradicts the kinetics studies that assign only two binding sites to TRK and HKT transporters (Haro and Rodríguez-Navarro, 2002, 2003; Garciadeblás et al., 2003). In light of our results, apparent stoichiometries different from 1 to 1 can be explained if the Na+-K+ symporter and the Na+ or K+ uniporter are operating in parallel and the uniporter transports more K+ than Na+. This can be expected because internal Na+ inhibits Na+ uptake (Fig. 6) and yeast cells expressing HKT1 transporters inevitably accumulate Na+ during the process of K+ starvation if special conditions are not designed (Garciadeblás et al., 2003). Similarly, a fairly high Na+ content can also be expected in most preparations of Xenopus oocytes (Guizouarn et al., 2001).
Apart from the possible physiological reasons that originated a diversity of functions for the product or products of the HvHKT1 mRNA, the finding of a dual function, uniport and symport, is not an outstanding result. HKT transporters bind two alkali cations (Garciadeblás et al., 2003), as do fungal TRK transporters (Haro and Rodríguez-Navarro, 2002, 2003), and the uniport and symport functions only differ as to whether the same cation binds the two sites and crosses the pore or whether two different cations have to be involved. This means that TRK-HKT transporters may be uniporters or symporters, depending on the physical characteristics of each transporter and the tested cations. Regarding the cations, the SLF construct of HvHKT1 exhibited uniporter function for Na+ or K+, but transported Rb+ exclusively in the form of Na+-Rb+ symport (Fig. 3), and, regarding the transporter, we have shown that the deletion of 15 amino acids in the N terminus of HvHKT1 (HvHKT1-19) produces the symport function (Fig. 4).
The variability of the functions obtained with the same cDNA in heterologous expressions (Fig. 2) reveals that several HKT1 proteins are probably produced from the same mRNA. The mutation of the first in-frame ATG triplet of the HvHKT1 and TaHKT1 cDNAs (mutant 27), which necessarily had to abolish the initiation of translation at that codon, did not affect the symport function (LLF constructs) but transformed the uniport function exhibited by the SLF constructs into symport. Two conclusions can be drawn from these and other experiments summarized in Figure 4: (1) The Na+ (or K+) uniport function exhibited by the SLF construct is mediated by a protein whose translation from the HvHKT1 mRNA is initiated at the first in-frame AUG codon; and (2) in the LLF construct, the HvHKT1 mRNA has an internal initiation of translation that produces a shorter protein and the symport function. Because initiation of translation at the second in-frame AUG codon did not produce an active transporter, it seems clear that a non-AUG codon between the first and second in-frame AUG codons provides the initiation for wild-type cDNA in the LLF construct, and that this produces the Na+-K+ symport function. The symport function of mutant HvHKT1-19, which encodes a shorter protein, supports this hypothesis.
The finding that a CUG codon was probably used for the initiation of translation in a deletion mutant (Fig. 4B, mutant 28), but that its mutation to CUC in wild-type cDNA did not abolish the symport function of the LLF construct (Fig. 4B, mutant 17) strongly suggests that there are more than one non-AUG codon involved in translation initiation. Due to this problem and the difficulties in purifying the protein, we have not been able to define exactly the HvHKT1 protein that mediates Na+-K+ symport in the SLF construct of HvHKT1 (i.e. the non-AUG codon that initiates translation). Considering the high number of triplets that might be involved, the possibility that the symporter has a heterotetrameric structure and even that different amounts of proteins may give rise to different functions, it seems clear that the problem needs to be tackled using another experimental approach. In any case, the identification of the involved codon in yeast cells is of less relevance from the physiological point of view of the plant and for this article, which aims to determine the function of HKT1 in roots.
Na+ Uniport Is the Plant Physiological Function of HKT1
Our experiments failed to detect high-affinity Na+ uptake that is activated by K+ in the roots of barley seedlings. These and previous results (Maathuis et al., 1996; Walker et al., 1996; Hayes et al., 2001; Garciadeblás et al., 2003) support the notion that an Na+-K+ symport, if it exists, is of negligible importance in the roots of most cereals. In contrast, a high-affinity Na+ uptake that is not activated by K+ was induced very early in the roots of barley seedlings that were exposed to a K+-free medium. The physiological relevance of the high-affinity Na+ uptake that is triggered by the absence of K+ is the alleviation of the K+ deficiency that this situation produces. It can be said that, for plants and fungi, Na+ is better than nothing (in fact, H+), as discussed elsewhere (Garciadeblás et al., 2003; Benito et al., 2004). It is worth observing that K+ deprivation may be frequent in natural conditions, for example, when young seedlings are exposed to a heavy rainfall in the field, especially in permeable poor soils.
High-affinity Na+ uptake in barley roots took place in two forms attending to the effect of K+, either K+ insensitive or inhibitable by low K+ concentrations. The former occurred at the beginning of K+ starvation, when the K+ content of the seedling roots is still normal, and the latter is characteristic of K+-depleted roots, although it appears before the roots show a deep K+ depletion. In previous reports, only the K+-inhibitable process had been detected (Rains and Epstein, 1967a, 1967b; Garciadeblás et al., 2003). The expression of HvHKT1 in yeast from the SLF construct mimicked the K+-insensitive Na+ uptake. This construct also mediated K+ uptake in yeast (Fig. 2), but only in cells with a very low K+ content (Fig. 6). The high sensitivity of the uniporter to the internal content of the transported cation suggests that, in roots that have an almost normal K+ content, HvHKT1 will mediate only Na+ uptake. Consistent with the notion that HvHKT1 does not mediate K+ uptake in barley, the Rb+ uptake tests that we carried out in roots throughout the process of K+ starvation also suggested that K+ uptake was always mediated by an HAK transporter, which does not discriminate between K+ and Rb+, and not by an HKT transporter, which does not transport Rb+, except as an Na+-Rb+ symport (Fig. 3).
A more difficult question to answer is whether HvHKT1 also mediates the form of high-affinity Na+ uptake that is strongly inhibited by K+. Our RT-PCR experiments failed to find HKT transcripts other than HvHKT1 that were highly expressed in seedlings under K+ starvation. The most likely hypothesis, therefore, is that HvHKT1 mediates both types of high-affinity Na+ uptake, insensitive and inhibitable by K+, and that barley does not have a second transporter very similar to HvHKT1, as in the case of OsHKT1 and OsHKT2 in rice (Horie et al., 2001; Garciadeblás et al., 2003). Because HvHKT1 binds K+ with high affinity (in fact, it is a high-affinity K+ transporter in some conditions), K+ and Na+ will compete for transport in certain conditions (note that the presence of two binding sites complicates the kinetic analysis) and that any mechanism of protein modification that decreases the Vmax of K+ influx could create a K+-inhibitable Na+ transporter (Garciadeblás et al., 2003). Considering the reported uniport-symport changes induced by a deletion in the HvHKT1 protein (SLF constructs of wild-type cDNA and mutant 19 in Fig. 4B), it can be expected that a small change in the structure of the HvHKT1 protein could also transform a K+-insensitive Na+ transporter into a transporter that is inhibited by K+.
Although our original experiments were carried out with the barley transporter HvHKT1, many experiments were later repeated with the wheat transporter TaHKT1, and all the results indicate that findings for the barley transporter apply to the wheat transporter.
The Na+-K+ Symport May Be a Nonfortuitous Artifact of Heterologous Expressions
We have proposed above that the uniport function expressed in yeast cells is the physiological function in roots, and this poses the question of whether the symport function is only a fortuitous artifact of heterologous expression or whether it reveals the existence of a physiological process performed by HKT1 transporters in plants that yeast cells and Xenopus oocytes fail to reproduce correctly. If the symport function of HvHKT1 and TaHKT1 in yeast cells is due to alternative initiations of translations of the HKT1 mRNAs, and another explanation for our results is difficult to imagine, the key to the answer is in this process. Alternative initiation of translation at a non-AUG codon has been described in a specific mRNA in yeast (Chang and Wang, 2004), but the process is very inefficient in mRNAs in which it does not occur naturally (Clements et al., 1988; Donahue and Cigan, 1988). Therefore, it seems unlikely that yeast cells force non-AUG initiations of translation by a fortuitous effect without any participation of the HKT1 mRNAs. It is more likely that the 5′ termini of the HvHKT1 and TaHKT1 mRNAs create a context effect that leads to alternative internal initiations of translation in plants in order to generate a diversity of transport functions with only one gene. This diversity may be the K+-insensitive and K+-inhibitable Na+ uptake, as discussed above.
That the yeast expression of the symporter is somehow related to a physiological process in the plant and is not fortuitous is further supported by the fact that the symporter is expressed exclusively in K+-starved cells of the LLF construct of HvHKT1. This conditional expression also applies to mutants 27 and 28, in which there is no AUG initiation codon (Table I). In contrast, the SLF construct of HvHKT1 and mutants with an AUG initiation codon (mutant 19) expressed either the uniporter or the symporter in all conditions (Table I). All these results point out that, when translation is initiated at an AUG codon, the expression of the transporter, either uniport or symport, is constitutive and when translation is initiated at a non-AUG codon, the expression of the transporter depends on the K+ status of the cells. To sum up, K+-starved roots express a K+-inhibitable Na+ uptake that yeast cells transformed with HvHKT1 do not express and K+-starved yeast cells transformed with certain constructs of HvHKT1 express a symport that cannot be detected in roots. Remarkably, both singular processes might be explained by alternative initiations of translation.
The simplest conclusion from all this is that, in K+-starved root cells, the HvHKT1 (and TaHKT1) mRNA is translated in an alternative form to produce the K+-inhibitable Na+ transporter and that K+-starved yeast cells try to reproduce it but fail to do so correctly. Perhaps yeast cells do not use the correct non-AUG initiation of translation. Although there are enough physiological differences between plant and yeast cells to explain this failure, the lack of most of the native 5′-nontranslated region of HvHKT1 in our constructs might be the cause of the expression of the artifactual symporter.
MATERIALS AND METHODS
Plant Seedlings
Barley seeds (Hordeum vulgare L. cv Albacete) were surface sterilized and germinated in filter paper that was wet with a 1.0 mm CaSO4 solution. Then the seeds were transferred to 5- to 10-L plastic containers with a 5 mm CaCl2 solution in which they were supported by cheesecloth stapled to floating frames. Seedlings were grown in the dark for 7 to 10 d at 28°C. The presence of root-associated bacteria or fungi was checked systematically with microbiological counts. In typical batches of plants, the number of bacteria was low and fungi were almost absent. Excised roots were cut approximately 5 mm below the seeds.
Strains, Media, and Growth Conditions
The Escherichia coli strain DH5α was routinely used for plasmidic DNA propagation. The yeast (Saccharomyces cerevisiae) strain WΔ3 (Mat a ade2 ura3 trp1 trk1Δ::LEU2 trk2Δ::HIS3; Haro et al., 1999) deficient in the endogenous K+ uptake systems TRK1 and TRK2 was used for functional complementation and transport assays. Yeast strains were grown in synthetic dextrose (Sherman, 1991) or Arg phosphate (AP) medium (Rodríguez-Navarro and Ramos, 1984) supplemented with 50 mm K+. For yeast growth experiments at low K+ concentrations, serial dilution drops of strains were inoculated on AP medium supplemented with the indicated K+ concentrations. K+-starved cells were obtained by transferring actively growing cells in 50 mm K+ AP medium into K+-free AP medium and incubating them for 4 h. NaN3-treated yeast cells were obtained by exposing actively growing cells in 50 mm K+ AP medium to 10 mm NaN3, K+-free AP medium for 10 min (Ramos and Rodríguez-Navarro, 1986; Haro and Rodríguez-Navarro, 2002, 2003). After washing the drug, the cells were preincubated in the assay buffer for 10 min before starting the cation uptake experiments.
Recombinant DNA Techniques
Manipulation of nucleic acids was performed by standard protocols or, when appropriate, according to the manufacturer's instructions. PCRs were performed in a Perkin-Elmer thermocycler, using the Expand-High-Fidelity PCR system (Roche Molecular Biochemicals). Some of the PCR fragments were first cloned into the PCR2.1-Topo vector using the TOPO TA cloning kit (Invitrogen). For yeast expression, the cDNAs were cloned into vector pYPGE15 and transformed into the trk1 trk2 yeast mutant, as described previously (Brunelli and Pall, 1993; Santa-María et al., 1997). The two constructs used in this article are described in Figure 2A. Total barley RNA was prepared using the RNeasy plant kit and DNeasy plant kit (Qiagen). PCR amplifications of HKT fragments were carried out on double-stranded cDNA synthesized from total RNA by using the cDNA synthesis system kit (Roche). The HvHKT1 full-length cDNA was obtained by using the 5′/3′-RACE kit (Roche) according to the manufacturer's instructions. The clone here reported is the longest that we could clone.
HvHKT1 Mutant Constructions
Oligonucleotide-directed site-specific mutants were constructed by PCR (Good and Nazar, 1992). Mutants with 1- or 2-nt changes were obtained by two-step PCR; the first reactions were carried out with two mutagenic primers (forward and reverse) that overlapped the mutation and two 5′ (forward) and 3′ (reverse) flanking primers. The forward flanking primer sequence, 5′- CCTCTAGATCGCACTCATATATAGCACCA-3′, contained the 20 nt of HvHKT1 preceding the ATG triplet and the XbaI site (see Fig. 2), and the reverse flanking primer sequence, 5′-GGGGTACCATTCTTCAGGCAGTACACTAGT-3′, corresponded to the 3′ noncoding sequence of HvHKT1 plus a KpnI site. The second PCR step, which was carried out using the products from the first step and the described flanking primers, produced mutated HvHKT1 cDNAs that could be cloned in the XbaI and KpnI sites of the pYPGE15 vector. Deletion mutants containing a new ATG triplet (see Figs. 4 and 5) were constructed in a single PCR reaction using the aforementioned reverse flanking primer and a 56-mer forward primer whose 5′ half (30 nt) was identical to the aforementioned forward primer, and the 3′ half (26 nt) started in ATG and continued with the sequence that corresponded in each case. The PCR products were cloned in XbaI and KpnI sites of the pYPGE15 vector, as in the point mutants described above. All constructs were sequenced to check that no random mutation had been introduced by the PCR reactions.
Cation Uptake Experiments in Roots and Yeast Cells
These experiments were carried out as described previously (Bañuelos et al., 2002). Excised roots were suspended in aerated 10 mm MES-Ca2+ buffer, pH 6.0, and yeast cells in the same buffer plus 2% Glc. After the addition of the tested cation, the depletion from the external medium was followed by atomic emission spectrophotometry. Root experiments were performed at 6 to 8 mg mL−1 of root dry weight and yeast experiments at 1.8 to 2.0 mg mL−1 of yeast dry weight.
During the induction period of high-affinity Na+ uptake, the exposure of the roots to a K+-free medium produced a permanent loss of K+, which prevented us from testing Na+ uptake in the absence of K+ and, consequently, to investigate whether Na+ uptake was enhanced by K+ (Rubio et al., 1995) or inhibited by K+ (Garciadeblás et al., 2003). To solve the problem, we carried out uptake experiments in the presence, when necessary, of yeast cells of a trk1 trk2 mutant expressing the Neurospora crassa NcHAK1 transporter. NcHAK1 mediates a very rapid K+ uptake, but does not mediate any appreciable Na+ uptake when Na+ is in the micromolar range of concentrations (Haro et al., 1999). In experiments that were performed as previously described (Bañuelos et al., 2002; Garciadeblás et al., 2003), the amount of yeast cells that it was necessary to add to keep the K+ concentration at 0.1 to 0.3 μm was low (0.5 mg mL−1) and the only special condition for these experiments was the presence of 2% Glc. The effect of the presence of Glc was tested in a series of control experiments of K+ and Na+ uptake with roots with different degrees of K+ starvation. The results proved that the only effect of Glc was to increase the Vmax of K+ and Na+ influxes (by approximately 30%), which is consistent with previous findings in sunflower roots (Quintero et al., 2001).
All experiments were repeated at least three times. The main source of variability of the results was the Na+ content of the medium where K+ starvation was carried out and the ratio of the mass of roots or yeast cells versus the volume of the starving medium. Both conditions affect the Na+ content of the K+-starved roots and yeast cells and consequently the rates of transport. In the reported experiments, the Na+ content was lower than 5 and 13 nmol mg−1 (dry weight) of roots or yeast cells, respectively. In well-standardized roots or yeast cells, the reproducibility of the results was high when the initial rates of uptake were high (with rates >4 nmol min−1 mg−1, sd <10% of the mean; with rates of approximately 2 nmol min−1 mg−1, sd ≤15% of the mean). In experiments with low initial rates of uptake, the reproducibility is lower (with rates <1 nmol min−1 mg−1, sd ≤30% of the mean).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AM000056 and AM000057.
Acknowledgments
We thank Ana Villa for her technical assistance.
This work was supported by the government of Spain (grant no. AGL2004–05153), Ramón y Cajal fellowship (to M.A.B.), Doctoral Fellowship (to J.B.); by the government of Argentina, CONICET Doctoral Fellowship (to M.E.S.); and by the European Regional Development Fund program of the European Union.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Alonso Rodríguez-Navarro (alonso.rodriguez@upm.es).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.067553.
References
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF (1992) Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 89: 3736–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285: 1256–1258 [DOI] [PubMed] [Google Scholar]
- Bañuelos MA, Garciadeblas B, Cubero B, Rodríguez-Navarro A (2002) Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol 130: 784–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito B, Garciadeblás B, Schreier P, Rodríguez-Navarro A (2004) Novel P-type ATPases mediate high-affinity potassium or sodium uptake in fungi. Eukaryot Cell 3: 359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito B, Rodríguez-Navarro A (2003) Molecular cloning and characterization of a sodium-pump ATPase of the moss Physcomitrella patens. Plant J 36: 382–389 [DOI] [PubMed] [Google Scholar]
- Bennetzen JL, Ma J (2003) The genetic colinearity of rice and other cereals on the basis of genomic sequence analysis. Curr Opin Plant Biol 6: 128–133 [DOI] [PubMed] [Google Scholar]
- Berthomieu P, Conejero G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, et al (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na(+) recirculation by the phloem is crucial for salt tolerance. EMBO J 22: 2004–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR, Slayman CL (1987) Role of “active” potassium transport in the regulation of cytoplasmic pH by non-animal cells. Proc Natl Acad Sci USA 84: 2737–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294: 1351–1362 [DOI] [PubMed] [Google Scholar]
- Brunelli JP, Pall ML (1993) A series of yeast/Escherichia coli l expression vectors designed for directional cloning of cDNAs and cre/lox-mediated plasmid excision. Yeast 9: 1309–1318 [DOI] [PubMed] [Google Scholar]
- Chang K-J, Wang C-C (2004) Translation initiation from a naturally occurring non-AUG codon in Saccharomyces cerevisiae. J Biol Chem 279: 13778–13785 [DOI] [PubMed] [Google Scholar]
- Clements JM, Laz TM, Sherman F (1988) Efficiency of translation initiation by non-AUG codons in Saccharomyces cerevisiae. Mol Cell Biol 8: 4533–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue TF, Cigan AM (1988) Genetic selection for mutations that reduce or abolish ribosomal recognition of the HIS4 translational initiator region. Mol Cell Biol 8: 2955–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabkin HJ, Rajbhandary UL (1998) Initiation of protein synthesis in mammalian cells with codons other than AUG and amino acids other than methionine. Mol Cell Biol 18: 5140–5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durell SR, Guy HR (1999) Structural models of the KtrB, TrkH and Trk1,2 symporters based on the structure of the KcsA K+ channel. Biophys J 77: 789–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciadeblás B, Senn ME, Bañuelos MA, Rodríguez-Navarro A (2003) Sodium transport and HKT transporters: the rice model. Plant J 34: 788–801 [DOI] [PubMed] [Google Scholar]
- Good L, Nazar RN (1992) An improved thermal cycle for two-step PCR-based targeted mutagenesis. Nucleic Acids Res 20: 4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizouarn H, Gabillat N, Motais R, Borgese F (2001) Multiple transport functions of a red blood cell anion exchanger, tAE1: its role in cell volume regulation. J Physiol 535: 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro R, Rodríguez-Navarro A (2002) Molecular analysis of the mechanism of potassium uptake through the TRK1 transporter of Saccharomyces cerevisiae. Biochim Biophys Acta 1564: 114–122 [DOI] [PubMed] [Google Scholar]
- Haro R, Rodríguez-Navarro A (2003) Functional analysis of the M2D helix of the TRK1 potassium transporter of Saccharomyces cerevisiae. Biochim Biophys Acta 1613: 1–6 [DOI] [PubMed] [Google Scholar]
- Haro R, Sainz L, Rubio F, Rodríguez-Navarro A (1999) Cloning of two genes encoding potassium transporters in Neurospora crassa and expression of the corresponding cDNAs in Saccharomyces cerevisiae. Mol Microbiol 31: 511–520 [DOI] [PubMed] [Google Scholar]
- Hayes DE, Smith FA, Walker NA (2001) High-affinity potassium transport into wheat roots involves sodium—a role for HKT1? Aust J Plant Physiol 28: 643–652 [Google Scholar]
- Horie T, Schroeder JI (2004) Sodium transporters in plants. Diverse genes and physiological functions. Plant Physiol 136: 2457–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Yoshida K, Nakayama H, Yamada K, Oiki S, Shinmyo A (2001) Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J 27: 115–128 [DOI] [PubMed] [Google Scholar]
- Kato Y, Sakaguchi M, Mori Y, Saito K, Nakamura T, Bakker EP, Sato Y, Goshima S, Uozumi N (2001) Evidence in support of a four transmembrane-pore-transmembrane topology model for the Arabidopsis thaliana Na+/K+ translocating AtHKT1 protein, a member of the superfamily of K+ transporters. Proc Natl Acad Sci USA 98: 6488–6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DA, Devos KM (2002) Trends in comparative genetics and their potential impacts on wheat and barley research. Plant Mol Biol 48: 729–740 [DOI] [PubMed] [Google Scholar]
- Laurie S, Feeney KA, Maathuis FJM, Heard PJ, Brown SJ, Leigh RA (2002) A role for HKT1 in sodium uptake by wheat roots. Plant J 32: 139–149 [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Verlin D, Smith FA, Sanders D, Fernández JA, Walker NA (1996) The physiological relevance of Na+-coupled K+-transport. Plant Physiol 112: 1609–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R, Gómez MJ, Ramos J, Rodríguez-Navarro A (1998) Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J Biol Chem 273: 14838–14844 [DOI] [PubMed] [Google Scholar]
- Mäser P, Eckelmann B, Vaidyanathan R, Horie T, Fairbairn DJ, Kubo M, Yamagami M, Yamaguchi K, Nishimura M, Uozumi N, et al (2002. a) Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett 531: 157–161 [DOI] [PubMed] [Google Scholar]
- Mäser P, Hosoo Y, Goshima S, Horie T, Eckelmann B, Yamada K, Yoshida K, Bakker EP, Shinmyo A, Oiki S, et al (2002. b) Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc Natl Acad Sci USA 99: 6428–6433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero JM, Molina R, Fournier JM, Benlloch M, Ramos J (2001) Glucose-induced activation of rubidium transport and water flux in sunflower root systems. J Exp Bot 52: 99–104 [PubMed] [Google Scholar]
- Rains DW, Epstein E (1967. a) Sodium absorption by barley roots: role of the dual mechanisms of alkali cation transport. Plant Physiol 42: 314–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains DW, Epstein E (1967. b) Sodium absorption by barley roots: its mediation by mechanism 2 of alkali cation transport. Plant Physiol 42: 319–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J, Haro R, Rodríguez-Navarro A (1990) Regulation of potassium fluxes in Saccharomyces cerevisiae. Biochim Biophys Acta 1029: 211–217 [DOI] [PubMed] [Google Scholar]
- Ramos J, Rodríguez-Navarro A (1986) Regulation and interconversion of the potassium transport systems of Saccharomyces cerevisiae as revealed by rubidium transport. Eur J Biochem 154: 307–311 [DOI] [PubMed] [Google Scholar]
- Rathjen J, Mellor J (1990) Characterization of sequences required for RNA initiation from the PGK promoter of Saccharomyces cerevisiae. Nucleic Acids Res 18: 3219–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades JD, Kandiah A, Mashali AM (1992) The Use of Saline Waters for Crop Production. Food and Agriculture Organization, Rome
- Rodríguez-Navarro A (2000) Potassium transport in fungi and plants. Biochim Biophys Acta 1469: 1–30 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro A, Ramos J (1984) Dual system for potassium transport in Saccharomyces cerevisiae. J Bacteriol 159: 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI (1995) Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270: 1660–1663 [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI (1996) High-affinity potassium uptake in plants. Response. Science 273: 978–979 [DOI] [PubMed] [Google Scholar]
- Santa-María GE, Rubio F, Dubcovsky J, Rodríguez-Navarro A (1997) The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 9: 2281–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI (1994) Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 370: 655–658 [DOI] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon J-M, Gaymard F, Grignon C (1992) Cloning and expression in yeast of a plant potassium ion transport system. Science 256: 663–665 [DOI] [PubMed] [Google Scholar]
- Sherman F (1991) Getting started with yeast. Methods Enzymol 194: 3–21 [DOI] [PubMed] [Google Scholar]
- Véry A-A, Sentenac H (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54: 575–603 [DOI] [PubMed] [Google Scholar]
- Walker NA, Sanders D, Maathuis FJM (1996) High-affinity potassium uptake in plants. Science 273: 977–978 [DOI] [PubMed] [Google Scholar]
- Wang T-B, Gassmann W, Rubio F, Schroeder JI, Glass ADM (1998) Rapid up-regulation of HKT1, a high-affinity potassium transporter gene, in roots of barley and wheat following withdrawal of potassium. Plant Physiol 118: 651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19: 765–768 [DOI] [PubMed] [Google Scholar]