Abstract
In cells of Saccharomyces cerevisiae, using ammonia as a source of nitrogen, Gln3p is sequestered in the cytoplasm by Ure2p but enters the nucleus when the cells are shifted to a nonpreferred source of nitrogen such as proline. The interpretation of recently published observations provides evidence for the view that Ure2p is the sensor for a drop in the intracellular concentration of glutamine, a signal that results in the polyubiquitination of the vesicle responsible for retaining the Gln3p–Ure2p complex in the cytoplasm. As a consequence of the drop in glutamine concentration, Gln3p is able to enter the nucleus and to activate the transcription of nitrogen-regulated genes.
Keywords: Gln3p, Npr1p, Rsp5p, Ure2p
In the last section of our review of nitrogen regulation in Saccharomyces cerevisiae in 2002 (1), we pointed out that “although we understand many of the molecular details of how transcription of nitrogen-regulated genes is controlled, we know relatively little about the nature and origin of the intracellular signals that govern the activity of the transcription factors.” In the meantime, a number of articles have appeared with results that make it possible to suggest a pathway of signal transduction for nitrogen regulation.
When our review was written, it had been known for a long time that the GATA factor Gln3p is responsible for the activation of transcription of nitrogen-regulated genes when the cells are shifted from a preferred source of nitrogen such as glutamine or ammonia to a nonpreferred nitrogen source such as proline (2, 3) and that Ure2p is responsible for preventing the activation of gene expression by Gln3p in cells grown on the preferred source of nitrogen (4). More recently it has been shown that Ure2p is capable of binding Gln3p and retaining it in the cytoplasm and that the shift from the preferred to the nonpreferred source of nitrogen results in the release of Gln3p and its entry into the nucleus, where it activates the transcription of nitrogen-regulated genes (5, 6).
It also has been known for a long time that the stimulus for the expression of nitrogen-regulated genes, the shift from a preferred to a nonpreferred source of nitrogen, results in a drop in the intracellular concentration of glutamine. It was shown that a mutant with diminished ability to produce glutamine because of a mutation in GLN1, the structural gene for glutamine synthetase, grown in the medium with ammonia as a source of nitrogen, expressed the nitrogen-regulated gene GDH2, the structural gene coding for the NAD-linked glutamate dehydrogenase, and the defective GLN1 gene at levels corresponding to those found in cells lacking the URE2 gene. The addition of glutamine to the medium prevented the activation of the expression of GDH2 and GLN1 by Gln3p (2, 7). More recently it has been shown that treatment with l-methionine sulfoximine, an inhibitor of glutamine synthetase, similarly leads to increased expression of nitrogen-regulated genes in cells grown on a preferred source of nitrogen, in this case, ammonia together with glutamate (8). It is, however, not known how the drop in the intracellular concentration of glutamine results in the release of Gln3p from its association with Ure2p and its entry into the nucleus.
One of the agents considered to be responsible for the ability of Ure2p to retain Gln3p in the cytoplasm in cells grown on a preferred source of nitrogen is the TOR (target of rapamycin) kinase complex, because inactivation of the TOR complex by treatment with rapamycin results in the transfer of Gln3p from the cytoplasm to the nucleus and the activation of the transcription of nitrogen-regulated genes (6). Furthermore it was found that in cells grown on a preferred source of nitrogen, Gln3p is phosphorylated, and that treatment with rapamycin results in its dephosphorylation. Accordingly, it was proposed that the shift to the nonpreferred source of nitrogen causes TOR to activate a phosphatase able to dephosphorylate Gln3p-phosphate and, in this manner, to release Gln3p from its association with Ure2p, facilitating its entry into the nucleus (6). However, recent observations militate against this view by demonstrating convincingly that the shift to a nonpreferred source of nitrogen results in the activation of the expression of nitrogen-regulated genes without reducing the phosphorylation of Gln3p. Because in cells grown on a nonpreferred source of nitrogen practically all of the Gln3p is nuclear, it appears that dephosphorylation of Gln3p-phosphate is not required for its detachment from Ure2p and its entry into the nucleus (9, 10).
These observations make it unlikely that the TOR complex plays a role in the transduction of the signal, which is a drop in the intracellular concentration of glutamine, to the transcription factor Gln3p. This conclusion receives additional support from the observation that treatment with latrunculin, an inhibitor of actin polymerization, blocks the transfer of Gln3p to the nucleus when the cells are shifted from a preferred to a nonpreferred source of nitrogen but not when the cells growing on the preferred source of nitrogen are treated with rapamycin (11).
The observation that the transfer of Gln3p to the nucleus requires an intact actin cytoskeleton is in good accord with the observation that the complex is not diffused in the cytoplasm but located in foci, presumably by association with intracellular membrane structures (11). Although the authors favor the idea that a functional cytoskeleton is required for Gln3p to move into the nucleus, it seems equally possible that the actin cytoskeleton is required to transduce the signal. It is possible to distinguish these possibilities by examining the effect of the loss of Ure2p on the gln3-like phenotype resulting from the treatment with latrunculin. In the latter case, but not in the former case, the lack of Ure2p should suppress the effect of latrunculin.
In this connection it is of interest to consider the results of an earlier study of gln3-like mutations (12, 13). It was found that mutations in five unlinked genes resulted in this phenotype, and that in each case the phenotype of the ure2 mutant, high expression of the nitrogen-regulated GLN1 gene coding for the expression of glutamine synthetase in cells growing on glutamine, was epistatic to the phenotype of the gln3-like mutants, the inability to activate the increased transcription of GLN1 in cells growing on glutamate, a nonpreferred source of nitrogen.
It is therefore apparent that the gln3-like mutations are in genes that code for products responsible for the transduction of the nitrogen regulation signal to the cytoplasmic Gln3p–Ure2p complex. The fact that no gln3-like mutants with a phenotype epistatic to that of the ure2 mutant were found makes it likely that the actin cytoskeleton is involved in the transduction of the nitrogen signal and not in the transfer of Gln3p from the cytoplasm to the nucleus. This conclusion is in good accord with the finding that, in cells growing on the nonpreferred nitrogen source proline, treatment with latrunculin results in the transfer of Gln3p from the nucleus to the cytoplasm (11).
The concept that the actin cytoskeleton plays a role in the transduction of the nitrogen regulation signal receives further support from the observation that Rsp5p, a ubiquitin protein ligase, which has been shown to play a role in endocytosis through the maintenance and remodeling of the actin cytoskeleton (14), also plays an essential role in the transduction of the nitrogen signal to the Gln3p–Ure2p vesicular complex (15). Rsp5p and Bul1,2p are required for the polyubiquitination of vesicular proteins and their transfer to the vacuole (16). It is therefore likely that the polyubiquitination of a vesicular component associated with the Gln3p–Ure2p complex is responsible for the release of Gln3p from its association with Ure2p and its transfer to the nucleus.
The polyubiquitination of this component by Rsp5, Bul1,2p is, as in the case of other vesicular components, antagonized by Npr1p (15, 17). Consequently, in cells lacking Npr1p, just as in cells lacking Ure2p, Gln3p enters the nucleus during growth on a preferred source of nitrogen and activates the expression of nitrogen-regulated genes; however, in contrast to the loss of Ure2p, the loss of Npr1p does not permit Gln3p to enter the nucleus in cells with defective Rsp5p or lacking Bul1,2p (15).
It will be interesting to discover whether the phenotype of cells treated with latrunculin, inability to permit the transfer of Gln3p to the nucleus, is epistatic to that of the npr1 mutant, inability to retain Gln3p in the cytoplasm. If this turns out to be the case, it would indicate that the ability of Rsp5p, Bul1,2p to interact with the vesicle that retains the Gln3p–Ure2p complex in the cytoplasm depends on an intact actin cytoskeleton.
The question remains how the decrease in the intracellular concentration of glutamine prevents Npr1p from antagonizing the polyubiquitination of the vesicular component associated with the Gln3p–Ure2p complex. It is unlikely that Npr1p itself is the sensor of this signal because, in another case, the polyubiquitination of the vesicular general amino acid permease, the signal that prevents Npr1p from antagonizing Rsp5p is an increase in the intracellular concentration of glutamine or of any other amino acid (18). It is therefore more likely that Ure2p senses the decline in the intracellular glutamine concentration. This idea is supported by the observation that the inactivation of glutamine synthetase by glutamine requires Ure2p (12, 19).
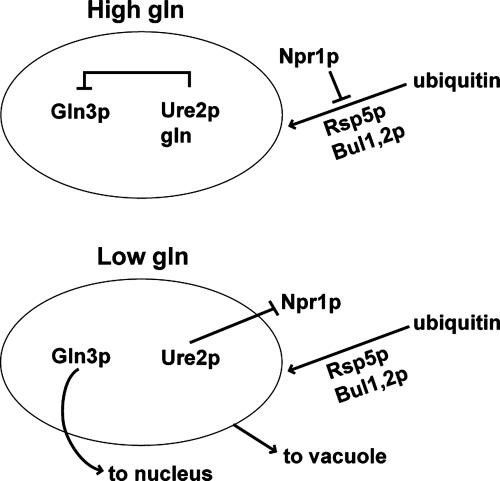
According to this view, the dissociation of glutamine from Ure2p enables Ure2p to inactivate the Npr1p associated with it in a vesicular complex. The resulting polyubiquitination and eventual destruction in the vacuole of this vesicle, which retains the Gln3p–Ure2p complex in the cytoplasm, enables Gln3p to enter the nucleus and to activate transcription of nitrogen-regulated genes (Fig. 1).
Fig. 1.
The release of Gln3p from the vesicle retaining the Gln3p–Ure2p complex in the cytoplasm in response to a drop in the intracellular concentration of glutamine (gln). See the text for more details.
Acknowledgments
I thank Hilda Harris-Ransom for the preparation of the manuscript.
Author contributions: B.M. wrote the paper.
Conflict of interest statement: No conflicts declared.
References
- 1.Magasanik, B. & Kaiser, C. A. (2002) Gene 290, 1–18. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell, A. P. & Magasanik, B. (1984) Mol. Cell. Biol. 4, 2758–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minehart, P. L. & Magasanik, B. (1991) Mol. Cell. Biol. 11, 6216–6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courchesne, W. E. & Magasanik, B. (1988) J. Bacteriol. 170, 708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blinder, D., Coschigano, P. W. & Magasanik, B. (1996) J. Bacteriol. 178, 4734–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck, T. & Hall, M. N. (1999) Nature 402, 689–692. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell, A. P. (1985) Genetics 111, 243–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crespo, J. L., Powers, T., Fowler, B. & Hall, M. N. (2002) Proc. Natl. Acad. Sci. USA 99, 6784–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, K. H., Kulkarni, A., Tate, J. J. & Cooper, T. G. (2005) J. Biol. Chem. 279, 10270–10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tate, J. J., Rai, R. & Cooper, T. G. (2005) J. Biol. Chem. 280, 27195–27204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox, K. H., Tate, J. J. & Cooper, T. G. (2004) J. Biol. Chem. 279, 19294–19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coschigano, P. W. & Magasanik, B. (1991) Mol. Cell. Biol. 11, 822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coschigano, P. W. (1991) Ph.D. thesis (Massachusetts Institute of Technology, Cambridge, MA).
- 14.Kamínska, J., Gajewski, B., Hopper, A. K. & Zoladek, T. (2002) Mol. Cell. Biol. 22, 6946–6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crespo, J. L., Helliwell, S. B., Wiederkehr, C., Demougin, P., Fowler, B., Primig, M. & Hall, M. N. (2004) J. Biol. Chem. 279, 37512–37517. [DOI] [PubMed] [Google Scholar]
- 16.Helliwell, S. B., Lasko, S. & Kaiser, C. A. (2001) J. Cell Biol. 153, 649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeCraene, J. O., Soetens, O. & André, B. (2001) J. Biol. Chem. 276, 43939.–43948. [DOI] [PubMed] [Google Scholar]
- 18.Chen, E. J. & Kaiser, C. A. (2002) Proc. Natl. Acad. Sci. USA 99, 14837–14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legrain, C., Vissers, S., Dubois, E., Legrain, M. & Wiame, J. M. (1982) Eur. J. Biochem. 123, 611–616. [DOI] [PubMed] [Google Scholar]