Abstract
Bacterial carbohydrates have long been considered T cell-independent antigens that primarily induce humoral immune responses. Recently, it has been demonstrated that bacterial capsules that possess a zwitterionic charge motif can activate CD4+ T cells after processing and presentation by antigen-presenting cells. Here we show that these zwitterionic polysaccharides can prevent T helper 1-mediated fibrosis by signaling for the release of IL-10 from CD4+ T cells in vivo. IL-10 production by these T cells and their ability to prevent fibrosis is controlled by the inducible costimulator (ICOS)-ICOS ligand pathway. These data demonstrate that the interaction of the zwitterionic polysaccharides with T cells results in modulation of surgical fibrosis in vivo and suggest a previously undescribed approach to “harnessing” T cell function to prevent inflammatory tissue disorders in humans.
Keywords: IL-10, microbial polysaccharides, inducible costimulator
Bacterial polysaccharides have been used with great success as components of traditional glycoconjugate vaccines that elicit protective humoral immune responses in humans. The T cell-independent nature of these polymers requires conjugation to protein antigens to induce T cell help and antibody class switching. However, a class of bacterial polysaccharides has been recently identified that can activate CD4+ T cells in vitro and in vivo and confer protection against intraabdominal abscess formation in a T cell-dependent manner (1, 2).
Structure-function studies revealed that capsular polysaccharides isolated from microorganisms such as Bacteroides fragilis, Streptococcus pneumoniae type 1, and Staphylococcus aureus type 8 possess these T cell-dependent properties based on the presence of positively and negatively charged groups associated with their repeating unit structures (1-3). These polymers, termed zwitterionic polysaccharides (ZPSs), are structurally distinct but share a common three-dimensional conformation characterized by a right-handed helix with repeated zwitterionically charged grooves (4).
These saccharides exhibit similar biologic activity, and T cell hybridomas specific for one type of ZPS recognize the other ZPSs but not polysaccharides lacking this charge motif (5). The ZPSs activate CD4+ αβ T cells by a recently described mechanism of processing and presentation by antigen-presenting cells (APCs) that requires nitric oxide-mediated degradation of these polymers in the MHCII pathway (6).
Based on these data, we hypothesized that the protective activity associated with ZPSs was attributable to the modulation of T cell function in vivo. This hypothesis was tested in an experimental rodent model of fibrosis, surgical adhesion formation. Adhesions are a common and severe fibrotic host response to trauma in the peritoneal or pelvic cavities that constitute a major clinical problem with few treatment options (7, 8). In response to intraabdominal or pelvic injury, overwhelming inflammation and fibrin deposition combine to create dense fibrous bands that can lead to major organ failure, bowel obstruction, and infertility in women (9). We have shown that experimental adhesion formation depends on fibrosis elicited by pathogenic CD4+ T helper 1 (Th1) cells (10).
Here we show that a distinct population of CD4+CD25-CD45RBlo T cells produces IL-10 in response to the ZPSs and are responsible for protection in this model. These data provide a rationale for the T cell-dependent properties elicited by a group of distinct carbohydrates, a class of molecules traditionally classified as T cell-independent antigens.
Materials and Methods
Animals. C57BL/6 WT control and IL-10-/- mice (male, 4-6 weeks old) were obtained from The Jackson Laboratory. WT and ICOS-/- 129S4/SvJae mice were bred and maintained as described (11). All animals were provided with food and water ad libitum and housed under pathogen-free conditions according to the Harvard Medical School animal management program.
Animal Model for Intraabdominal Abscess Formation. Mice were injected i.p. with 0.2 ml of B. fragilis (1 × 108 colony-forming units) mixed with sterile cecal contents (1:1 vol/vol). Six days later, animals were examined at necropsy for the presence of one or more abscesses within the peritoneal cavity by an observer blinded to the identity of the experimental groups (2).
Mouse Model of Surgical Adhesion Formation. Abdominal adhesions were induced by abrasion of the cecum and abdominal wall as described (12). Severity of adhesions was evaluated 6 days later by an observer blinded to the identity of the experimental groups according to a standard scoring system (10, 13-15): 0, no adhesion; 1, one thin filmy adhesion; 2, more than one thin adhesion; 3, thick adhesion with focal point; 4, thick adhesion with plantar attachment or more than one thick adhesion with focal point; 5, very thick vascularized adhesions or more than one plantar adhesion. The scores for the various groups were compared by Mann-Whitney U test or the Kruskal-Wallis with Dunn's Multiple Comparisons test.
Polysaccharides. S. pneumoniae type 1 capsular polysaccharide (CP1) was obtained from American Type Culture Collection and purified as described (2). Capsular polysaccharide A (PSA) from B. fragilis was purified as described (16). Polygalacturonic acid (PG), a nonzwitterionic polymer used as a control polysaccharide, was obtained from Sigma. Polysaccharides were prepared in sterile, pyrogen-free saline.
Adoptive T Cell Transfer Experiments. WT C57BL/6 mice were treated with saline, PG, or CP1 (50 μg) on three successive days before the harvest of spleens and purification of CD4+ T cells (R & D Systems columns) (>95% CD4+). For some experiments, CD4+ T cell populations were further divided in CD45RBlo or CD45RBhi cell populations by sorting using a Coulter EPICS XL cytometer (Beckman Coulter) and a mAb specific for CD45RB (BD Biosciences). The brightest 40-50% and the dimmest 20-30% were sorted as high and low populations, respectively. Intermediate CD45RB stained populations were discarded. In some cases, CD4+CD45RBlo populations were stained with a mAb specific for CD25, and CD4+CD45RBlo CD25- and CD4+CD45RBlo CD25+ were as described. Purity >95% for each population was achieved. Cell populations were transferred to naïve WT animals via the intracardiac route (i.c.) 24 h before adhesion surgery.
Antibodies and Treatments. For experiments investigating the role of IL-10 in adhesion prevention, mice were treated with 200 μg of IL-10-specific mAb or the respective isotype control (R & D Systems) via the i.p. route at t = 0, 24, 48, and 72 h after cecal abrasion. This Ab is described by the manufacturer to neutralize the bioactivity of IL-10 in vivo. To study the effect of inducible costimulator ligand (ICOSL) blockade on adhesion prevention, a rat IgG2a mAb (HK5.3) specific for murine ICOSL (17) (400 μg per mouse) or an isotype control were administered to mice i.p. 0, 48, and 96 h after surgery.
Flow Cytometry and Analysis. Spleen cells were collected at designated time points, and T cells were purified by using nylon wool columns. After preincubation with rat anti-mouse CD16/CD32 to block Fc receptors, T cells were stained with FITC-, phycoerythrin (PE)-Cy5-, or PE-labeled mAbs to CD4, CD45RB, CD25, or ICOS or the corresponding isotype control antibodies. For intracellular cytokine analysis cells were incubated for 4 h with phorbol 12-myristate 13-acetate (PMA)/ionomycin/brefeldin A (BD Biosciences), permeabilized with Cytofix/Cytoperm (BD Biosciences) and stained with PE-, Cy5-, or PE-conjugated monoclonal antibodies specific for IL-4, IFN-γ, or IL-10 or the corresponding isotype controls. Stained cells were analyzed on a Coulter EPICS XL cytometer (BD Biosciences).
Ex Vivo ZPS-Specificity Studies. WT and ICOS-/- mice were treated with CP1 (50 μg); 10 days later, splenic CD4+CD45RBlo T cells were isolated and cocultured with irradiated autologous APCs. Cells were stimulated with CP1 or PG (20 μg/ml), and culture supernatants were harvested 6 or 8 days after culture for IL-10 quantitation by ELISA.
Results
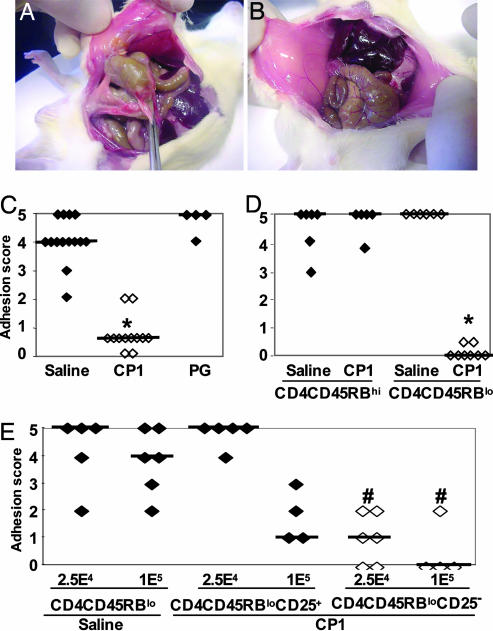
Prevention of Postsurgical Fibrosis by ZPS Treatment. To assess the T cell-dependent properties of ZPSs, we first determined whether they could prevent surgical adhesion formation in a mouse model. Rodent models closely approximate the clinical presentation of this Th1-mediated inflammatory tissue response (10, 12-14), and adhesion formation involves the cecum, large bowel, and abdominal wall (Fig. 1A). Mice were treated s.c. with saline, the zwitterionic polysaccharide CP1 (S. pneumoniae type 1 capsular polysaccharide), the control nonzwitterionic polysaccharide PG, or saline at -24, 0, and +24 h relative to surgery. This treatment regimen with ZPSs has been successful in preventing the development of intraabdominal abscess formation in rodents (1). Severity of adhesions was scored from 0 to 5 (5 being most severe) 6 days later. CP1 treatment significantly reduced the degree of adhesion formation compared with saline or PG (Fig. 1 B and C). Another ZPS, B. fragilis PSA, also protected against adhesion formation. Mice treated with PSA had a median adhesion score of 0, whereas saline-treated animals had a score of 5 (n = 12 for each group; P = 0.001, Mann-Whitney test).
Fig. 1.
T cells mediated protection against surgical adhesion by ZPS. (A) Male C57BL/6 mice were injected s.c. with saline or CP1 (50 μg per mouse) -24, 0, and +24 h relative to cecal abrasion surgery. Six days later, animals were examined and scored for the severity of adhesions as described in Materials and Methods. Saline-treated animals exhibited dense vascularized adhesions involving the cecum and opposing abdominal wall. (B) CP1-treated mice exhibited fewer and less severe adhesions. (C) Mice were treated as above with saline (filled symbols), CP1 (open symbols), or PG (filled symbols) and subjected to cecal abrasion. Animals treated with CP1 had significantly lower adhesion scores than saline- or PG-treated animals (*, P < 0.001, Kruskal-Wallis test). (D) CD4+CD45RBlo T cells from CP1-treated mice transfer protection against adhesion formation. Groups of mice were treated with saline or CP1 (50 μg per mouse) on three successive days, and splenic CD4+ T cells were isolated 1 day after the final treatment. Cells were stained with CD45RB-specific Ab and high- and low-expressing populations isolated by FACS. A total of 5 × 104 cells of each population were then transferred i.c., and animals were subjected to cecal abrasion 24 h later. Animals receiving CD4+CD45RBhi T cells (filled symbols) from saline or CP1-treated animals developed severe adhesions. Mice receiving CD4+CD45RBlo T cells (open symbols) from saline-treated mice developed severe adhesions, whereas mice receiving CD4+CD45RBlo T cells from animals treated with CP1 had significantly lower adhesion scores (*, P < 0.001 compared with CD4+CD45RBlo T cells from saline-treated mice). (E) CP1 protective effect is preferentially transferred by CD4+CD45RBloCD25- T cells. Groups of mice were treated with saline or CP1 on three successive days as described above. Splenic CD4+ T cells were isolated and double-stained with CD45RB- and CD25-specific antibodies, and CD4+CD45RBloCD25+ and CD4+CD45RBloCD25- were purified by FACS. A total of 2.5 × 104 or 1 × 105 cells of each population were then transferred i.c. to animals subjected to cecal abrasion 24 h later. Mice receiving 2.5 × 104 CD4+CD45RBloCD25+ T cells from animals treated with CP1 developed severe adhesions, whereas mice receiving more (1 × 105) such cells had lower adhesion scores but were not significantly different from animals given CD4+CD45RBlo T cells from saline-treated mice. Animals receiving 2.5 × 104 or 1 × 105CD4+CD45RBloCD25- T cells (open symbols) had significantly lower adhesion scores compared with CD4+CD45RBlo T cells from saline-treated mice (#, P < 0.005). All animal experiments were performed at least two times, and the results were combined.
Transfer of CD4+CD45RBlo T Cells from ZPS-Treated Mice Protects Against Th1-Driven Fibrosis. ZPSs can activate CD4+ T cells (2, 6), and the transfer of these cells to naïve animals prevents experimental intraabdominal abscess formation (1-3). To determine whether the protection observed in the adhesion model was also T cell-dependent, similar adoptive transfer experiments were performed.
Mice were treated with saline or CP1 on three successive days, and splenic CD4+ T cells were harvested and sorted based on the level of expression of the surface glycoprotein CD45RB. Subpopulations of CD4+ T cells expressing high and low levels of CD45RB have different cytokine secretion profiles and mediate distinct immune functions (18). Transfer of CD4+CD45RBhi T cells to lymphocyte-deficient mice can induce colitis, whereas CD4+CD45RBlo T cells can transfer protection in the same model (19-21). Therefore, CD4+CD45RBlo and CD4+CD45RBhi T cells from CP1- and saline-treated groups were transferred to different groups of naïve recipient mice that underwent cecal abrasion surgery 24 h later. All animals receiving CD4+CD45RBhi T cells (from CP1- or saline-treated mice) developed severe adhesions (Fig. 1D). In addition, mice receiving CD4+CD45RBlo T cells from saline-treated animals developed adhesions. However, the transfer of CD4+CD45RBlo T cells from CP1-treated mice resulted in a significant reduction in adhesion formation (P < 0.001). Transfer of the same number of CD4+CD45RBlo T cells from PG-treated mice did not confer protection (data not shown).
A second model of Th1-driven fibrosis, intraabdominal abscess formation, was used to confirm the role of CD4+CD45RBlo T cells in ZPS-mediated protection. A total of 5 × 104 CD4+CD45RBlo T cells from PG-, CP1-, or saline-treated mice were transferred to animals 4 h before i.p. injection of B. fragilis mixed with sterile cecal contents. Transfer of CD4+CD45RBlo T cells from CP1-treated mice prevented the formation of intraabdominal abscesses (one of nine mice developed abscesses six days later, 11% abscess rate). Transfer of the same number of cells from saline-treated or PG-treated mice did not prevent abscess formation (10 of 10 and 9 of 10 mice developed abscesses, respectively) (P value < 0.01, χ2 analysis).
CD4+CD45RBloCD25- T Cells Confer Protection. It has been shown that CD4+CD25+ T cells, generally called naturally occurring T regulatory cells, are important in the control of inflammatory processes in mice (22). To investigate the possibility that the presence of CD4+CD25+ T cells in the CD4+CD45RBlo populations isolated from CP1-treated animals was responsible for protection, transfer experiments were repeated with CD4+CD45RBlo cells depleted of the CD4+CD25+ subpopulations. CD4+CD45RBlo cells isolated from animals treated with ZPS or saline were further sorted into CD4+CD45RBloCD25- and CD4+CD45RBloCD25+ and transferred to the recipients as described above. Protection was observed when 2.5 × 104 or 1 × 105 CD4+CD45RBloCD25- T cells were transferred (Fig. 1E). In contrast, animals receiving 2.5 × 104 CD4+CD45RBloCD25+ T cells from CP1-treated mice developed severe adhesions. When more CD4+CD45RBloCD25+ T cells (1 × 105) from CP1-treated animals were transferred, some protection was observed, but this was not as effective as the transfer of an equal number of CD4+CD45RBloCD25- T cells. These data indicate that the T cell-dependent protective effect elicited by ZPS mainly resides in the CD4+CD45RBloCD25- T cell population.
To further determine whether CP1 had any effect on CD4+CD25+ T cells, in vivo expression of CD25 on CD4+CD45RBlo T cells after ZPS administration was evaluated. Mice were treated with saline, PG, or CP1 on three successive days, and splenic CD4+ T cells were harvested daily after treatment commenced and stained for CD45RB and CD25. ZPS treatment increased the proportion of CD4+CD45RBlo T cells in vivo. In animals treated with CP1, the proportion of CD4+CD45RBlo T cells increased with time, peaking at 36% of the total CD4+CD45RB population 4 days after the beginning of treatment; saline- and PG-treated mice showed 21% and 25%, respectively. The total number of CD4+CD45RBlo T cells per spleen per mouse was 4.2 × 105 in the saline-treated group, 5.3 × 105 in the PG group, and 7.6 × 105 in the CP1 group. (Fig. 5A, which is published as supporting information on the PNAS web site). In contrast, no significant differences were observed in the expression of CD25 on gated CD4+CD45RBlo T cells from any treated mice, indicating that ZPS treatment does not result in an increase in this surface marker (Fig. 5B).
Protection by ZPS-Induced CD4+CD45RBlo Cells Depends on IL-10. Several T cell populations that produce IL-10 have been shown to regulate immune responses (22), and protection conferred by CD4+CD45RBlo T cells in colitis is mediated by this cytokine (23). To determine whether IL-10 was involved in the protective activity conferred by CD4+CD45RBlo T cells, we investigated whether ZPS treatment elicited IL-10 production by these cells in vivo. Mice were treated with saline, PG, or CP1 on three successive days. Splenic CD4+ T cells were harvested daily after treatment commenced, incubated in vitro with phorbol 12-myristate 13-acetate (PMA)/ionomicin/brefeldin A, and stained for intracellular cytokines and surface CD45RB. Analysis by flow cytometry revealed that CP1 treatment resulted in an increased proportion of CD4+CD45RBlo T cells producing IL-10 compared with saline or PG treatment (Fig. 2A, gated on CD4+CD45RBlo T cells). Treatment with CP1 did not affect the proportion of CD4+CD45RBhi T cells that produce IL-10 (data not shown). Similar analysis of CD4+CD45RBlo T cells for IL-4 and IFN-γ showed few cells positive for these cytokines for all treatments and time points (Fig. 2 A), suggesting that treatment with ZPS specifically elicits IL-10 production by CD4+CD45RBlo T cells.
Fig. 2.
Role of IL-10 in ZPS-mediated protection against adhesion formation. (A) ZPS induce IL-10 production in vivo by CD4+CD45RBlo T cells. Groups of mice were treated with saline, CP1, or PG (50 μg per dose s.c. on three successive days), and splenic CD4+ T cells were harvested daily after the first dose. CD4+ T cells were incubated in vitro for 4 h with phorbol 12-myristate 13-acetate (PMA)/ionomicin/brefeldin A and stained for CD4 and CD45RB surface markers and intracellular IL-10, IL-4, and IFN-γ. The percentage of CD4+CD45RBlo cells that produced IL-10 increased after the first dose, peaking at day 4, when the percentage of CD4+CD45RBlo cells producing IL-10 was 7.4% in the saline-treated group, 5.1% in the PG-treated group, and 24.9% in the CP1-treated group. Treatment with CP1 or PG did not increase production of IL-4 or IFN-γ (day 4 shown). Results are representative of three separate experiments. (B) Protection by CP1 is abrogated by anti-IL-10 treatment. Mice were treated with saline (filled symbols) or CP1 (open symbols) as described above and treated i.p. with a mAb specific for IL-10 (200 μg at t = 0, 24, 48, and 72 h with respect to cecal abrasion surgery). Treatment of CP1-treated animals with the IL-10-specific mAb resulted in significantly higher adhesion scores than treatment with the isotype control Ab (*, P < 0.001, Mann-Whitney test). (C) Treatment with IL-10-specific mAb abrogates protection transferred by CD4+CD45RBlo T cells harvested from CP1-treated animals. CD4+CD45RBlo T cells from CP1-treated mice were transferred to naïve recipient animals that were treated 1 day later with a mAb specific for IL-10 (open symbols) or an isotype control Ab (filled symbols) (200 μgat t = 0, 24, 48, and 72 h with respect to cecal abrasion surgery). Mice receiving CD4+CD45RBlo T cells that were treated with the isotype control Ab had few adhesions. However, the protection conferred by the transfer of CD4+CD45RBlo T cells to mice was abrogated by treatment with IL-10-specific Ab (*, P = 0.0002 compared with isotype control treatment, Mann-Whitney test). (D) CP1 fails to protect IL-10-/- mice from adhesion formation. WT (filled symbols) or IL-10-/- mice (open symbols) treated with saline developed severe adhesions. CP1 treatment of WT mice yielded a reduction in adhesion scores, whereas treatment of IL-10-/- mice with CP1 did not result in protection (*, P = 0.003, Kruskal-Wallis test). (E) CD4+CD45RBlo cells harvested from IL-10-/- CP1-treated animals fail to protect against adhesion formation. CD4+CD45RBlo T cells from WT or IL-10-/- ZPS-treated mice were transferred to naïve recipient animals. CD4+CD45RBlo T cells from WT mice conferred protection, whereas CD4+CD45RBlo T cells from IL-10-/- mice did not (*, P = 0.0002, Mann-Whitney test). All animal experiments were performed at least two times, and the results were combined.
The specific role of IL-10 production in ZPS-mediated protection against adhesion formation was directly assessed in antibody blocking studies in vivo. Groups of mice were treated with saline or CP1 as described above and treated with a mAb specific for IL-10 or an isotype control Ab at 0, 24, 48, and 72 h with respect to cecal abrasion. Mice treated with saline and given the IL-10-specific Ab or an isotype control Ab developed adhesions (Fig. 2B). In contrast, mice treated with CP1 and the isotype control Ab were protected against adhesion formation. However, administration of the IL-10-specific mAb to CP1-treated mice abrogated this protective effect (P < 0.001).
We then determined the dependency of the protective activity of CD4+CD45RBlo T cells on IL-10. CD4+CD45RBlo T cells from CP1-treated mice were transferred to naïve animals subsequently treated with an IL-10-specific or an isotype control Ab at times indicated above with respect to cecal abrasion. Animals that received cells and were treated with an Ab specific for IL-10 developed more adhesions than animals treated with the control Ab (Fig. 2C). The ability to block the protective activity of CD4+CD45RBlo T cells taken from CP1-treated mice with an IL-10-specific Ab demonstrated the singular role of this cytokine in the cell-mediated protection.
We also performed experiments using IL-10-/- mice to confirm that CD4+CD45RBlo T cells from ZPSs animals are the source of the protective IL-10. Groups of WT control littermate or IL-10-/- mice were treated with saline or CP1 before cecal abrasion. As expected, WT and IL-10-/- mice treated with saline developed severe adhesions (Fig. 2D). In contrast, IL-10-/- mice treated with CP1 had a significantly higher median adhesion score than similarly treated WT mice (P < 0.003). Failure of the IL-10-/- mice to be protected by ZPS treatment confirmed our observation that IL-10 is an essential mediator of protective activity. In a separate experiment, WT and IL-10-/- mice were treated with CP1, and CD4+CD45RBlo T cells were isolated and transferred to naïve recipients. CD4+CD45RBlo T cells from IL-10-/- mice did not confer protection (Fig. 2E).
ZPS-Mediated Protection Is Controlled by the ICOS-ICOSL Pathway. The T cell costimulatory molecule ICOS has been shown to superinduce IL-10 production via the ICOS-ICOSL signaling pathway (24, 25). Therefore, the role of this pathway in IL-10-mediated protection elicited by ZPS was investigated. Mice were treated with CP1 or saline and with an ICOSL-specific blocking Ab or an isotype control Ab and subjected to cecal abrasion. Administration of an ICOSL-specific blocking Ab to mice treated with CP1 diminished protection against adhesion formation compared with CP1-treated mice receiving the isotype control (Fig. 3A, P = 0.0003). This result was corroborated in studies in which littermate control WT or ICOS-/- mice were treated with saline or CP1 and subjected to cecal abrasion. As expected, both groups treated with saline developed adhesions, whereas WT mice treated with CP1 had fewer and less severe adhesions (Fig. 3B). However, ICOS-/- mice treated with CP1 were not protected against adhesion formation (P = 0.002, compared with CP1-treated WT animals).
Fig. 3.
Protection against adhesion formation by ZPS-specific CD4+CD45RBlo cells is controlled by ICOS-ICOSL interactions. (A) ICOSL-specific Ab abrogates protection by CP1. Mice were treated with saline or CP1 before the induction of adhesions. CP1-treated mice were also given a mAb specific for ICOSL (400 μg oer mouse) or an isotype control Ab i.p. 0, 48, and 96 h after surgery. Mice treated with CP1 and the isotype control Ab (open symbols) had significantly fewer and less severe adhesions than mice treated with the mAb specific for ICOSL (filled symbols, P = 0.0003 compared with isotype control treatment, Kruskal-Wallis test). (B) ICOS-/- mice are not protected by CP1 treatment. WT (filled symbols) and ICOS-/- (open symbols) mice were treated with saline or CP1 as described above before the induction of adhesions. ICOS-/- animals treated with CP1 had higher adhesion scores than similarly treated WT animals (P = 0.002, Kruskal-Wallis test). (C) ICOSL Ab abrogates protection by CD4+CD45RBlo T cells from CP1-treated mice. Animals were treated with CP1 as described above, and CD4+CD45RBlo T cells were harvested from spleens 1 day later by FACS and transferred to two groups of naïve mice i.c. Twenty-four hours later, animals were subjected to cecal abrasion surgery. Recipient animals received the ICOSL-specific Ab (open symbols) or the isotype control Ab (filled symbols, 400 μg per mouse for each group) i.p. 0, 48, and 96 h after surgery. Treatment with ICOSL Ab abrogated protection conferred by the transfer of CD4+CD45RBlo T cells compared with treatment with an isotype control Ab (P = 0.0006, Kruskal-Wallis test). Each experiment was performed twice, and the results were combined.
To verify the relationship between the protective effect of CD4+CD45RBlo T cells and the ICOS-ICOSL pathway, experiments were performed in which CD4+CD45RBlo T cells from CP1-treated mice were isolated and adoptively transferred to naïve animals. These animals were subsequently treated with the ICOSL-blocking Ab or the isotype control Ab and underwent cecal abrasion. Mice receiving cells from CP1-treated mice that were treated with control Ab were protected against adhesion formation (Fig. 3C). However, mice receiving the cells that were treated with the ICOSL blocking Ab developed severe adhesions (P = 0.0006), indicating that blockade of ICOS-ICOSL signaling abrogated CP1-mediated protection. These data demonstrate the dependence of CP1-responsive CD4+CD45RBlo T cells upon the ICOS-ICOSL costimulatory pathway in the prevention of adhesion formation.
The effect of ZPS treatment on ICOS and IL-10 expression from CD4+CD45RBlo T cells was assessed. Animals were treated with CP1 or PG as described above, and CD4+ T cells were isolated daily after treatment began. Cells were stained for CD45RBlo and ICOS surface expression and intracellular IL-10 production. Treatment with CP1 elicited IL-10 production and ICOS expression on CD4+CD45RBlo T cells compared to PG treatment (Fig. 4A). Although ZPS treatment elicited IL-10 production in both CD4+CD45RBloICOS+ and CD4+CD45RBloICOS- T cells, the number of IL-10 producing cells was higher in the former population.
Fig. 4.
IL-10 production by CD4+CD45RBlo cells is specific for CP1 and depends on ICOS. (A) Treatment with CP1 increases IL-10 production from CD4+CD45RBlo cells that expressed ICOS, whereas PG does not. CD4+CD45RBlo cells from animals treated with CP1 or PG were analyzed for ICOS and IL-10 by flow cytometry as described above. Both ICOS expression and intracellular IL-10 levels on these cells peaked 4 days after the first dose of CP1 (shown). The percentage of CD4+CD45RBlo T cells that expressed ICOS and produced IL-10 was 5.6 in the PG-treated group and 14.0 in the CP1-treated group. (B) CP1 elicits IL-10 from CD4+CD45RBlo T cells in an antigen-specific manner. WT and ICOS-/- mice were treated with CP1 (50 μg s.c.), and 10 days later, splenic CD4+CD45RBlo T cells were isolated and cocultured with irradiated autologous APCs in vitro. Cells were stimulated with CP1 or PG (20 μg/ml), and culture supernates were harvested 6 or 8 days after culture for IL-10 quantitation by ELISA. CD4+CD45RBlo T cells from WT mice stimulated with CP1 yielded higher levels of IL-10 than did CD4+CD45RBlo T cells from ICOS-/- mice. This response was specific to CP1, because PG did not elicit IL-10 from WT or ICOS-/- CD4+CD45RBlo T cells.
To demonstrate the specificity of CD4+CD45RBlo T cells for ZPS and the role of the ICOS-ICOSL pathway in antigen recognition, WT littermate control and ICOS-/- mice were treated with CP1 (a single 50-μg s.c. dose) and splenic CD4+CD45RBlo T cells isolated and cocultured ex vivo with irradiated autologous APCs. After stimulation in vitro with CP1 or PG (20 μg/ml), culture supernates were harvested 6 or 8 days after culture, and IL-10 production was assessed by ELISA. CD4+CD45RBlo T cells from WT mice stimulated with CP1 yielded 7-fold higher levels of IL-10 than those from ICOS-/- mice at day 8 after culture (Fig. 4B). These cells did not produce IL-4 or IFN-γ as assessed by ELISA (data not shown). In contrast, PG did not elicit IL-10 from WT or ICOS-/- CD4+CD45RBlo T cells. In separate experiments, CD4+CD45RBlo T cells harvested from animals treated with PG and subsequently stimulated ex vivo by this polymer also did not produce IL-10 (data not shown).
Discussion
Previous studies have shown that ZPSs exhibit T cell-dependent properties, and as such, do not fit the conventional paradigm for immune responses to bacterial carbohydrates (2, 6). A rationale for their ability to activate T cells was provided by the finding that ZPSs interact with CD4+ T cells after processing and presentation by APCs in a nitric oxide-dependent mechanism via the MHC II pathway (6). Importantly, chemically neutralized ZPSs or polysaccharides lacking a zwitterionic motif do not possess this ability and thus do not exhibit these T cell-dependent properties (2, 3).
In the present study, a postsurgical fibrosis model of adhesion formation was used to investigate the mechanism of protection by ZPSs in vivo (1, 2). Our group has previously used this model to determine the involvement of T cells, which play a central role in the pathogenesis of numerous autoimmune and fibrotic tissue disorders, in adhesiogenesis. Th1 CD4+T cells are required for the development of postsurgical adhesions, and these T cells produce IL-17, which stimulates the production of neutrophil-specific chemokines MIP-2 and KC (10, 26). These T cells home directly to the site of surgically induced adhesions and control local chemokine production (27). Here we show that treatment of mice with ZPS prevented adhesion formation as did the transfer of CD4+CD45RBlo T cells from ZPS-treated animals. The finding that this cell population also conferred protection against another Th1-mediated fibrotic host response, abscess formation, corroborated the in vivo activity of ZPSs.
These studies clearly demonstrate that the mechanism of protection induced by ZPS treatment depends on IL-10 production from CD4+CD45RBlo T cells. ZPS induced IL-10 production from these cells, but not IFN-γ or IL-4. This conclusion was confirmed by experiments in which CD4+CD45RBlo T cells taken from ZPS-treated IL-10-/- mice failed to transfer protection. It should be noted that this conclusion is based on our assumption that the phenotype of these cells is the same (other than their ability to produce IL-10) in WT and IL-10-/- mice. These data are in agreement with previous studies that demonstrate that frequent administration of high doses of recombinant IL-10 prevent surgical adhesions in mice (28-30). The use of ZPSs to induce endogenous IL-10 production in vivo may obviate the many problems associated with direct administration of recombinant IL-10. Clinical trials of this cytokine's ability to prevent inflammatory diseases failed because sufficient serum concentrations cannot be maintained or targeted to sites of inflammation (31).
ICOS-ICOSL interactions are critical to the generation of IL-10 producing T cells and ultimately in the protective activity elicited by ZPS. ZPS treatment in vivo results in an increase in ICOS+ IL-10+ CD4+CD45RBlo T cells. Importantly, IL-10 production by CD4+CD45RBlo T cells ex vivo was antigen-specific and dependent on ICOS-ICOSL signaling. These data were corroborated by in vivo experiments clearly showing that ICOS-ICOSL interactions are necessary for protection.
Demonstration of the importance of this interaction in the generation of IL-10-producing CD4+ T cells in vivo confirms previous studies (25, 32, 33). In vivo-derived ovalbumin-specific IL-10 producing CD4+ T cells have been described that mediate the acquisition of tolerance in an experimental model of airway hyperreactivity using transgenic mice expressing the T cell receptor specific for this protein (25). The generation of these cells in vivo depended on ICOS-ICOSL interactions. Most recently, this group has demonstrated the existence of regulatory CD4+ T cells distinct from CD4+CD25+ T cells that produce IL-10 and IFN-γ (33).
T cells that control or suppress a range of inflammatory disorders such as autoimmune reactions, transplant rejection, and inflammatory bowel disease in experimental models have been described in detail (reviewed in ref. 22). These cells, generally termed T regulatory (Treg) cells, have been identified by using surface markers such as CD25. However, it is now clear that Treg cells comprise a heterogeneous population, and that CD4+ T cells, irrespective of CD25 expression, can exhibit suppressor activity (34). This finding is corroborated by previous studies that demonstrate that both CD25- and CD25+ CD4+CD45RBlo T cells can equally transfer protection not only against colitis induced by CD4+CD45RBhi T cells in SCID mice (18, 35) but also against colitis induced by Helicobacter hepaticus in recombination activating gene (RAG) knockout mice (36). In these models, production of IL-10 by the transferred cell population is necessary and sufficient for protection.
The goal of this study was to determine the mechanism by which ZPSs induce protection against Th1-mediated inflammatory diseases. These data show that this mechanism depends on IL-10 production by CD4+CD45RBlo T cells in vivo. There are few instances of T cells being induced in vivo to produce IL-10 after short-term antigenic exposure of unmanipulated WT mice. This finding is particularly striking given the fact that ZPSs are carbohydrate polymers traditionally considered T cell-independent antigens. We hypothesize that the common conformational structure associated with ZPSs likely provides a rationale for their interaction with a subset of naïve CD4+ T cells that are induced to become IL-10 producing cells or an existing population of CD4+CD25-CD45RBlo T cells that are induced to expand. The molecular mechanism underlying expansion of these cells requires investigation. In addition to ICOS-ICOSL interactions, signals through other members of the B7:CD28 family may be involved in directing IL-10 production by CD4+CD25-CD45RBlo cells in response to ZPSs. CTLA-4 and PD-1 are the predominant inhibitory pathways that regulate T cell responses (37); moreover, our group has described the role of PD-1 on controlling incidence and severity of adhesions (27).
We have shown that IL-2 produced by CD4+ T cells taken from ZPS treated animals plays a role in the protection against abscess induction. In the present study, we demonstrate that IL-10 production by CD4+CD25-CD45RBlo T cells is responsible for prevention of another form of postsurgical fibrosis. Further studies are necessary to determine they relationship between IL-2 and IL-10 production by CD4+ T cells and whether they are part of the same pathway leading to protection by ZPSs.
In summary, these data show that ZPSs are a class of microbial antigens with distinct antiinflammatory properties mediated by their ability to induce IL-10 production by T cells in vivo. Bacterial polysaccharides have been used with great success as components of traditional vaccines that elicit protective humoral immune responses in humans. Here, we show that certain bacterial capsules have the ability to modulate host immune responses in a T cell-dependent manner and offer an approach for preventing or treating deleterious inflammatory host tissue responses such as adhesions and inflammatory bowel disease in humans.
Supplementary Material
Acknowledgments
We thank Dr. S. Mazmanian for critical review of this manuscript and R. J. Panzo and M. Adamowicz for expert technical assistance. This work was supported in part by National Institutes of Health Grants R01 GM64805-01 (to A.O.T.) and R01 AI039576 (to D.L.K.).
Author contributions: B.R.-P., D.R.C., D.L.K., and A.O.T. designed research; B.R.-P., D.R.C., and A.O.T. performed research; B.R.-P., D.R.C., A.H.S., H.Y., M.H.S., and A.O.T. contributed new reagents/analytic tools; B.R.-P., D.R.C., A.H.S., H.Y., W.K.-M., M.H.S., D.L.K., and A.O.T. analyzed data; and B.R.-P. and A.O.T. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ZPS, zwitterionic polysaccharides; APC, antigen-presenting cell; Th1, T helper 1; CP1, type 1 capsular polysaccharide; PSA, capsular polysaccharide A; PG, polygalacturonic acid; ICOS, inducible costimulator; ICOSL, ICOS ligand.
References
- 1.Tzianabos, A. O., Kasper, D. L., Cisneros, R. L., Smith, R. S. & Onderdonk, A. B. (1995) J. Clin. Invest. 96, 2727-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzianabos, A. O., Finberg, R. W., Wang, Y., Chan, M., Onderdonk, A. B., Jennings, H. J. & Kasper, D. L. (2000) J. Biol. Chem. 275, 6733-6740. [DOI] [PubMed] [Google Scholar]
- 3.Tzianabos, A. O., Wang, J. Y. & Lee, J. C. (2001) Proc. Natl. Acad. Sci. USA 98, 9365-9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, Y. H., Roehrl, M. H., Kasper, D. L. & Wang, J. Y. (2002) Biochemistry 41, 15144-15151. [DOI] [PubMed] [Google Scholar]
- 5.Stingele, F., Corthesy, B., Kusy, N., Porcelli, S. A., Kasper, D. L. & Tzianabos, A. O. (2004) J. Immunol. 172, 1483-1490. [DOI] [PubMed] [Google Scholar]
- 6.Cobb, B. A., Wang, Q., Tzianabos, A. O. & Kasper, D. L. (2004) Cell 117, 677-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis, H. (1997) Eur. J. Surg. Suppl. 577, 5-9. [PubMed] [Google Scholar]
- 8.Ellis, H., Moran, B. J., Thompson, J. N., Parker, M. C., Wilson, M. S., Menzies, D., McGuire, A., Lower, A. M., Hawthorn, R. J., O'Brien, F., et al. (1999) Lancet 353, 1476-1480. [DOI] [PubMed] [Google Scholar]
- 9.Chegini, N. (2002) Front. Biosci. 7, e91-e115. [DOI] [PubMed] [Google Scholar]
- 10.Chung, D. R., Chitnis, T., Panzo, R. J., Kasper, D. L., Sayegh, M. H. & Tzianabos, A. O. (2002) J. Exp. Med. 195, 1471-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenwald, R. J., McAdam, A. J., Van der Woude, D., Satoskar, A. R. & Sharpe, A. H. (2002) J. Immunol. 168, 991-995. [DOI] [PubMed] [Google Scholar]
- 12.Krause, T. J., Zazanis, G. A. & Mckinnon, R. D. (1996) Surgery 4, 53-57. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy, R., Costain, D. J., McAlister, V. C. & Lee, T. D. (1996) Surgery 120, 866-870. [DOI] [PubMed] [Google Scholar]
- 14.Kocak, I., Unlu, C., Akcan, Y. & Yakin, K. (1999) Fertil. Steril. 72, 873-878. [DOI] [PubMed] [Google Scholar]
- 15.Nagelschmidt, M. & Saad, S. (1997) J. Surg. Res. 67, 113-118. [DOI] [PubMed] [Google Scholar]
- 16.Tzianabos, A. O., Onderdonk, A. B., Rosner, B., Cisneros, R. L. & Kasper, D. L. (1993) Science 262, 416-419. [DOI] [PubMed] [Google Scholar]
- 17.Harada, H., Salama, A. D., Sho, M., Izawa, A., Sandner, S. E., Ito, T., Akiba, H., Yagita, H., Sharpe, A. H., Freeman, G. J. & Sayegh, M. H. (2003) J. Clin. Invest. 112, 234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powrie, F., Leach, M. W., Mauze, S., Caddle, L. B. & Coffman, R. L. (1993) Int. Immunol. 5, 1461-1471. [DOI] [PubMed] [Google Scholar]
- 19.Read, S., Malmstrom, V. & Powrie, F. (2000) J. Exp. Med. 192, 295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foussat, A., Cottrez, F., Brun, V., Fournier, N., Breittmayer, J. P. & Groux, H. (2003) J. Immunol. 171, 5018-5026. [DOI] [PubMed] [Google Scholar]
- 21.Annacker, O., Pimenta-Araujo, R., Burlen-Defranoux, O., Barbosa, T. C., Cumano, A. & Bandeira, A. (2001) J. Immunol. 166, 3008-3018. [DOI] [PubMed] [Google Scholar]
- 22.Powrie, F. & Maloy, K. J. (2003) Science 299, 1030-1031. [DOI] [PubMed] [Google Scholar]
- 23.Asseman, C., Mauze, S., Leach, M. W., Coffman, R. L. & Powrie, F. (1999) J. Exp. Med. 190, 995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohning, M., Hutloff, A., Kallinich, T., Mages, H. W., Bonhagen, K., Radbruch, A., Hamelmann, E. & Kroczek, R. A. (2003) J. Exp. Med. 197, 181-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akbari, O., Freeman, G. J., Meyer, E. H., Greenfield, E. A., Chang, T. T., Sharpe, A. H., Berry, G., DeKruyff, R. H. & Umetsu, D. T. (2002) Nat. Med. 8, 1024-1032. [DOI] [PubMed] [Google Scholar]
- 26.Chung, D. R., Kasper, D. L., Panzo, R. J., Chtinis, T., Grusby, M. J., Sayegh, M. H. & Tzianabos, A. O. (2003) J. Immunol. 170, 1958-1963. [DOI] [PubMed] [Google Scholar]
- 27.Holsti, M. A., Chitnis, T., Panzo, R. J., Bronson, R. T., Yagita, H., Sayegh, M. H. & Tzianabos, A. O. (2004) J. Immunol. 172, 5774-5781. [DOI] [PubMed] [Google Scholar]
- 28.Montz, F. J., Holschneider, C. H., Bozuk, M., Gotlieb, W. H. & Martinez-Maza, O. (1994) Fertil. Steril. 61, 1136-1140. [DOI] [PubMed] [Google Scholar]
- 29.Holschneider, C. H., Cristoforoni, P. M., Ghosh, K., Punyasavatsut, M., Abed, E. & Montz, F. J. (1997) J. Surg. Res. 70, 138-143. [DOI] [PubMed] [Google Scholar]
- 30.Holschneider, C. H., Nejad, F. & Montz, F. J. (1999) Fertil. Steril. 71, 67-73. [DOI] [PubMed] [Google Scholar]
- 31.Stokkers, P. C. & Hommes, D. W. (2004) Cytokine 28, 167-173. [DOI] [PubMed] [Google Scholar]
- 32.Kohyama, M., Sugahara, D., Sugiyama, S., Yagita, H., Okumura, K. & Hozumi, N. (2004) Proc. Natl. Acad. Sci. USA 101, 4192-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stock, P., Akbari, O., Berry, G., Freeman, G. J., Dekruyff, R. H. & Umetsu, D. T. (2004) Nat. Immunol. 5, 1149-1156. [DOI] [PubMed] [Google Scholar]
- 34.Fontenot, J. D., Rasmussen, J. P., Williams, L. M., Dooley, J. L., Farr, A. G. & Rudensky, A. Y. (2005) Immunity 22, 329-341. [DOI] [PubMed] [Google Scholar]
- 35.Groux, H. & Powrie, F. (1999) Immunol. Today 20, 442-445. [DOI] [PubMed] [Google Scholar]
- 36.Kullberg, M. C., Jankovic, D., Gorelick, P. L., Caspar, P., Letterio, J. J., Cheever, A. W. & Sher, A. (2002) J. Exp. Med. 196, 505-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenwald, R. J., Freeman, G. J. & Sharpe, A. H. (2005) Annu. Rev. Immunol. 23, 515-548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.