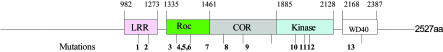
The drama of kinases, mitochondrial dysfunction, and Parkinson's disease (PD) has introduced a new player: leucine-rich repeat kinase 2 (LRRK2), a complex protein with multiple domains, including a leucine-rich repeat (LRR), a ROC-COR GTPase, a mitogen-activated protein kinase kinase kinase, and WD40 domains. Mutations have been found in all domains in LRRK2 and have been identified as the cause of the late-onset, autosomal-dominant Park8 type of Parkinson's disease (1–4). The mutations are found in 5–6% of patients with familial PD. Importantly, mutations have also been associated with sporadic PD with unprecedented 1–2% prevalence (5) (Fig. 1).
Fig. 1.
LRRK2 domain structures and mutations. LRRK2 has 2,527 amino acids and contains a LRR domain (for protein/protein interaction), Ras-in-complex and C-terminal of ROC domain (GTPase), Ser/Thr kinase domain, and WD40 domain (for protein/protein interactions). Numbers above the protein line indicate the boundaries of each domain. Numbers below the protein line indicate mutations. G2019S (mutation 11) and R1441C (mutation 4) are the two mutations that West et al. (6) used in their experiments.
In this issue of PNAS, West et al. (6) present exciting data and insight into the biochemical properties of LRRK2 and its possible role in PD pathogenesis. West et al. (6) show that LRRK2 is indeed a kinase and that it is associated with the mitochondrial outer membrane. Moreover, the autosomal-dominant PARK8-associated mutations (G2019S and R1441C) increased LRRK2 kinase activity. These three results have laid the groundwork on which new hypotheses can be generated and tested.
A protein with sequences that encode kinase domains cannot be taken for granted as an authentic kinase. For example, transmembrane guanylyl cyclases contain a conserved kinase-like domain that assumes a regulatory function rather than kinase activity (7). To demonstrate the kinase activity of LRRK2, West et al. (6) first showed that myelin basic protein can serve as an artificial substrate. Subsequently, the researchers demonstrated that LRRK2 is autophosphorylated. These two sets of experiments not only confirm that LRRK2 is an authentic kinase but also raise an interesting possibility that autophosphorylation may be a regulatory mechanism of LRRK2 function.
The autosomal-dominant mode of Park8 has two potential causes. First, the mutations in LRRK2 may cause a hyperactivity or gain-of-function of LRRK2. Alternatively, mutations could cause a loss of LRRK2 function, and the haploinsufficiency would lead to a dominant disease inheritance. West et al. (6) demonstrated that two disease mutations in human Park8, G2019S and R1441C, augment LRRK2 kinase activity. This result favors the hyperactivity/gain-of-function hypothesis, assuming that kinase activity is the main biological function of LRRK2.
The G2019S mutation is within the conserved kinase activation segment. Interestingly, there is another Park8 mutation, I2020T, immediately adjacent to the G2019S mutation, indicating the importance of this structural moiety in determining kinase activity and pathogenicity. These two mutations are at a peptide hinge to a loop structure that normally covers the kinase catalytic site and prevents substrate access. It is conceivable that mutations at the loop structure may enhance substrate access to LRRK2 kinase. Interestingly, a mutation at the same structure in the Ser/Thr kinase BRAF causes kinase hyperactivity and malignant melanomas (8).
The R1441C mutation is in the ROC-COR GTPase domain. West et al. (6) found that this mutation also increased LRRK2 kinase activity. It will be very important to test whether LRRK2, with its highly conserved ROC-COR structure, is an authentic GTPase. There is a large body of evidence that GTPases have a critical role in regulating Tyr kinases (9–11) as well as Ser/Thr kinases (12). The special feature of LRRK2, however, is that the GTPase and kinase are in the same protein. This unique feature is shared by a family of “Roco” proteins that are highly conserved through evolution (13). Because this family of proteins was only recently identified, it remains to be explored whether intramolecular signal transduction is a new mechanism of regulation for this class of protein kinases. It is also worth noting that DAPK (death-associated protein kinase), a Ca2+/calmodulin-dependent kinase in this superfamily, has a proapoptotic function that is strictly dependent on the kinase activity (14).
The LRR and WD40 domains also contain human Park8 mutations, yet they have not been explored thus far. LRR is a conserved protein/protein interaction domain (15). There is evidence that LRR of the Ran-GTPase-activating protein is required for binding and activating Ran GTPase (16). Because the LRR domain of LRRK2 is adjacent to the GTPase domain, it will be interesting to examine whether the human Park8 mutations in the LRR domain have an effect on GTPase activity and whether it in turn may regulate kinase activity of LRRK2.
WD40 is also a conserved protein/protein interaction domain. WD40 proteins are involved in a wide range of cellular functions, including signal transduction, mRNA processing, transcription, cytoskeletal assembly, and mitochondrial fission (17). Mitochondrial fission/fusion has been implicated in apoptosis, neurodegeneration, and aging (18). Intriguingly, both GTPases (Drp1 and OPA1) and WD40 proteins (Caf4p and Mdv1p) have been identified as components of the mitochondrial fission/fusion machinery (18–20). A mutation in OPA1 causes dominant optic atrophy (21, 22).
At the molecular level, how can we conceptualize that so many mutations in different domains all cause a dominant gain-of-function of LRRK2? Our speculation is that LRRK2 kinase activity may have several inhibitory regulations, ranging from intramolecular mechanisms to recruitment of other interacting proteins as negative regulators. Any mutations affecting these inhibitory regulations will manifest in hyperactive kinase activity. In fact, many tyrosine kinases and Ser/Thr kinases have intrinsic autoinhibition structures and mechanisms, such as c-Abl (23) and α-p21-activated protein kinase (24). In the case of c-Abl, the disruption of the autoinhibition leads to chronic myelogeneous leukemia.
The third important finding from West et al. (6) is that although most of LRRK2 is localized in the cytosol, ≈10% is clearly associated with the mitochondrial outer membrane. Could hyperactive kinase activity of LRRK2 lead to mitochondrial dysfunction? This question is of immediate interest, because mitochondrial dysfunction has been emerging as a common theme in recent PD studies.
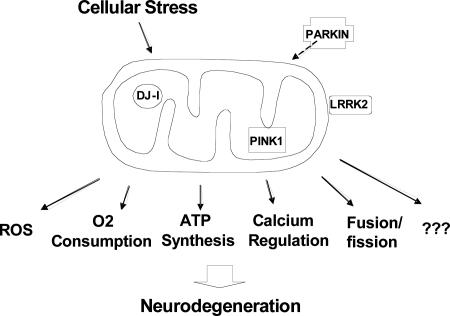
Mitochondrial dysfunction is implicated in normal aging as well as in neurodegenerative diseases such as Alzheimer's disease, Huntington's disease, and PD (25–28). As to PD patients, first, a 30–40% decrease of mitochondrial electron transport complex I activity was observed in the substantia nigra, and complex I subunits are selectively reduced (29–32). Second, in both human and non-human primates, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and rotenone, which are respiratory complex I inhibitors, induce a clinical syndrome that replicates the hallmarks of PD (33, 34). Third, mitochondrial dysfunction has been implicated in several familial PD models. (i) A decrease of mitochondrial respiratory function and an increase in oxidative damage were observed in Parkin knockout models in both mouse and Drosophila (35–37). (ii) Overexpression of DJ-1, a protein localized to the mitochondria (38, 39), protects against the oxidative stress response and mitochondrial damage, whereas reduction has the opposite effect (38, 40). (iii) The mutations leading to the autosomal-recessive Park6 type of PD are in PINK1, a nuclear-encoded kinase that is physically localized to mitochondria. Taken as a whole, four familial PD gene products are either localized to mitochondria (PINK1, DJ-1, and LRRK2) or lead to a functional deficit in mitochondria (Parkin and DJ-1), supporting a central role of mitochondria in PD pathogenesis (Fig. 2).
Fig. 2.
A model of mitochondria and PD pathogenesis. Cellular damage by mutations in DJ-1, PINK1, LRRK2, and PARKIN may converge on mitochondria. Identifying the specific aspects of mitochondrial dysfunction is the next challenge.
In summary, the initial characterization of LRRK2 protein by West et al. (6) has provided important information for future studies. Several crucial lines of investigation are expected to emerge. In vivo models will be needed to study LRRK2 and its pathogenesis pathways, especially in the dopaminergic neurons of substantia nigra. Also, it will be important to identify substrates of LRRK2 kinase and LRRK2 interacting proteins to understand the molecular components and pathways of signaling, not only for mechanistic studies but also for therapeutic development, because kinase pathways and components are highly “drug-able” targets. Finally, it will be crucial to understand whether mitochondrial dysfunction is caused by LRRK2 mutations and whether there are converging points of deficits caused by mutations in DJ-1, Parkin, and PINK1.
The stage has four players, more may be coming, and the plot will unfold.
Acknowledgments
We sincerely thank Drs. Wencheng Liu, Yanping Li, and Ai Yamamoto for insightful discussions. M.F.B. is supported by the Parkinson's Disease Foundation, the M. J. Fox Foundation, and the Department of Defense.
C.L. and M.F.B. wrote the paper.
Conflict of interest statement: No conflicts declared.
See companion article on page 16842.
References
- 1.Farrer, M., Stone, J., Mata, I. F., Lincoln, S., Kachergus, J., Hulihan, M., Strain, K. J. & Maraganore, D. M. (2005) Neurology 65, 738–740. [DOI] [PubMed] [Google Scholar]
- 2.Zimprich, A., Muller-Myhsok, B., Farrer, M., Leitner, P., Sharma, M., Hulihan, M., Lockhart, P., Strongosky, A., Kachergus, J., Calne, D. B., et al. (2004) Am. J. Hum. Genet. 74, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paisan-Ruiz, C., Jain, S., Evans, E. W., Gilks, W. P., Simon, J., van der Brug, M., de Munain, A. L., Aparicio, S., Gil, A. M., Khan, N., et al. (2004) Neuron 44, 595–600. [DOI] [PubMed] [Google Scholar]
- 4.Nichols, W. C., Pankratz, N., Hernandez, D., Paisan-Ruiz, C., Jain, S., Halter, C. A., Michaels, V. E., Reed, T., Rudolph, A., Shults, C. W., et al. (2005) Lancet 365, 410–412. [DOI] [PubMed] [Google Scholar]
- 5.Gilks, W. P., Abou-Sleiman, P. M., Gandhi, S., Jain, S., Singleton, A., Lees, A. J., Shaw, K., Bhatia, K. P., Bonifati, V., Quinn, N. P., et al. (2005) Lancet 365, 415–416. [DOI] [PubMed] [Google Scholar]
- 6.West, A. B., Moore, D. J., Biskup, S., Bugayenko, A., Smith, W. W., Ross, C. A., Dawson, V. L. & Dawson, T. M. (2005) Proc. Natl. Acad. Sci. USA 102, 16842–16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhandari, R., Srinivasan, N., Mahaboobi, M., Ghanekar, Y., Suguna, K. & Visweswariah, S. S. (2001) Biochemistry 40, 9196–9206. [DOI] [PubMed] [Google Scholar]
- 8.Davies, H., Bignell, G. R., Cox, C., Stephens, P., Edkins, S., Clegg, S., Teague, J., Woffendin, H., Garnett, M. J., Bottomley, W., et al. (2002) Nature 417, 949–954. [DOI] [PubMed] [Google Scholar]
- 9.Bence, K., Ma, W., Kozasa, T. & Huang, X. Y. (1997) Nature 389, 296–299. [DOI] [PubMed] [Google Scholar]
- 10.Jiang, Y., Ma, W., Wan, Y., Kozasa, T., Hattori, S. & Huang, X. Y. (1998) Nature 395, 808–813. [DOI] [PubMed] [Google Scholar]
- 11.Ma, Y. C., Huang, J., Ali, S., Lowry, W. & Huang, X. Y. (2000) Cell 102, 635–646. [DOI] [PubMed] [Google Scholar]
- 12.Manser, E., Loo, T. H., Koh, C. G., Zhao, Z. S., Chen, X. Q., Tan, L., Tan, I., Leung, T. & Lim, L. (1998) Mol. Cell 1, 183–192. [DOI] [PubMed] [Google Scholar]
- 13.Bosgraaf, L. & Van Haastert, P. J. (2003) Biochim. Biophys. Acta 1643, 5–10. [DOI] [PubMed] [Google Scholar]
- 14.Cohen, O., Feinstein, E. & Kimchi, A. (1997) EMBO J. 16, 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobe, B. & Kajava, A. V. (2001) Curr. Opin. Struct. Biol. 11, 725–732. [DOI] [PubMed] [Google Scholar]
- 16.Haberland, J. & Gerke, V. (1999) Biochem. J. 343, Part 3, 653–662. [PMC free article] [PubMed] [Google Scholar]
- 17.Smith, T. F., Gaitatzes, C., Saxena, K. & Neer, E. J. (1999) Trends Biochem. Sci. 24, 181–185. [DOI] [PubMed] [Google Scholar]
- 18.Bossy-Wetzel, E., Barsoum, M. J., Godzik, A., Schwarzenbacher, R. & Lipton, S. A. (2003) Curr. Opin. Cell Biol. 15, 706–716. [DOI] [PubMed] [Google Scholar]
- 19.Griffin, E. E., Graumann, J. & Chan, D. C. (2005) J. Cell Biol. 170, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tieu, Q. & Nunnari, J. (2000) J. Cell Biol. 151, 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander, C., Votruba, M., Pesch, U. E., Thiselton, D. L., Mayer, S., Moore, A., Rodriguez, M., Kellner, U., Leo-Kottler, B., Auburger, G., et al. (2000) Nat. Genet. 26, 211–215. [DOI] [PubMed] [Google Scholar]
- 22.Delettre, C., Lenaers, G., Griffoin, J. M., Gigarel, N., Lorenzo, C., Belenguer, P., Pelloquin, L., Grosgeorge, J., Turc-Carel, C., Perret, E., et al. (2000) Nat. Genet. 26, 207–210. [DOI] [PubMed] [Google Scholar]
- 23.Nagar, B., Hantschel, O., Young, M. A., Scheffzek, K., Veach, D., Bornmann, W., Clarkson, B., Superti-Furga, G. & Kuriyan, J. (2003) Cell 112, 859–871. [DOI] [PubMed] [Google Scholar]
- 24.Zhao, Z. S., Manser, E., Chen, X. Q., Chong, C., Leung, T. & Lim, L. (1998) Mol. Cell. Biol. 18, 2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen, J. K. (2004) Nat. Med. 10, Suppl., S18–S25. [DOI] [PubMed] [Google Scholar]
- 26.Melov, S. (2004) Trends Neurosci. 27, 601–606. [DOI] [PubMed] [Google Scholar]
- 27.Beal, M. F. (2002) Free Radical Biol. Med. 32, 797–803. [DOI] [PubMed] [Google Scholar]
- 28.Beal, M. F. (2005) Ann. Neurol. 58, 495–505. [DOI] [PubMed] [Google Scholar]
- 29.Bindoff, L. A., Birch-Machin, M., Cartlidge, N. E., Parker, W. D., Jr., & Turnbull, D. M. (1989) Lancet 2, 49. [DOI] [PubMed] [Google Scholar]
- 30.Schapira, A. H., Cooper, J. M., Dexter, D., Clark, J. B., Jenner, P. & Marsden, C. D. (1990) J. Neurochem. 54, 823–827. [DOI] [PubMed] [Google Scholar]
- 31.Mann, V. M., Cooper, J. M., Krige, D., Daniel, S. E., Schapira, A. H. & Marsden, C. D. (1992) Brain 115, 333–342. [DOI] [PubMed] [Google Scholar]
- 32.Hattori, N., Tanaka, M., Ozawa, T. & Mizuno, Y. (1991) Ann. Neurol. 30, 563–571. [DOI] [PubMed] [Google Scholar]
- 33.Beal, M. F. (2003) Ann. N.Y. Acad. Sci. 991, 120–131. [DOI] [PubMed] [Google Scholar]
- 34.Dauer, W. & Przedborski, S. (2003) Neuron 39, 889–909. [DOI] [PubMed] [Google Scholar]
- 35.Palacino, J. J., Sagi, D., Goldberg, M. S., Krauss, S., Motz, C., Wacker, M., Klose, J. & Shen, J. (2004) J. Biol. Chem. 279, 18614–18622. [DOI] [PubMed] [Google Scholar]
- 36.Greene, J. C., Whitworth, A. J., Kuo, I., Andrews, L. A., Feany, M. B. & Pallanck, L. J. (2003) Proc. Natl. Acad. Sci. USA 100, 4078–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore, D. J., Zhang, L., Troncoso, J., Lee, M. K., Hattori, N., Mizuno, Y., Dawson, T. M. & Dawson, V. L. (2005) Hum. Mol. Genet. 14, 71–84. [DOI] [PubMed] [Google Scholar]
- 38.Canet-Aviles, R. M., Wilson, M. A., Miller, D. W., Ahmad, R., McLendon, C., Bandyopadhyay, S., Baptista, M. J., Ringe, D., Petsko, G. A. & Cookson, M. R. (2004) Proc. Natl. Acad. Sci. USA 101, 9103–9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, L., Shimoji, M., Thomas, B., Moore, D. J., Yu, S. W., Marupudi, N. I., Torp, R., Torgner, I. A., Ottersen, O. P., Dawson, T. M. & Dawson, V. L. (2005) Hum. Mol. Genet. 14, 2063–2073. [DOI] [PubMed] [Google Scholar]
- 40.Yokota, T., Sugawara, K., Ito, K., Takahashi, R., Ariga, H. & Mizusawa, H. (2003) Biochem. Biophys. Res. Commun. 312, 1342–1348. [DOI] [PubMed] [Google Scholar]